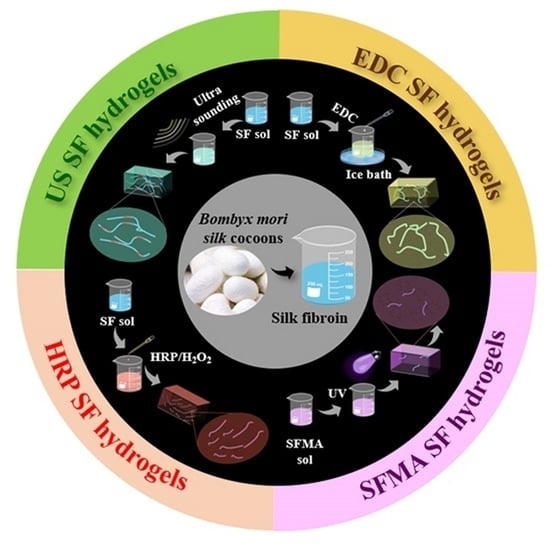
Comparison of Silk Hydrogels Prepared via Different Methods
Abstract
:1. Introduction
2. Materials and Methods
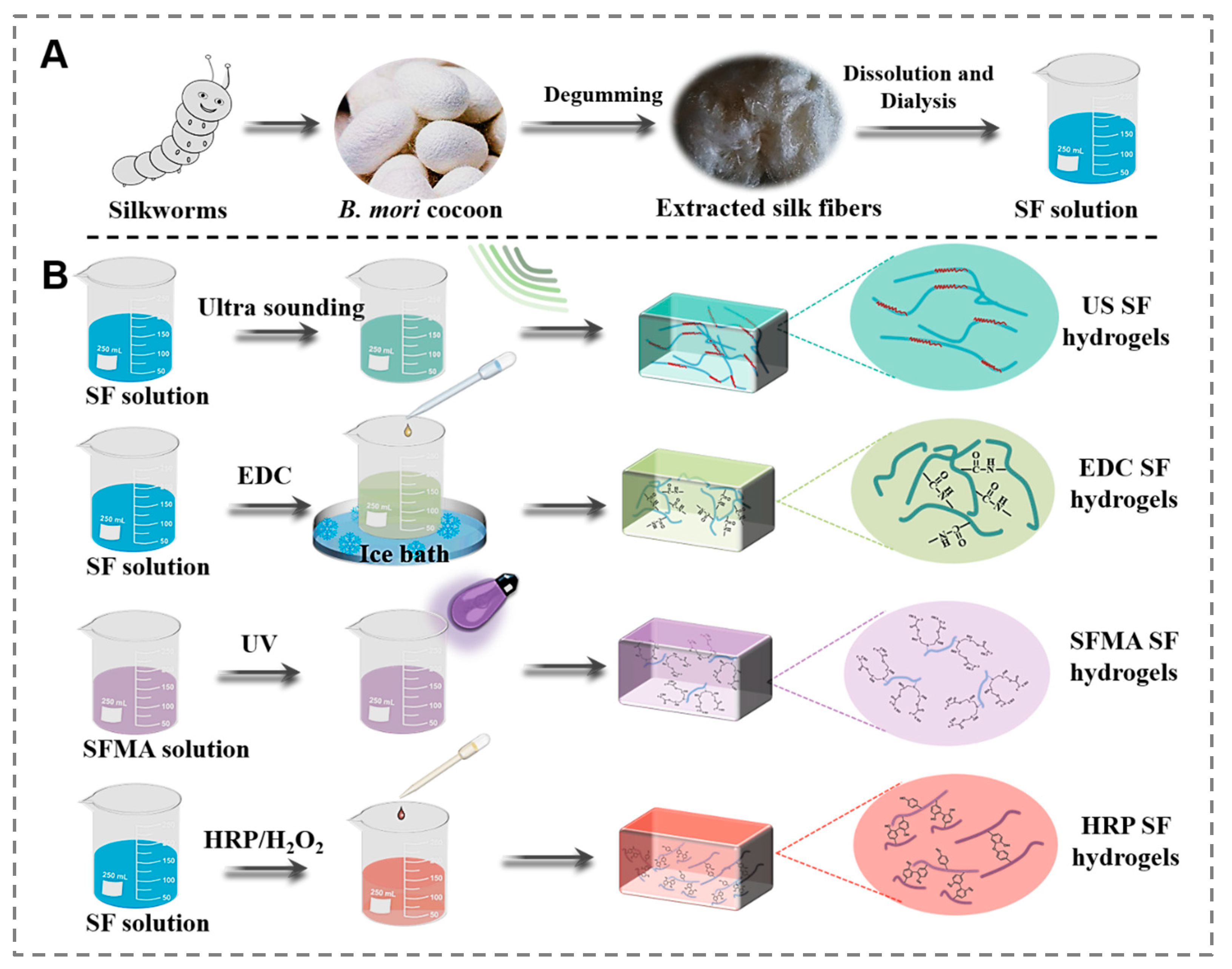
2.1. Preparation of Different SF Hydrogels
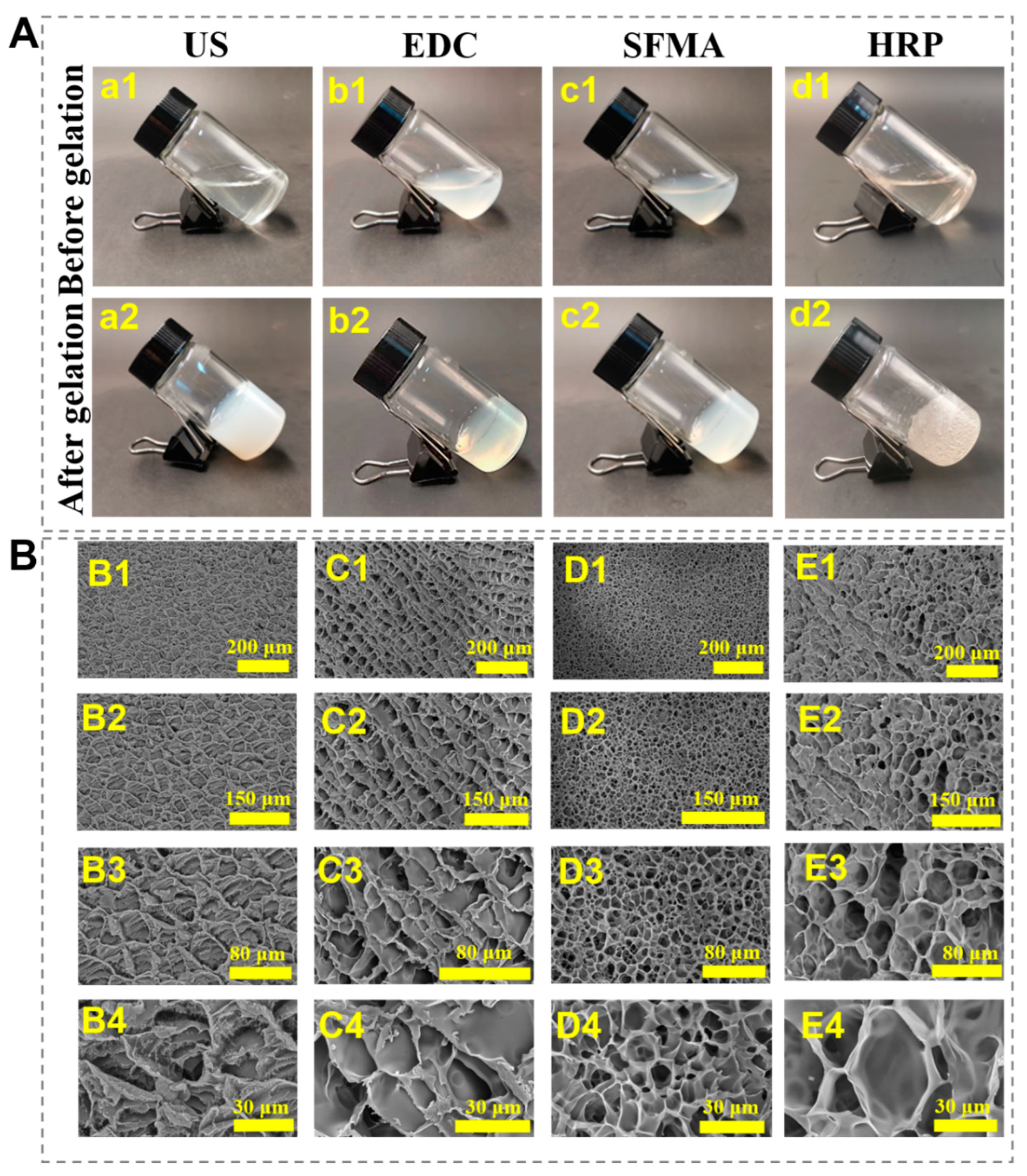
2.2. Morphology Observation of the SF Hydrogels
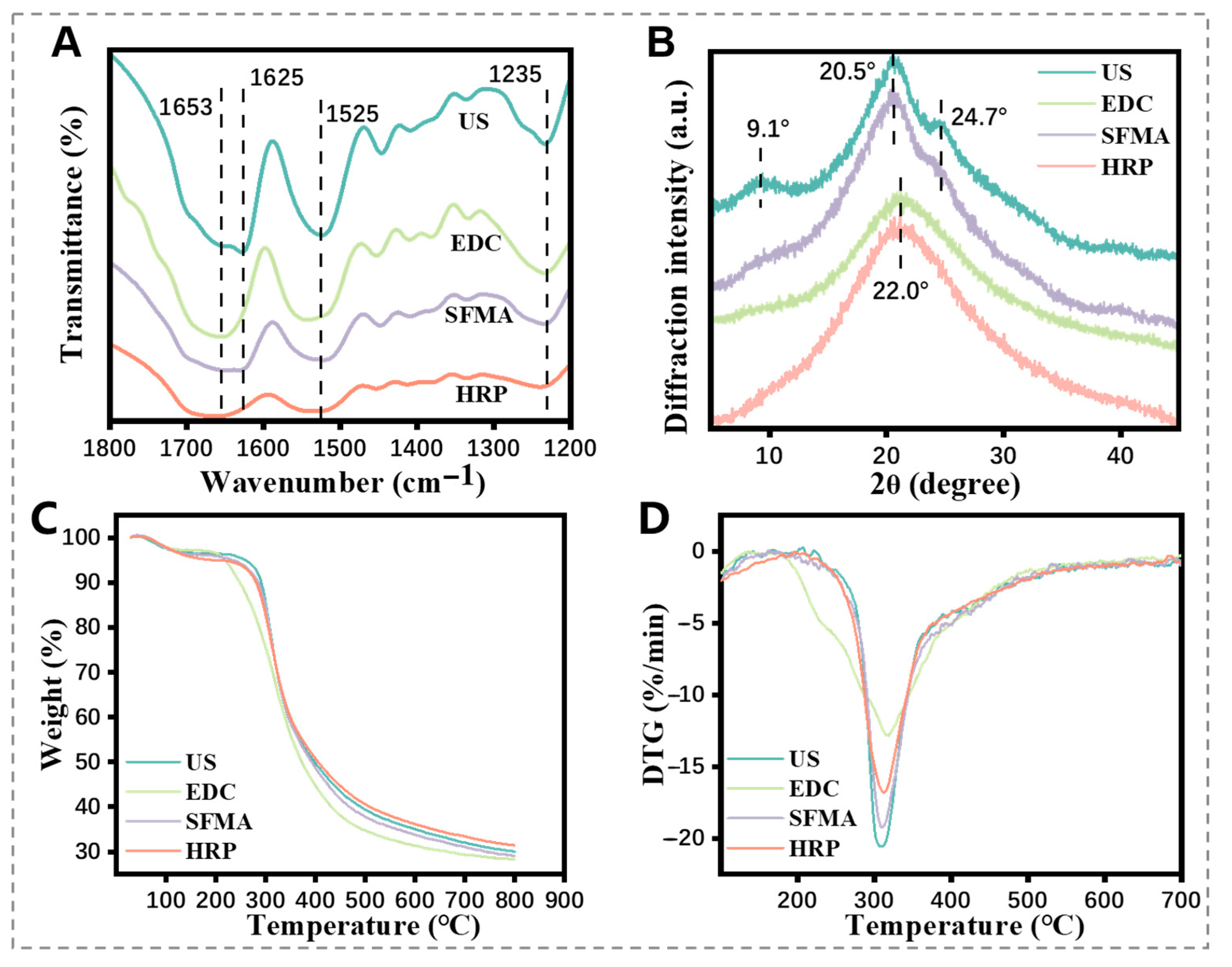
2.3. Structure and Stability Analysis of the SF Hydrogels
2.4. Physical Properties of the SF Hydrogels
2.5. Cell Culture
2.6. Statistical Analysis
3. Results and Discussion
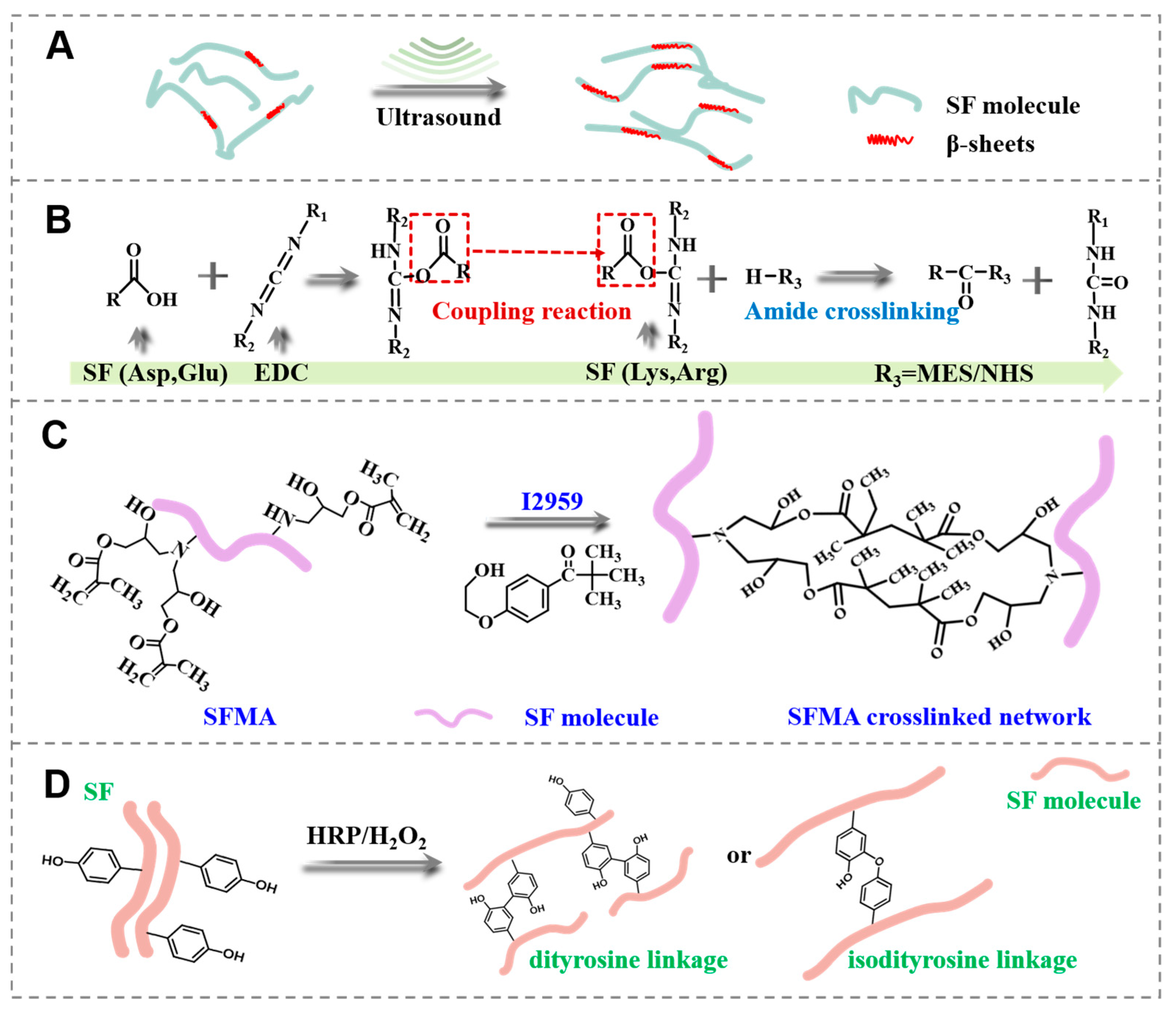
3.1. Design and Preparation of the SF Hydrogels
3.2. Morphology Analysis of the SF Hydrogels
3.3. Structures Analysis of the SF Hydrogels
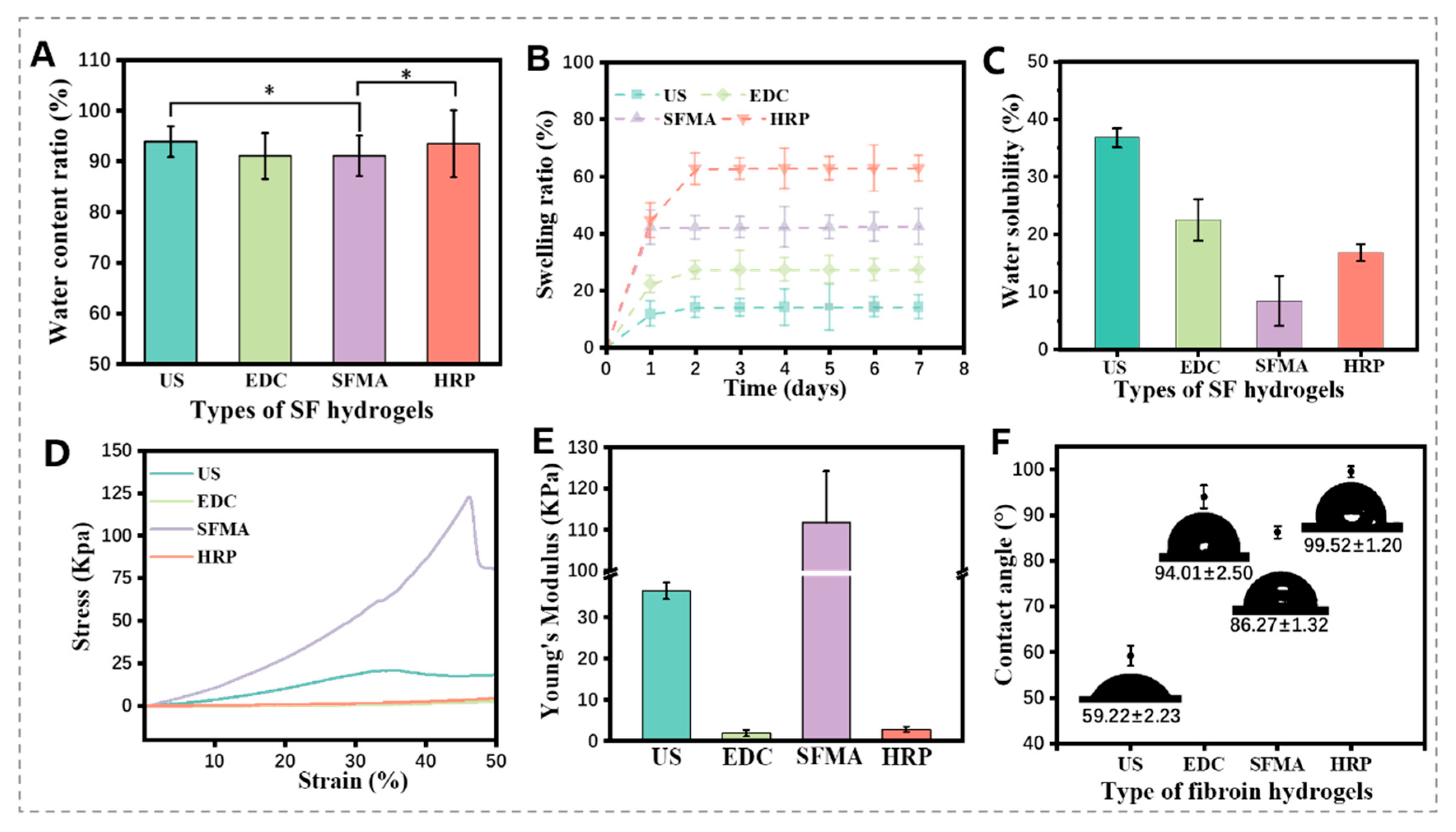
3.4. Physical Properties of the SF Hydrogels
3.5. Gelation Mechanism Analysis
3.6. Cytocompatibility of the SF Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Prince, E.; Kumacheva, E. Design and applications of man-made biomimetic fibrillar hydrogels. Nat. Rev. Mater. 2019, 4, 99–115. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Truskey, G.A.; Fernandez, C.E. Tissue-engineered blood vessels as promising tools for testing drug toxicity. Expert Opin. Drug Metab. Toxicol. 2015, 11, 1021–1024. [Google Scholar] [CrossRef]
