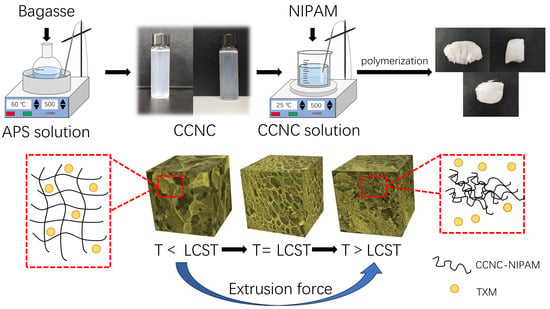
Temperature-Sensitive Aerogel Using Bagasse Carboxylated Cellulose Nanocrystals/N-Isopropyl Acrylamide for Controlled Release of Pesticides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CCNCs
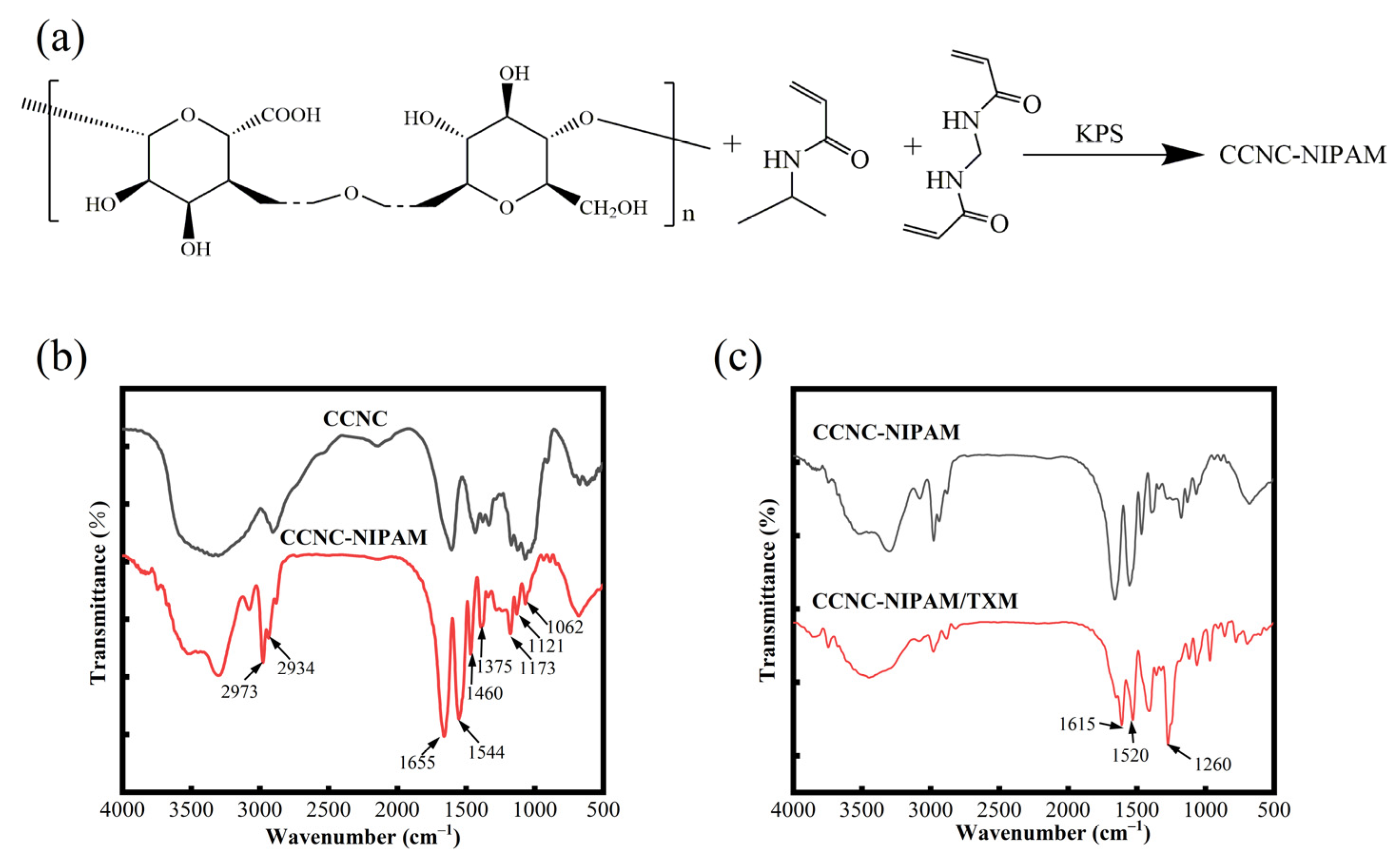
2.3. Preparation of CCNC-NIPAM
2.4. Characterizations
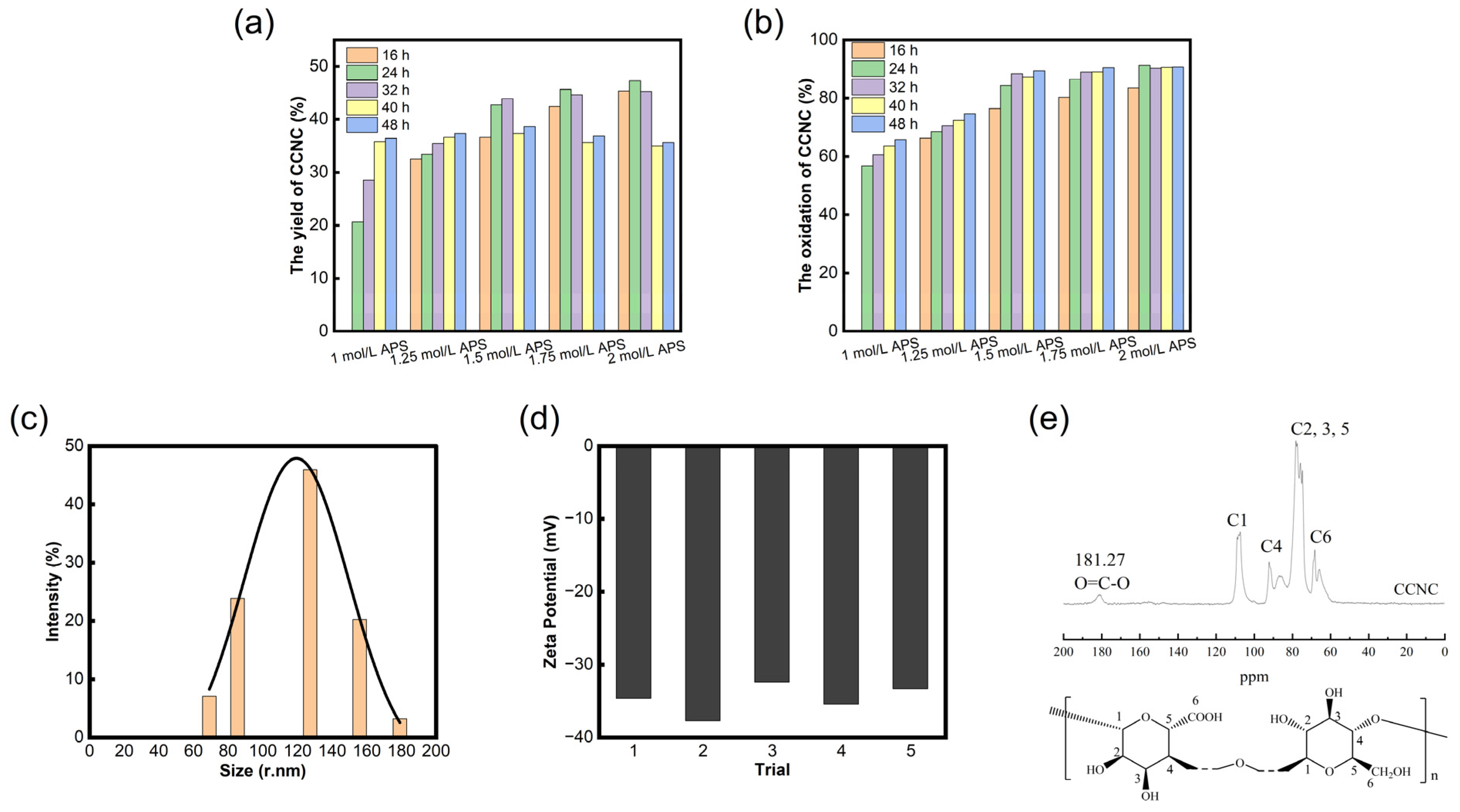
2.5. The Yield of CCNCs
2.6. The Oxidation Degree of CCNCs
2.7. The Porosity of the CCNC-NIPAM
2.8. Swelling and Deswelling Properties of CCNC-NIPAM
2.9. The Loading and Encapsulation Efficiency of CCNC-NIPAM
2.10. Drug Release Behavior
2.11. The Release Kinetics of the CCNC-NIPAM
3. Results
3.1. Characterization of CCNCs
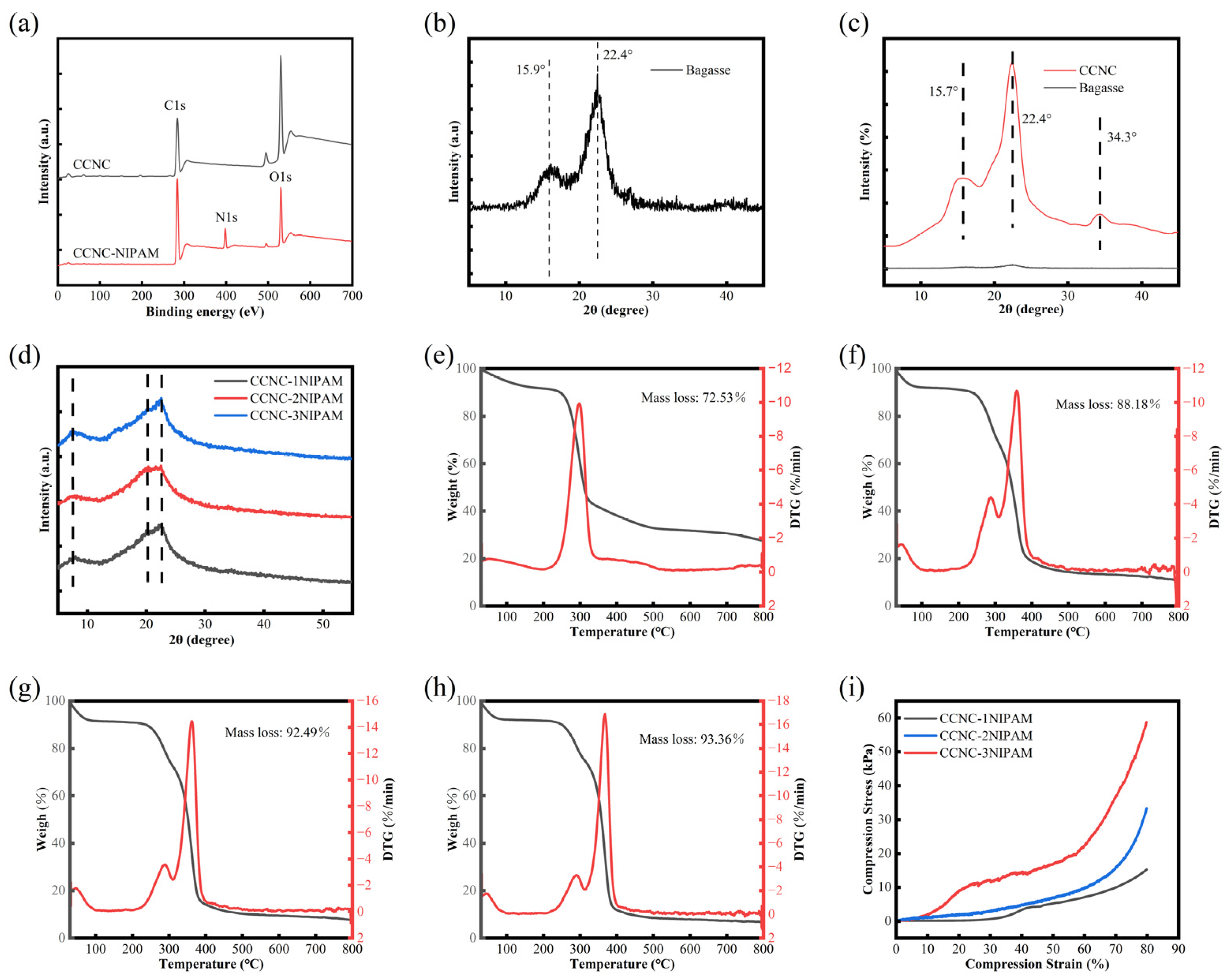
3.2. SEM Analysis
3.3. XPS Analysis
3.4. XRD Analysis
3.5. TG Analysis
3.6. Porosity Analysis
3.7. Compression Performance Analysis
3.8. FTIR Analysis
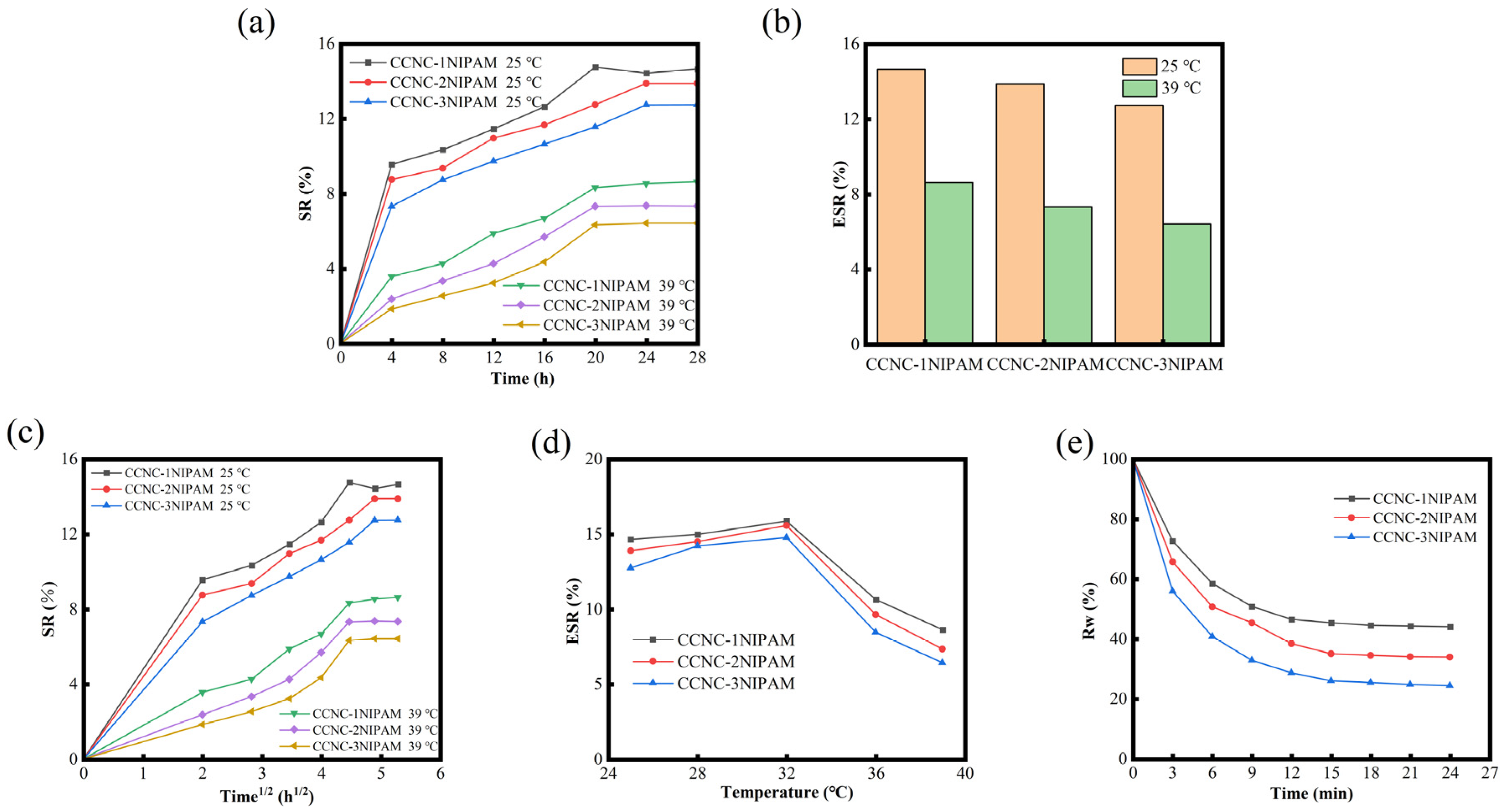
3.9. Swelling and Deswelling Property of CCNC-NIPAM
3.10. Load Efficiency (LE) and Encapsulation Efficiency (EE)
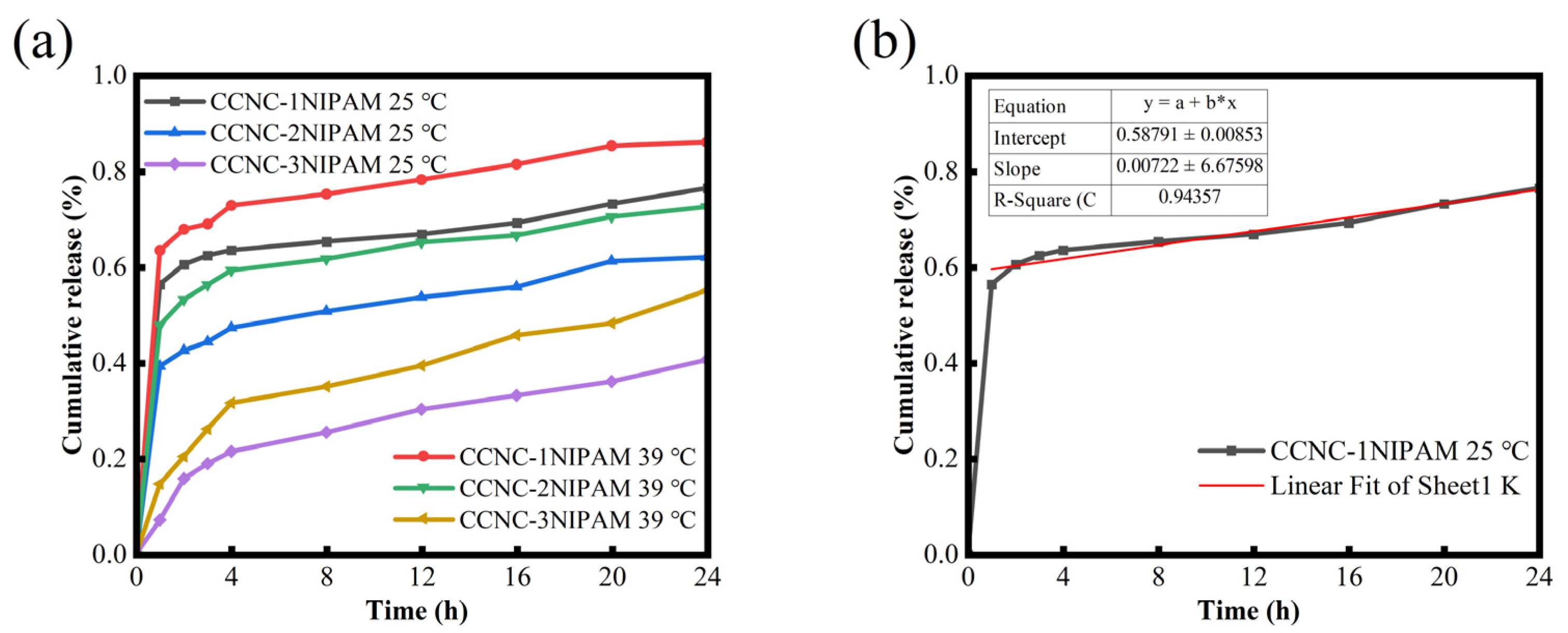
3.11. Drug Release Behavior Analysis
3.12. Analysis of Fitting Results of Release Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aruna; Bagotia, N.; Sharma, A.K.; Kumar, S. A review on modified sugarcane bagasse biosorbent for removal of dyes. Chemosphere 2021, 268, 129309. [Google Scholar] [CrossRef] [PubMed]
