Development of Plum Seed-Derived Carboxymethylcellulose Bioink for 3D Bioprinting
Abstract
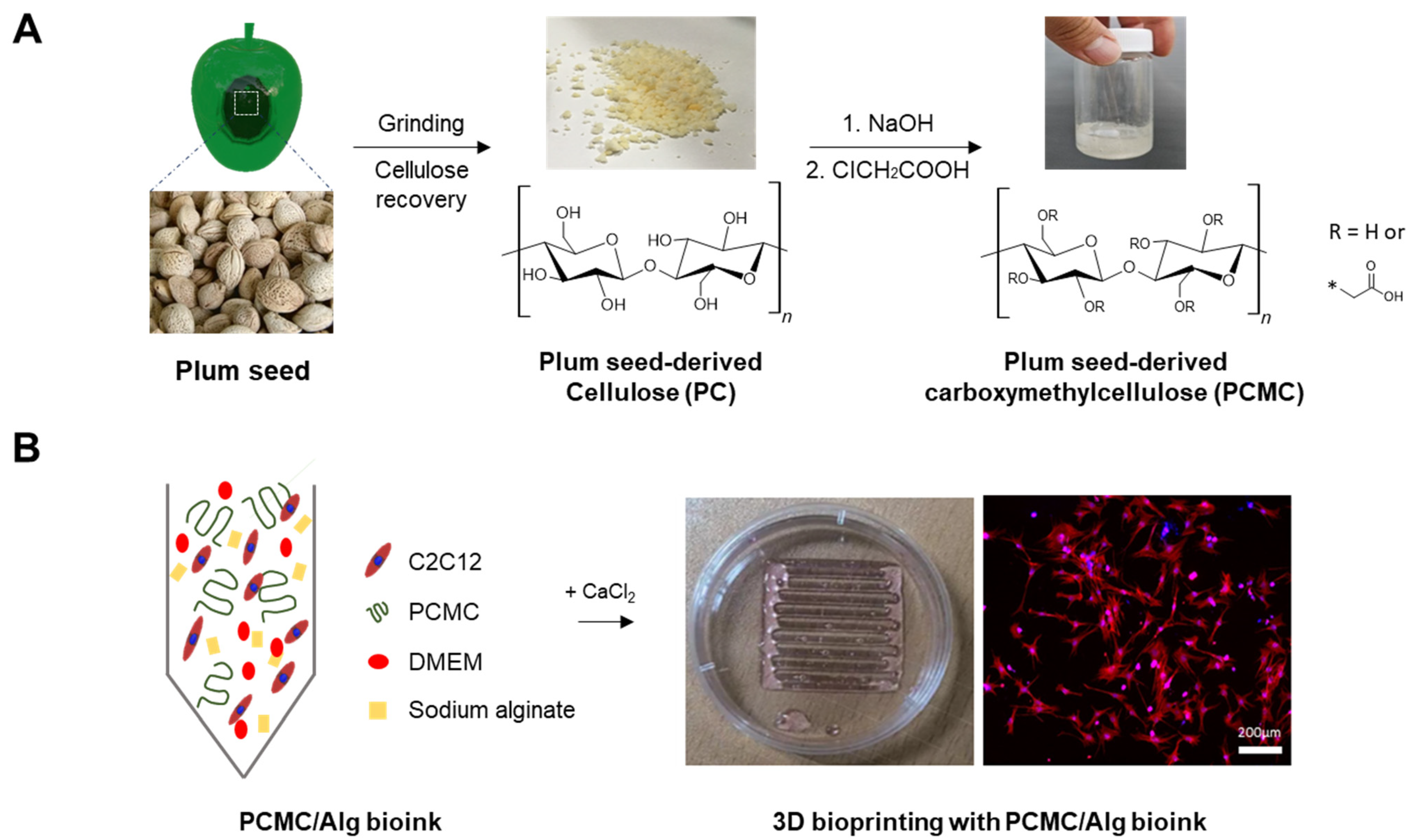
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Recovery of Cellulose
2.3. Synthesis of PCMC
2.4. Characterization of PCMC
2.5. Bioinks Preparation
2.6. Rheological Properties
2.7. Three-Dimensional Printing Ability
2.8. Cell Viability
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of PCMC
3.2. Characterization of PCMC/Alg Bioink
3.3. Three-Dimensional Printing Ability
3.4. Cell Viability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Ambrosi, A.; Pumera, M. 3D-printing technologies for electrochemical applications. Chem. Soc. Rev. 2016, 45, 2740–2755. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- DeBari, M.K.; Keyser, M.N.; Bai, M.A.; Abbott, R.D. 3D printing with silk: Considerations and applications. Connect. Tissue Res. 2020, 61, 163–173. [Google Scholar] [CrossRef]
- Panda, S.; Hajra, S.; Mistewicz, K.; Nowacki, B.; In-Na, P.; Krushynska, A.; Mishra, Y.K.; Kim, H.J. A focused review on three-dimensional bioprinting technology for artificial organ fabrication. Biomater. Sci. 2022, 10, 5054–5080. [Google Scholar] [CrossRef]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J. Pharm. Sci. 2020, 15, 529–557. [Google Scholar] [CrossRef]
- Ferris, C.J.; Gilmore, K.J.; Beirne, S.; McCallum, D.; Wallace, G.G. Bio-ink for on-demand printing of living cells. Biomater. Sci. 2013, 1, 224–230. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Ahadian, S.; Xu, C.; Montazerian, H.; Ko, H.; Nasiri, R.; Barros, N.; Khademhosseini, A. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Mater. Today Bio. 2019, 1, 100008. [Google Scholar] [CrossRef]
- Ho, C.M.B.; Ng, S.H.; Yoon, Y. A review on 3D printed bioimplants. Int. J. Precis. Eng. Manuf. 2015, 16, 1035–1046. [Google Scholar] [CrossRef]
- Semba, J.A.; Mieloch, A.A.; Rybka, J.D. Introduction to the state-of-the-art 3D bioprinting methods, design, and applications in orthopedics. Bioprinting 2020, 18, e00070. [Google Scholar] [CrossRef]
- Kačarević, Ž.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An introduction to 3D bioprinting: Possibilities, challenges and future aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef]
- Mobaraki, M.; Ghaffari, M.; Yazdanpanah, A.; Luo, Y.; Mills, D.K. Bioinks and bioprinting: A focused review. Bioprinting 2020, 18, e00080. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef]
- Lee, S.C.; Gillispie, G.; Prim, P.; Lee, S.J. Physical and chemical factors influencing the printability of hydrogel-based extrusion bioinks. Chem. Rev. 2020, 120, 10834–10886. [Google Scholar] [CrossRef]