- Ayub, Z.H.; Arai, M.; Hirabayashi, K. Mechanism of the Gelation of Fibroin Solution. Biosci. Biotechnol. Biochem. 1993, 57, 1910–1912. [Google Scholar] [CrossRef]
- Nagarkar, S.; Patil, A.; Lele, A.; Bhat, S.; Bellare, J.; Mashelkar, R.A. Some Mechanistic Insights into the Gelation of Regenerated Silk Fibroin Sol. Ind. Eng. Chem. Res. 2009, 48, 8014–8023. [Google Scholar] [CrossRef]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for biomaterials applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Aly, A.S. Self dissolving chitosan, Self-dissolving chitosan, I. preparation, characterization, and evaluation for drug delivery system. Angew. Makromol. Chem. 1998, 259, 13–18. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels—A review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Venezia, V.; Verrillo, M.; Avallone, P.R.; Silvestri, B.; Cangemi, S.; Pasquino, R.; Grizzuti, N.; Spaccini, R.; Luciani, G. Waste to Wealth Approach: Improved Antimicrobial Properties in Bioactive Hydrogels through Humic Substance–Gelatin Chemical Conjugation. Biomacromolecules 2023, 24, 2691–2705. [Google Scholar] [CrossRef]
- Kaewprasit, K.; Takaomi, K.; Siriporn, D. Thai silk fibroin gelation process enhancing by monohydric and polyhydric alcohols. Int. J. Biol. Macromol. 2018, 118, 1726–1735. [Google Scholar] [CrossRef]
- Cui, X.; Soliman, B.G.; Alcala Orozco, C.R. Rapid photocrosslinking of silk hydrogels with high cell density and enhanced shape fidelity. Adv. Healthc. Mater. 2020, 9, 1901667. [Google Scholar] [CrossRef]
- Stoppel, W.L.; Gao, A.E.; Greaney, A.M.; Partlow, B.P.; Bretherton, R.C.; Kaplan, D.L.; Black, L.D. Elastic, silk-cardiac extracellular matrix hydrogels exhibit time-dependent stiffening that modulates cardiac fibroblast response. J. Biomed. Mater. Res. A 2016, 104, 3058–3072. [Google Scholar] [CrossRef]
- Nezhad-Mokhtari, P.; Ghorbani, M.; Roshangar, L.; Rad, J.S. Chemical gelling of hydrogels-based biological macromolecules for biomaterials: Photo- and enzymaticcrosslinking methods. Int. J. Biol. Macromol. 2019, 139, 760–772. [Google Scholar] [CrossRef]
