Progress on a Novel, 3D-Printable Heart Valve Prosthesis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gott, V.L.; Daggett, R.L.; Young, W.P. Development of a carbon-coated, central-hinging, bileaflet valve. Ann. Thorac. Surg. 1989, 48 (Suppl. 3), S28–S30. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, N.S.; Cooper, T.; Morrow, A.G. Complete replacement of the mitral valve. Successful clinical application of a flexible polyurethane prosthesis. J. Thorac. Cardiovasc. Surg. 1960, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- DeWall, R.A.; Qasim, N.; Carr, L. Evolution of mechanical heart valves. Ann. Thorac. Surg. 2000, 69, 1612–1621. [Google Scholar] [CrossRef]
- Young, W.P.; Daggett, R.L.; Gott, V.L. Long-term follow-up of patients with a hinged leaflet prosthetic heart valve. Prosthet. Heart Valves 1969, 1, 622–632. [Google Scholar]
- Ghanbari, H.; de Mel, A.; Seifalian, A.M. Cardiovascular application of polyhedral oligomeric silsesquioxane nanomaterials: A glimpse into prospective horizons. Int. J. Nanomed. 2011, 6, 775–786. [Google Scholar] [CrossRef]
- Ghanbari, H.; Viatge, H.; Kidane, A.G.; Burriesci, G.; Tavakoli, M.; Seifalian, A.M. Polymeric heart valves: New materials, emerging hopes. Trends Biotechnol. 2009, 27, 359–367. [Google Scholar] [CrossRef]
- Simmons, A.; Hyvarinen, J.; Odell, R.A.; Martin, D.J.; Gunatillake, P.A.; Noble, K.R.; Poole-Warren, L.A. Long-term in vivo biostability of poly(dimethylsiloxane)/poly(hexamethylene oxide) mixed macrodiol-based polyurethane elastomers. Biomaterials 2004, 25, 4887–4900. [Google Scholar] [CrossRef]
- Wheatley, D.J.; Raco, L.; Bernacca, G.M.; Sim, I.; Belcher, P.R.; Boyd, J.S. Polyurethane: Material for the next generation of heart valve prostheses? Eur. J. Cardiothorac. Surg. 2000, 17, 440–448. [Google Scholar] [CrossRef]
- Jiang, H.; Campbell, G.; Boughner, D.; Wan, W.K.; Quantz, M. Design and manufacture of a polyvinyl alcohol (PVA) cryogel tri-leaflet heart valve prosthesis. Med. Eng. Phys. 2004, 26, 269–277. [Google Scholar] [CrossRef]
- Rhamani, B.; Tzamtzis, S.; Ghanbari, H.; Burriesci, G.; Seifalian, A.M. Manufacturing and hydrodynamic assessment of a novel aortic valve made of a new nanocomposite polymer. J. Biomech. 2012, 45, 1205–1211. [Google Scholar] [CrossRef]