- Mokhena, T.C.; Mochane, M.J.; Motaung, T.E.; Linganiso, L.Z.; Songca, S.P. Sugarcane Bagasse and Cellulose Polymer Composites; Sugarcane—Technology and Research: Houma, LA, USA, 2018. [Google Scholar]
- Syverud, K. Tissue Engineering Using Plant-Derived Cellulose Nanofibrils (CNF) as Scaffold Materials. In Proceedings of the Anselme Payen Award Symposium in honor of Akira Isogai on Nanocelluloses, Their Preparation, Properties and Applications/Spring ACS National Meeting, San Diego, CA, USA, 23 October 2016; pp. 171–189. [Google Scholar]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Z.; Zhang, J.; Wang, G.; Zhang, R.; Suo, D. Preparation and Applications of the Cellulose Nanocrystal. Int. J. Polym. Sci. 2019, 2019, 767028. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Zhu, S.; Wu, Q.; Li, J.; Gao, Y.; Wang, B.; Li, J.; Gao, W.; Zeng, J.; et al. Preparation of nanocellulose in high yield via chemi-mechanical synergy. Carbohydr. Polym. 2021, 251, 117094. [Google Scholar] [CrossRef] [PubMed]
- Kandhola, G.; Djioleu, A.; Rajan, K.; Labbé, N.; Sakon, J.; Carrier, D.J.; Kim, J.-W. Maximizing production of cellulose nanocrystals and nanofibers from pre-extracted loblolly pine kraft pulp: A response surface approach. Bioresour. Bioprocess. 2020, 7, 19. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2020, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Jia, S.; Zhang, L.; Zhou, Y.; Lv, Y.; Zhang, X.; Shao, Z. Preparation of treelike and rodlike carboxymethylated nanocellulose and their effect on carboxymethyl cellulose films. J. Appl. Polym. Sci. 2021, 138, 50092. [Google Scholar] [CrossRef]
- Fraschini, C.; Chauve, G.; Bouchard, J. TEMPO-mediated surface oxidation of cellulose nanocrystals (CNCs). Cellulose 2017, 24, 2775–2790. [Google Scholar] [CrossRef]
- He, J.; Liu, S.; Li, L.; Piao, G. Lyotropic liquid crystal behavior of carboxylated cellulose nanocrystals. Carbohydr. Polym. 2017, 164, 364–369. [Google Scholar] [CrossRef]
- Leung, A.C.W.; Hrapovic, S.; Lam, E.; Liu, Y.; Male, K.B.; Mahmoud, K.A.; Luong, J.H.T. Characteristics and Properties of Carboxylated Cellulose Nanocrystals Prepared from a Novel One-Step Procedure. Small 2011, 7, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M.; Dong, W.; Zhang, J. Redox/pH dual stimuli-responsive degradable Salecan-g-SS-poly(IA-co-HEMA) hydrogel for release of doxorubicin. Carbohydr. Polym. 2017, 155, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Z.; Dong, L.; Waterhouse, G.I.; Zhang, Q.; Li, L. A remarkable thermosensitive hydrogel cross-linked by two inorganic nanoparticles with opposite charges. J. Colloid Interface Sci. 2019, 538, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, G.; Leroux, J.