- Zhou, J.; Allonas, X.; Ibrahim, A.; Liu, X. Progress in the development of polymeric and multifunctional photoinitiators. Prog. Polym. Sci. 2019, 99, 101165. [Google Scholar] [CrossRef]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for biomaterials. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lin, R.Z.; Qi, H. Functional human vascular network generated in photocross-linkable gelatin methacrylate hydrogels. Adv. Funct. Mater. 2012, 22, 2027–2039. [Google Scholar] [CrossRef]
- Hu, Z.; Yan, S.; Li, X.; You, R.; Zhang, Q.; Kaplan, D.L. Natural Silk Nanofibril Aerogels with Distinctive Filtration Capacity and Heat-Retention Performance. ACS Nano 2021, 15, 8171–8183. [Google Scholar] [CrossRef]
- Xiao, W.; Li, J.; Qu, X.; Wang, L.; Tan, Y.; Li, K.; Li, H.; Yue, X.; Li, B.; Liao, X. Cell-laden interpenetrating network hydrogels formed from methacrylated gelatin and silk fibroin via a combination of sonication and photocrosslinking approaches. Mater. Sci. Eng. C 2019, 99, 57–67. [Google Scholar] [CrossRef]
- Yan, S.; Wang, L.; Fan, H.; Li, X.; You, H.; You, R.; Zhang, Q.; Xu, W.; Zhang, Y. Biomimetic Natural Silk Nanofibrous Microspheres for Multifunctional Biomedical Applications. ACS Nano 2022, 16, 15115–15123. [Google Scholar] [CrossRef]