- Schroter, F.; Hartrumpf, M.; Kuehnel, R.U.; Ostovar, R.; Albes, J.M. Further Evolution of a New Nonbiological Transcatheter Valvular Prosthesis. Thorac. Cardiovasc. Surg. 2021, 69, 43–48. [Google Scholar] [CrossRef]
- Mohammadi, H.; Boughner, D.; Millon, L.E.; Wan, W.K. Design and simulation of a poly(vinyl alcohol)-bacterial cellulose nanocomposite mechanical aortic heart valve prosthesis. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2009, 223, 697–711. [Google Scholar] [CrossRef]
- Rahmani, B.; Tzamtzis, S.; Sheridan, R.; Mullen, M.J.; Yap, J.; Seifalian, A.M.; Burriesci, G. A new transcatheter heart valve concept (the TRISKELE): Feasibility in an acute preclinical model. EuroIntervention 2016, 12, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Tschorn, P.; Schroter, F.; Hartrumpf, M.; Kuhnel, R.U.; Ostovar, R.; Albes, J.M. Engineering a New Polymeric Heart Valve Using 3D Printing-TRISKELION. Medicina 2022, 5, 1695. [Google Scholar] [CrossRef] [PubMed]
- Kabirian, F.; Mela, P.; Heying, R. 4D Printing Applications in the Development of Smart Cardiovascular Implants. Front. Bioeng. Biotechnol. 2022, 10, 873453. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Yang, W.; Sun, L.; Cai, S.; Yang, R.; Liang, W.; Yu, H.; Liu, L. 4D Printing: A Review on Recent Progresses. Micromachines 2020, 11, 796. [Google Scholar] [CrossRef]
- Miao, S.; Castro, N.; Nowicki, M.; Xia, L.; Cui, H.; Zhou, X.; Zhu, W.; Lee, S.J.; Sarkar, K.; Vozzi, G.; et al. 4D printing of polymeric materials for tissue and organ regeneration. Mater Today 2017, 20, 577–591. [Google Scholar] [CrossRef]
- Gladman, A.S.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef]
- Hua, L.; Xie, M.; Jian, Y.; Wu, B.; Chen, C.; Zhao, C. Multiple-Responsive and Amphibious Hydrogel Actuator Based on Asymmetric UCST-Type Volume Phase Transition. ACS Appl. Mater. Interfaces 2019, 11, 43641–43648. [Google Scholar] [CrossRef]
- Kapyla, E.; Delgado, S.M.; Kasko, A.M. Shape-Changing Photodegradable Hydrogels for Dynamic 3D Cell Culture. ACS Appl. Mater. Interfaces 2016, 8, 17885–17893. [Google Scholar] [CrossRef]
- Rahmatabadi, D.; Aberoumand, M.; Soltanmohammadi, K.; Soleyman, E.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Bodaghi, M.; Baghani, M. Toughening PVC with Biocompatible PCL Softeners for Supreme Mechanical Properties, Morphology, Shape Memory Effects, and FFF Printability. Macromol. Mater. Eng. 2023, 308, 2300114. [Google Scholar] [CrossRef]