-C.; Damas, J.; Adam, A. Biocompatibility of thermosensitive chitosan-based hydrogels: An in vivo experimental approach to injectable biomaterials. Biomaterials 2002, 23, 2717–2722. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.; Bucatariu, S.-M.; Doroftei, F.; Fundueanu, G. Smart composite materials based on chitosan microspheres embedded in thermosensitive hydrogel for controlled delivery of drugs. Carbohydr. Polym. 2017, 157, 493–502. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, S.-H.; Mao, S.-H.; Tsai, M.-J.; Chou, P.-Y.; Liao, C.-H.; Chen, J.-P. Injectable thermosensitive hydrogel containing hyaluronic acid and chitosan as a barrier for prevention of postoperative peritoneal adhesion. Carbohydr. Polym. 2017, 173, 721–731. [Google Scholar] [CrossRef]
- Sun, C.; Boluk, Y.; Ayranci, C. Investigation of nanofiber nonwoven meshes produced by electrospinning of cellulose nanocrystal suspensions in cellulose acetate solutions. Cellulose 2015, 22, 2457–2470. [Google Scholar] [CrossRef]
- Hu, S.; Qin, Z.; Cheng, M.; Chen, Y.; Liu, J.; Zhang, Y. Improved properties and drug delivery behaviors of electrospun cellulose acetate nanofibrous membranes by introducing carboxylated cellulose nanocrystals. Cellulose 2018, 25, 1883–1898. [Google Scholar] [CrossRef]