- Hu, Z.; Das, S.K.; Yan, S.; You, R.; Li, X.; Luo, Z.; Li, M.; Zhang, Q.; Kaplan, D.L. Stability and biodegradation of silk fibroin/hyaluronic acid nerve conduits. Compos. Part B Eng. 2020, 200, 108222. [Google Scholar] [CrossRef]
- Oliveira, J.T.; Crawford, A.; Mundy, J.L. Novel melt-processable chitosanpolybutylene succinate fiber scaffolds for cartilage tissue engineering. J. Biomater. Sci. Polym. Ed. 2012, 22, 773–788. [Google Scholar] [CrossRef]
- Costa, J.B.; Silva-Correia, J.; Ribeiro, V.P.; da Silva Morais, A.; Oliveira, J.M.; Reis, R.L. Engineering patient-specific bioprinted constructs for treatment of degenerated intervertebral disc. Mater. Today Commun. 2019, 19, 506–512. [Google Scholar] [CrossRef]
- Benjamin, P.; Hanna, C.W.; Kovacina, R.-J.; Moreau, J.E.; Applegate, M.B.; Burke, K.A.; Marelli, B.; Mitropoulos, A.N.; Omenetto, F.G.; Kaplan, D.L. Highly tunable elastomeric silk biomaterials. Adv. Funct. Mater. 2014, 24, 4615–4624. [Google Scholar]