- Guvendiren, M.; Molde, J.; Soares, R.M.; Kohn, J. Designing biomaterials for 3D printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef]
- Zandi, N.; Sani, E.S.; Mostafavi, E.; Ibrahim, D.M.; Saleh, B.; Shokrgozar, M.A.; Tamjid, E.; Weiss, P.S.; Simchi, A.; Annabi, N. Nanoengineered shear-thinning and bioprintable hydrogel as a versatile platform for biomedical applications. Biomaterials 2021, 267, 120476. [Google Scholar] [CrossRef]
- Ouyang, L.; Highley, C.B.; Rodell, C.B.; Sun, W.; Burdick, J.A. 3D printing of shear-thinning hyaluronic acid hydrogels with secondary cross-linking. ACS Biomater. Sci. Eng. 2016, 2, 1743–1751. [Google Scholar] [CrossRef]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced bioinks for 3D printing: A materials science perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef]
- Chang, C.C.; Boland, E.D.; Williams, S.K.; Hoying, J.B. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98, 160–170. [Google Scholar] [CrossRef]
- Hacker, M.C.; Krieghoff, J.; Mikos, A.G. Synthetic polymers. In Principles of Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 559–590. [Google Scholar]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurface Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef]
- Sionkowska, A. Collagen blended with natural polymers: Recent advances and trends. Prog. Polym. Sci. 2021, 122, 101452. [Google Scholar] [CrossRef]
- Bhatia, S.; Bhatia, S. Natural polymers vs synthetic polymer. In Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae; Springer: Cham, Germany, 2016; pp. 95–118. [Google Scholar]
- Mandal, B.B.; Kundu, S.C. Osteogenic and adipogenic differentiation of rat bone marrow cells on non-mulberry and mulberry silk gland fibroin 3D scaffolds. Biomaterials 2009, 30, 5019–5030. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef]
- Yeo, M.G.; Kim, G.H. A cell-printing approach for obtaining hASC-laden scaffolds by using a collagen/polyphenol bioink. Biofabrication 2017, 9, 025004. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, S.; Kuss, M.A.; Streubel, P.N.; Duan, B. Effects of hydroxyapatite and hypoxia on chondrogenesis and hypertrophy in 3D bioprinted ADMSC laden constructs. ACS Biomater. Sci. Eng. 2017, 3, 826–835. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Kamel, S.; Ali, N.; Jahangir, K.; Shah, S.M.; El-Gendy, A.A. Pharmaceutical significance of cellulose: A review. Express Polym. Lett. 2008, 2, 758–778. [Google Scholar] [CrossRef]
- Béguin, P.; Aubert, J. The biological degradation of cellulose. FEMS Microbiol. Rev. 1994, 13, 25–58. [Google Scholar] [CrossRef]
- Marchessault, R.H.; Sundararajan, P.R. Cellulose. In The Polysaccharides; Elsevier: Amsterdam, The Netherlands; Academic press: Cambridge, MA, USA, 1983; pp. 11–95. [Google Scholar]