- Schichl, K.; Affeld, K. A computer controlled versatile pulse duplicator for precision testing of artificial heart valves. Int. J. Artif. Organs 1993, 16, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Kuehnel, R.U.; Pohl, A.; Puchner, R.; Wendt, M.O.; Hartrumpf, M.; Pohl, M.; Albes, J.M. Opening and closure characteristics of different types of stented biological valves. Thorac. Cardiovasc. Surg. 2006, 54, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Kuehnel, R.U.; Puchner, R.; Pohl, A.; Wendt, M.O.; Hartrumpf, M.; Pohl, M.; Albes, J.M. Characteristic resistance curves of aortic valve substitutes facilitate individualized decision for a particular type. Eur. J. Cardiothorac. Surg. 2005, 27, 450–455; discussion 455. [Google Scholar] [CrossRef] [PubMed]
- Kuehnel, R.U.; Wendt, M.O.; Jainski, U.; Hartrumpf, M.; Pohl, M.; Albes, J.M. Suboptimal geometrical implantation of biological aortic valves provokes functional deficits. Interact. Cardiovasc. Thorac. Surg. 2010, 10, 971–975; discussion 975. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- ISO 5840-2:2021; Cardiovascular implants—Cardiac Valve Prostheses—Part 2: Surgically Implanted Heart Valve Substitutes. ISO: Geneva, Switzerland, 2021.
- Wan, W.K.; Campbell, G.; Zhang, Z.F.; Hui, A.J.; Boughner, D.R. Optimizing the tensile properties of polyvinyl alcohol hydrogel for the construction of a bioprosthetic heart valve stent. J. Biomed. Mater. Res. 2002, 63, 854–861. [Google Scholar] [CrossRef]
- Kannan, R.Y.; Salacinski, H.J.; Edirisinghe, M.J.; Hamilton, G.; Seifalian, A.M. Polyhedral oligomeric silsequioxane-polyurethane nanocomposite microvessels for an artificial capillary bed. Biomaterials 2006, 27, 4618–4626. [Google Scholar] [CrossRef]
- Kannan, R.Y.; Salacinski, H.J.; Ghanavi, J.E.; Narula, A.; Odlyha, M.; Peirovi, H.; Butler, P.E.; Seifalian, A.M. Silsesquioxane nanocomposites as tissue implants. Plast. Reconstr. Surg. 2007, 119, 1653–1662. [Google Scholar] [CrossRef]
- Kiraly, R.; Yozu, R.; Hillegass, D.; Harasaki, H.; Murabayashi, S.; Snow, J.; Nose, Y. Hexsyn trileaflet valve: Application to temporary blood pumps. Artif. Organs 1982, 6, 190–197. [Google Scholar] [CrossRef]