- Yi, P.; Wang, Y.; He, P.; Zhan, Y.; Sun, Z.; Li, Y.; Zhang, Y. Study on β-cyclodextrin-complexed nanogels with improved thermal response for anticancer drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 773–779. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, J.-J.; Han, C.-R.; Duan, J.-F.; Xu, F.; Sun, R.-C. Tough nanocomposite hydrogels from cellulose nanocrystals/poly(acrylamide) clusters: Influence of the charge density, aspect ratio and surface coating with PEG. Cellulose 2013, 21, 541–551. [Google Scholar] [CrossRef]
- Araki, J. Electrostatic or steric?—Preparations and characterizations of well-dispersed systems containing rod-like nanowhiskers of crystalline polysaccharides. Soft Matter 2013, 9, 4125–4141. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, C.; He, X.; Zhang, X.; Zhang, W.; Zhang, X. Polyethylenimine-Grafted Cellulose Nanofibril Aerogels as Versatile Vehicles for Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 2607–2615. [Google Scholar] [CrossRef]
- Xu, Y.; Qiu, C.; Zhang, X.; Zhang, W. Crosslinking chitosan into H3PO4/HNO3–NANO2 oxidized cellulose fabrics as antibacterial-finished material. Carbohydr. Polym. 2014, 112, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wu, P. A one-step strategy for thermal- and pH-responsive graphene oxide interpenetrating polymer hydrogel networks. J. Mater. Chem. 2011, 21, 4095–4097. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, S.L.; Park, S.N. Properties and in vitro drug release of pH- and temperature-sensitive double cross-linked interpenetrating polymer network hydrogels based on hyaluronic acid/poly (N-isopropylacrylamide) for transdermal delivery of luteolin. Int. J. Biol. Macromol. 2018, 118, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Liu, Y.; Lai, W.; Huang, F.; Ou, A.; Qin, R.; Liu, X.; Wang, X. Ester Crosslinking Enhanced Hydrophilic Cellulose Nanofibrils Aerogel. ACS Sustain. Chem. Eng. 2018, 6, 11979–11988. [Google Scholar] [CrossRef]
- Shao, L.; Cao, Y.; Li, Z.; Hu, W.; Li, S.; Lu, L. Dual responsive aerogel made from thermo/pH sensitive graft copolymer alginate-g-P(NIPAM-co-NHMAM) for drug controlled release. Int. J. Biol. Macromol. 2018, 114, 1338–1344. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Zhao, J.; Xiao, M.; Zhang, W.; Lu, C. Mechanically Strong and Thermally Responsive Cellulose Nanofibers/Poly(N-isopropylacrylamide) Composite Aerogels. ACS Sustain. Chem. Eng. 2016, 4, 4321–4327. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, J.; Cai, P.; Xiao, H. Dual-responsive IPN hydrogel based on sugarcane bagasse cellulose as drug carrier. Int. J. Biol. Macromol. 2018, 118, 132–140. [Google Scholar] [CrossRef]