- Sahoo, J.K.; Hasturk, O.; Falcucci, T.; Kaplan, D.L. Silk chemistry and biomedical material designs. Nat. Rev. Chem. 2023, 7, 302–318. [Google Scholar] [CrossRef]
- Kundu, J.; Poole-Warren, L.A.; Martens, P.; Kundu, S.C. Silk fibroin/poly(vinyl alcohol) photo cross-linked hydrogels for delivery of macromolecular drugs. Acta Biomater. 2012, 8, 1720–1729. [Google Scholar] [CrossRef]
- Xiao, W.; He, J.; Nichol, J.W.; Wang, L.; Hutson, C.B.; Wang, B.; Du, Y.; Fan, H.; Khademhosseini, A. Synthesis and characterization of photocrosslinkable gelatin and silk fibroin interpenetrating polymer network hydrogels. Acta Biomater. 2011, 7, 2384–2393. [Google Scholar] [CrossRef]
- Raia, N.R.; Partlow, B.P.; McGill, M.; Kimmerling, E.P.; Ghezzi, C.E.; Kaplan, D.L. Enzymatically crosslinked silk-hyaluronic acid hydrogels. Biomaterials 2017, 131, 58–67. [Google Scholar] [CrossRef]
- Omenetto, F.G.; Kaplan, D.L. A new route for silk. Nat. Photonics 2008, 2, 641–643. [Google Scholar] [CrossRef]
- Wu, X.; Hou, J.; Li, M.; Wang, J.; Kaplan, D.L.; Lu, S. Sodium dodecyl sulfate-induced rapid gelation of silk fibroin. Acta Biomater. 2012, 8, 2185–2192. [Google Scholar] [CrossRef]