- Roe, B.B.; Kelly, P.B., Jr.; Myers, J.L.; Moore, D.W. Tricuspid leaflet aortic valve prosthesis. Circulation 1966, 33 (Suppl. 4), I124–I130. [Google Scholar] [CrossRef]
- Hinghofer-Szalkay, H.; Greenleaf, J.E. Continuous monitoring of blood volume changes in humans. J. Appl. Physiol. 1987, 63, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Pop, G.A.; Duncker, D.J.; Gardien, M.; Vranckx, P.; Versluis, S.; Hasan, D.; Slager, C.J. The clinical significance of whole blood viscosity in (cardio)vascular medicine. Neth. Heart J. 2002, 10, 512–516. [Google Scholar] [PubMed]
- Nader, E.; Skinner, S.; Romana, M.; Fort, R.; Lemonne, N.; Guillot, N.; Gauthier, A.; Antoine-Jonville, S.; Renoux, C.; Hardy-Dessources, M.D.; et al. Blood Rheology: Key Parameters, Impact on Blood Flow, Role in Sickle Cell Disease and Effects of Exercise. Front. Physiol. 2019, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- Connes, P.; Alexy, T.; Detterich, J.; Romana, M.; Hardy-Dessources, M.D.; Ballas, S.K. The role of blood rheology in sickle cell disease. Blood Rev. 2016, 30, 111–118. [Google Scholar] [CrossRef]
- Pohl, M.; Wendt, M.O.; Werner, S.; Koch, B.; Lerche, D. In vitro testing of artificial heart valves: Comparison between Newtonian and non-Newtonian fluids. Artif. Organs 1996, 20, 37–46. [Google Scholar] [CrossRef]
- Vlastos, G.; Lerche, D.; Koch, B.; Samba, O.; Pohl, M. The effect of parallel combined steady and oscillatory shear flows on blood and polymer solutions. Rheol. Acta 1997, 36, 160–172. [Google Scholar] [CrossRef]
- Campo-Deano, L.; Dullens, R.P.; Aarts, D.G.; Pinho, F.T.; Oliveira, M.S. Viscoelasticity of blood and viscoelastic blood analogues for use in polydymethylsiloxane in vitro models of the circulatory system. Biomicrofluidics 2013, 7, 34102. [Google Scholar] [CrossRef]
- Sousa, P.C.; Pinho, F.T.; Oliveira, M.S.; Alves, M.A. Extensional flow of blood analog solutions in microfluidic devices. Biomicrofluidics 2011, 5, 14108. [Google Scholar] [CrossRef]
- Mei, X.; Zhu, D.; Li, J.; Huang, K.; Hu, S.; Li, Z.; Lopez de Juan Abad, B.; Cheng, K. A fluid-powered refillable origami heart pouch for minimally invasive delivery of cell therapies in rats and pigs. Med 2021, 2, 1253–1268. [Google Scholar] [CrossRef]