- Veres, P.; Kéri, M.; Bányai, I.; Lázár, I.; Fábián, I.; Domingo, C.; Kalmár, J. Mechanism of drug release from silica-gelatin aerogel—Relationship between matrix structure and release kinetics. Colloids Surfaces B Biointerfaces 2017, 152, 229–237. [Google Scholar] [CrossRef]

| Sample | I002 | Iam | Cr. I (%) |
|---|---|---|---|
| bagasse | 60 | 20 | 50.0 |
| CCNC | 5591 | 2080 | 62.8 |
| Sample | Porosity (%) |
|---|---|
| CCNC-1NIPAM | 90.29 |
| CCNC-2NIPAM | 87.23 |
| CCNC-3NIPAM | 85.84 |
| Sample | 30 wt% | 50 wt% | 70 wt% | |
|---|---|---|---|---|
| CCNC-1NIPAM | LE (%) | 14.57 | 20.23 | 20.56 |
| EE (%) | 48.57 | 40.46 | 29.37 | |
| CCNC-2NIPAM | LE (%) | 15.93 | 21.48 | 21.99 |
| EE (%) | 53.10 | 42.96 | 31.41 | |
| CCNC-3NIPAM | LE (%) | 16.32 | 22.57 | 23.00 |
| EE (%) | 54.40 | 45.14 | 32.86 |
| Kinetic Models | Coefficient Correlation | CCNC-1NIPAM | CCNC-2NIPAM | CCNC-3NIPAM | |||
|---|---|---|---|---|---|---|---|
| 25 °C | 39 °C | 25 °C | 39 °C | 25 °C | 39 °C | ||
| Zero-order | k0 | 0.0072 | 0.0091 | 0.0094 | 0.0092 | 0.0116 | 0.0153 |
| R2 | 0.9436 | 0.9368 | 0.9543 | 0.8989 | 0.9359 | 0.9266 | |
| First-order | k1 | 0.0225 | 0.0405 | 0.0197 | 0.0245 | 0.0160 | 0.0245 |
| R2 | 0.9550 | 0.9762 | 0.9715 | 0.9444 | 0.9596 | 0.9590 | |
| Korsmeyer -Peppas | k2 (n) | 0.0936 | 0.0863 | 0.1123 | 0.1496 | 0.4051 | 0.3829 |
| R2 | 0.9955 | 0.9840 | 0.9993 | 0.9993 | 0.9755 | 0.9729 | |