- Håkansson, K.M.; Henriksson, I.C.; de la Peña Vázquez, C.; Kuzmenko, V.; Markstedt, K.; Enoksson, P.; Gatenholm, P. Solidification of 3D printed nanofibril hydrogels into functional 3D cellulose structures. Adv. Mater. Technol. 2016, 1, 1600096. [Google Scholar] [CrossRef]
- Saravanakumar, T.; Park, H.; Mo, A.; Choi, M.; Kim, D.; Park, S. Detoxification of furanic and phenolic lignocellulose derived inhibitors of yeast using laccase immobilized on bacterial cellulosic nanofibers. J. Molec Catal. B 2016, 134, 196–205. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L. Developing fibrillated cellulose as a sustainable technological material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef]
- Abbaspoor, S.; Ashrafi, A.; Salehi, M. Synthesis and characterization of ethyl cellulose micro/nanocapsules using solvent evaporation method. Colloid. Polym. Sci. 2018, 296, 1509–1514. [Google Scholar] [CrossRef]
- Benhamou, A.A.; Kassab, Z.; Boussetta, A.; Salim, M.H.; Ablouh, E.; Nadifiyine, M.; Moubarik, A.; El Achaby, M. Beneficiation of cactus fruit waste seeds for the production of cellulose nanostructures: Extraction and properties. Int. J. Biol. Macromol. 2022, 203, 302–311. [Google Scholar] [CrossRef]
- Sharip, N.S.; Ariffin, H. Cellulose nanofibrils for biomaterial applications. Mater. Today Proc. 2019, 16, 1959–1968. [Google Scholar] [CrossRef]
- Abeer, M.M.; Mohd Amin, M.C.I.; Martin, C. A review of bacterial cellulose-based drug delivery systems: Their biochemistry, current approaches and future prospects. J. Pharm. Pharmacol. 2014, 66, 1047–1061. [Google Scholar] [CrossRef]
- de Amorim, J.D.P.; da Silva Junior, C.J.G.; de Medeiros, A.D.M.; do Nascimento, H.A.; Sarubbo, M.; de Medeiros, T.P.M.; Costa, A.F.d.S.; Sarubbo, L.A. Bacterial Cellulose as a Versatile Biomaterial for Wound Dressing Application. Molecules 2022, 27, 5580. [Google Scholar] [CrossRef]
- da Costa Lopes, A.M.; Bogel-Łukasik, R. Acidic ionic liquids as sustainable approach of cellulose and lignocellulosic biomass conversion without additional catalysts. ChemSusChem 2015, 8, 947–965. [Google Scholar] [CrossRef]
- Habibi, Y.; Mahrouz, M.; Vignon, M.R. Microfibrillated cellulose from the peel of prickly pear fruits. Food Chem. 2009, 115, 423–429. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and characterization of cellulose from different fruit and vegetable pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.K.; Kim, B.; Chung, Y.; Lee, K. Analysis of ethyl carbamate in plum wines produced in Korea. Food Sci. Biotechnol. 2018, 27, 277–282. [Google Scholar] [CrossRef]