- Rahmatabadi, D.; Soltanmohammadi, K.; Pahlavani, M.; Aberoumand, M.; Soleyman, E.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Bodaghi, M.; Baghani, M. Shape memory performance assessment of FDM 3D printed PLA-TPU composites by Box-Behnken response surface methodology. Int. J. Adv. Manuf. Technol. 2023, 127, 935–950. [Google Scholar] [CrossRef]
- Rahmatabadi, D.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Baghani, M. 4D printing of PLA-TPU blends: Effect of PLA concentration, loading mode, and programming temperature on the shape memory effect. J. Mater. Sci. 2023, 58, 7227–7243. [Google Scholar] [CrossRef]
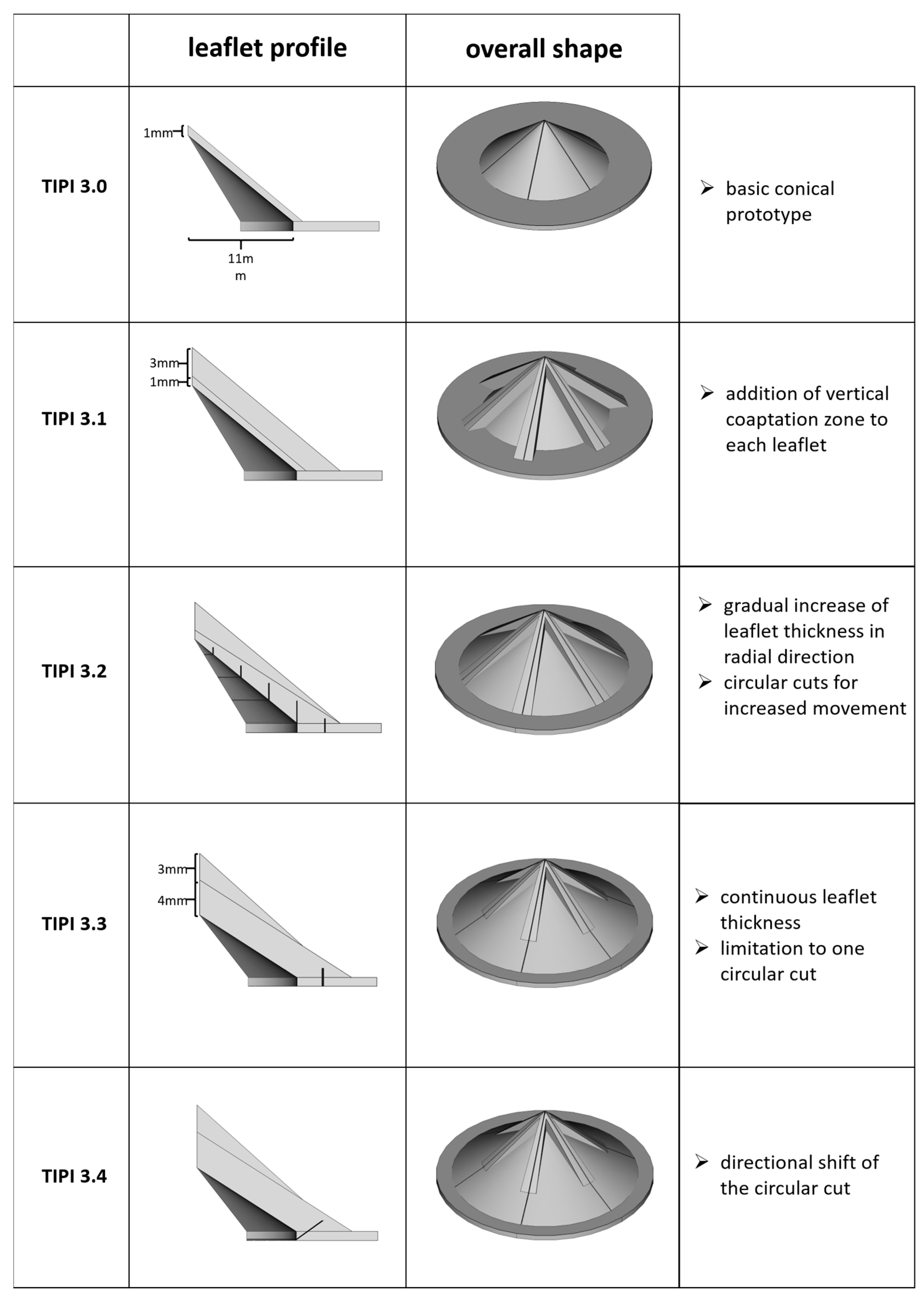

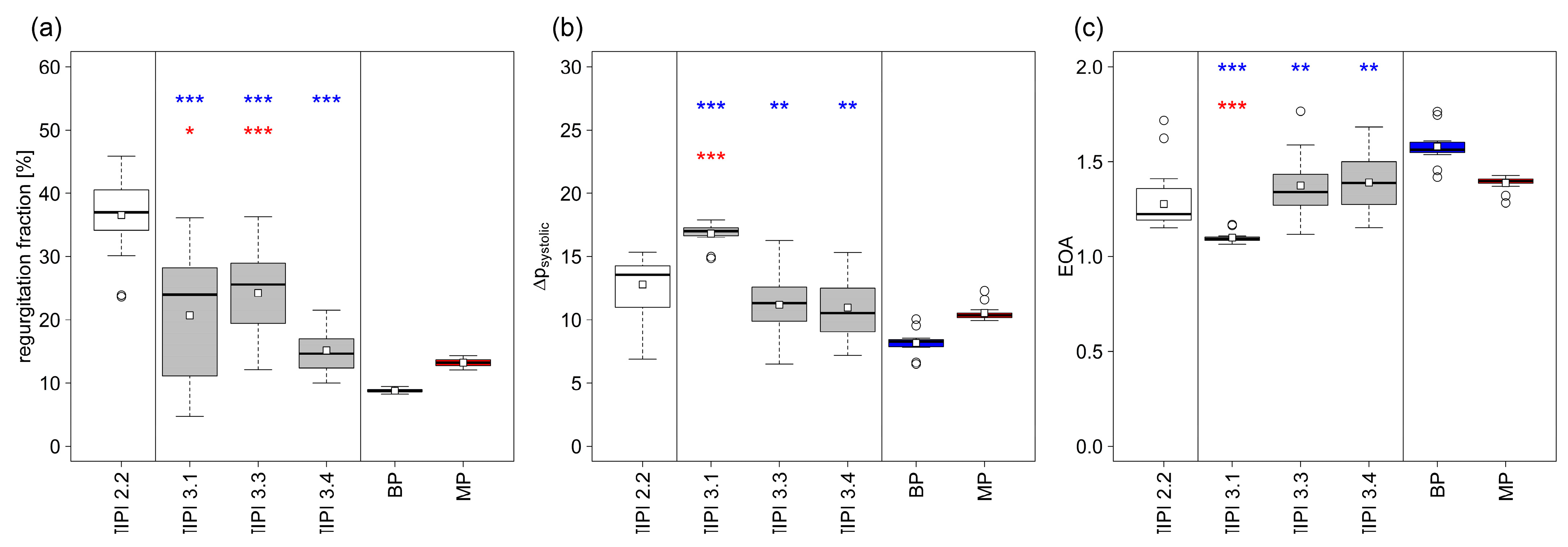
| Closing Time (ms) | Closing Volume (mL) | Leakage (mL) | Cardiac Output (L/min) | Regurgitation Fraction (%) | Δpsystolic (mmHg) | EOA (cm2) | |
|---|---|---|---|---|---|---|---|
| TIPI 2.2 | 102.3+/−25.25 | 9.6+/−2.71 | 15.91+/−2.38 | 3.1+/−0.25 | 36.56+/−5.04 | 12.78+/−2.2 | 1.28 |
| TIPI 3.1 | 39.4+/−5.07 | 3.23+/−0.33 | 11.23+/−7.57 | 3.87+/−0.53 | 20.72+/−10.89 | 16.82+/−0.86 | 1.10 |
| TIPI 3.3 | 89.43+/−21.08 | 8.67+/−2.65 | 8.24+/−3.44 | 3.7+/−0.35 | 24.24+/−7.2 | 10.36+/−3.94 | 1.37 |
| TIPI 3.4 | 80.53+/−20.48 | 7.25+/−2.26 | 3.48+/−1.61 | 4.22+/−0.34 | 15.17+/−3.67 | 10.96+/−2.68 | 1.39 |
| BP | 39.8+/−4.75 | 2.58+/−0.31 | 3.54+/−0.32 | 4.45+/−0.02 | 8.79+/−0.3 | 8.18+/−0.9 | 1.58 |
| MP | 42+/−5.32 | 2.73+/−0.63 | 6.48+/−0.47 | 4.23+/−0.03 | 13.23+/−0.66 | 10.53+/−0.63 | 1.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schröter, F.; Kühnel, R.-U.; Hartrumpf, M.; Ostovar, R.; Albes, J.M. Progress on a Novel, 3D-Printable Heart Valve Prosthesis. Polymers 2023, 15, 4413. https://doi.org/10.3390/polym15224413
Schröter F, Kühnel R-U, Hartrumpf M, Ostovar R, Albes JM. Progress on a Novel, 3D-Printable Heart Valve Prosthesis. Polymers. 2023; 15(22):4413. https://doi.org/10.3390/polym15224413
Chicago/Turabian StyleSchröter, Filip, Ralf-Uwe Kühnel, Martin Hartrumpf, Roya Ostovar, and Johannes Maximilian Albes. 2023. "Progress on a Novel, 3D-Printable Heart Valve Prosthesis" Polymers 15, no. 22: 4413. https://doi.org/10.3390/polym15224413
APA StyleSchröter, F., Kühnel, R.-U., Hartrumpf, M., Ostovar, R., & Albes, J. M. (2023). Progress on a Novel, 3D-Printable Heart Valve Prosthesis. Polymers, 15(22), 4413. https://doi.org/10.3390/polym15224413