| Higuchi | k3 | 0.0433 | 0.0557 | 0.0596 | 0.0566 | 0.0712 | 0.0933 |
| R2 | 0.9514 | 0.9810 | 0.9868 | 0.9609 | 0.9827 | 0.9720 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, N.; Qin, Z.; Li, W.; Xiang, N.; Luo, X.; Ji, H.; Wang, Z.; Xie, X. Temperature-Sensitive Aerogel Using Bagasse Carboxylated Cellulose Nanocrystals/N-Isopropyl Acrylamide for Controlled Release of Pesticides. Polymers 2023, 15, 4451. https://doi.org/10.3390/polym15224451
Dong N, Qin Z, Li W, Xiang N, Luo X, Ji H, Wang Z, Xie X. Temperature-Sensitive Aerogel Using Bagasse Carboxylated Cellulose Nanocrystals/N-Isopropyl Acrylamide for Controlled Release of Pesticides. Polymers. 2023; 15(22):4451. https://doi.org/10.3390/polym15224451
Chicago/Turabian StyleDong, Ni, Zuzeng Qin, Wang Li, Nian Xiang, Xuan Luo, Hongbing Ji, Zhiwei Wang, and Xinling Xie. 2023. "Temperature-Sensitive Aerogel Using Bagasse Carboxylated Cellulose Nanocrystals/N-Isopropyl Acrylamide for Controlled Release of Pesticides" Polymers 15, no. 22: 4451. https://doi.org/10.3390/polym15224451
APA StyleDong, N., Qin, Z., Li, W., Xiang, N., Luo, X., Ji, H., Wang, Z., & Xie, X. (2023). Temperature-Sensitive Aerogel Using Bagasse Carboxylated Cellulose Nanocrystals/N-Isopropyl Acrylamide for Controlled Release of Pesticides. Polymers, 15(22), 4451. https://doi.org/10.3390/polym15224451