- Soc, J. Book Review of Bioconjugate Techniques. Second Edition. J. Med. Chem. 2008, 51, 6619. [Google Scholar]
- Matsumoto, A.; Chen, J.; Collette, A.L.; Kim, U.-J.; Altman, G.H.; Cebe, P.; Kaplan, D.L. Mechanisms of Silk Fibroin Sol−Gel Transitions. J. Phys. Chem. B 2006, 110, 21630–21638. [Google Scholar] [CrossRef] [PubMed]
- Sgalla, S.; Fabrizi, G.; Cacchi, S.; Macone, A.; Bonamore, A.; Boffi, A. Horseradish peroxidase in ionic liquids: Reactions with water insoluble phenolic substrates. J. Mol. Catal. B Enzym. 2007, 44, 144–148. [Google Scholar] [CrossRef]
- Şenol, D. Synthesis, Structural Characterization, Enzymatic and Oxidative Polymerization of 2,6-Diaminopyridine. J. Fluoresc. 2020, 30, 157–174. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Shibata, K.; Uyama, H.; Kobayashi, S. Synthesis of ultrahigh molecular weight phenolic polymers by enzymatic polymerization in the presence of amphiphilic triblock copolymer in water. Polymer 2008, 49, 4791–4795. [Google Scholar] [CrossRef]
- Mu, Q.; Cui, K.; Wang, Z.J.; Matsuda, T.; Cui, W.; Kato, H.; Namiki, S.; Yamazaki, T.; Frauenlob, M.; Nonoyama, T.; et al. Force-triggered rapid microstructure growth on hydrogel surface for on-demand functions. Nat. Commun. 2022, 13, 6213. [Google Scholar] [CrossRef]
- Xu, X.; Jerca, V.V.; Hoogenboom, R. Bioinspired double network hydrogels: From covalent double network hydrogels via hybrid double network hydrogels to physical double network hydrogels. Mater. Horizons 2021, 8, 1173–1188. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, J.; Huang, R.; Huang, Y.; Yan, S.; Zhang, Q. Comparison of Silk Hydrogels Prepared via Different Methods. Polymers 2023, 15, 4419. https://doi.org/10.3390/polym15224419
Hua J, Huang R, Huang Y, Yan S, Zhang Q. Comparison of Silk Hydrogels Prepared via Different Methods. Polymers. 2023; 15(22):4419. https://doi.org/10.3390/polym15224419
Chicago/Turabian StyleHua, Jiahui, Renyan Huang, Ying Huang, Shuqin Yan, and Qiang Zhang. 2023. "Comparison of Silk Hydrogels Prepared via Different Methods" Polymers 15, no. 22: 4419. https://doi.org/10.3390/polym15224419
APA StyleHua, J., Huang, R., Huang, Y., Yan, S., & Zhang, Q. (2023). Comparison of Silk Hydrogels Prepared via Different Methods. Polymers, 15(22), 4419. https://doi.org/10.3390/polym15224419