- Kwon, J.; Nam, E.; Jun, J.; Chung, K.; Yun, S.; Kim, S.; Do, Y. Asian plum diversity based on phenotypic traits in republic of Korea. Korean J. Plant Resour. 2018, 31, 254–267. [Google Scholar]
- Lakshmi, D.S.; Trivedi, N.; Reddy, C. Synthesis and characterization of seaweed cellulose derived carboxymethyl cellulose. Carbohydr. Polym. 2017, 157, 1604–1610. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, W.; Wu, M.; Rahmaninia, M.; Xu, C.; Li, B. Tailoring Functionality of Nanocellulose: Current Status and Critical Challenges. Nanomaterials 2023, 13, 1489. [Google Scholar] [CrossRef]
- Habib, A.; Sathish, V.; Mallik, S.; Khoda, B. 3D printability of alginate-carboxymethyl cellulose hydrogel. Materials 2018, 11, 454. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, Y.J.; Kang, P.H.; Jeun, J.P. Synthesis and Adsorption Properties of Carboxymethyl Lignin with Irradiated Lignin by Electron Beam. Polymer 2016, 40, 70–76. [Google Scholar] [CrossRef]
- Yadollahi, M.; Gholamali, I.; Namazi, H.; Aghazadeh, M. Synthesis and Characterization of Antibacterial Carboxymethylcellulose/CuO Bio-Nanocomposite Hydrogels. Int. J. Biol. Macromol. 2015, 73, 109–114. [Google Scholar] [CrossRef]
- Salimi, E.; Nigje, A.K. Investigating the Antibacterial Activity of Carboxymethyl Cellulose Films Treated with Novel Ag@ GO Decorated SiO2 Nanohybrids. Carbohydr. Polym. 2022, 298, 120077. [Google Scholar] [CrossRef]
- Yadollahi, M.; Gholamali, I.; Namazi, H.; Aghazadeh, M. Synthesis and Characterization of Antibacterial Carboxymethyl Cellulose/ZnO Nanocomposite Hydrogels. Int. J. Biol. Macromol. 2015, 74, 136–141. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Lee, S.; Lim, J.W.; Byun, I.; Jang, K.-J.; Kim, J.-W.; Chung, J.H.; Kim, J.; Seonwoo, H. Development of Plum Seed-Derived Carboxymethylcellulose Bioink for 3D Bioprinting. Polymers 2023, 15, 4473. https://doi.org/10.3390/polym15234473
Lee J, Lee S, Lim JW, Byun I, Jang K-J, Kim J-W, Chung JH, Kim J, Seonwoo H. Development of Plum Seed-Derived Carboxymethylcellulose Bioink for 3D Bioprinting. Polymers. 2023; 15(23):4473. https://doi.org/10.3390/polym15234473
Chicago/Turabian StyleLee, Juo, Sungmin Lee, Jae Woon Lim, Iksong Byun, Kyoung-Je Jang, Jin-Woo Kim, Jong Hoon Chung, Jungsil Kim, and Hoon Seonwoo. 2023. "Development of Plum Seed-Derived Carboxymethylcellulose Bioink for 3D Bioprinting" Polymers 15, no. 23: 4473. https://doi.org/10.3390/polym15234473
APA StyleLee, J., Lee, S., Lim, J. W., Byun, I., Jang, K.-J., Kim, J.-W., Chung, J. H., Kim, J., & Seonwoo, H. (2023). Development of Plum Seed-Derived Carboxymethylcellulose Bioink for 3D Bioprinting. Polymers, 15(23), 4473. https://doi.org/10.3390/polym15234473






