Determination of Tetracycline Antibiotics in Milk by Solid-Phase Extraction Using a Coordination Polymer Based on Cobalt Trimesinate as a Sorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Cobalt Trimesinate
2.3. Characterization
2.4. Tetracycline Adsorption Study
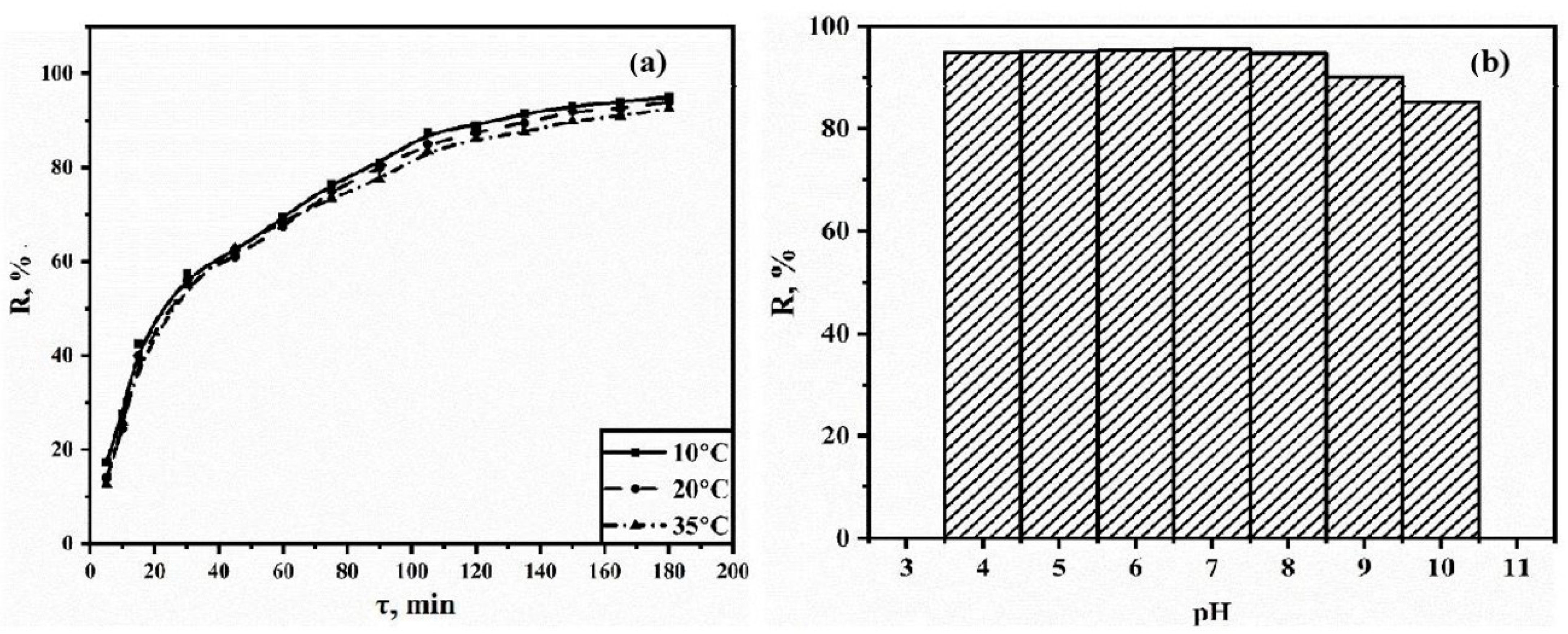
2.4.1. Dependence of Adsorption on the pH of the Medium
2.4.2. Dependence of Adsorption on Tetracycline Concentration
2.4.3. Number of Working Cycles
2.4.4. Study of Adsorption on a Real Object
3. Results and Discussion
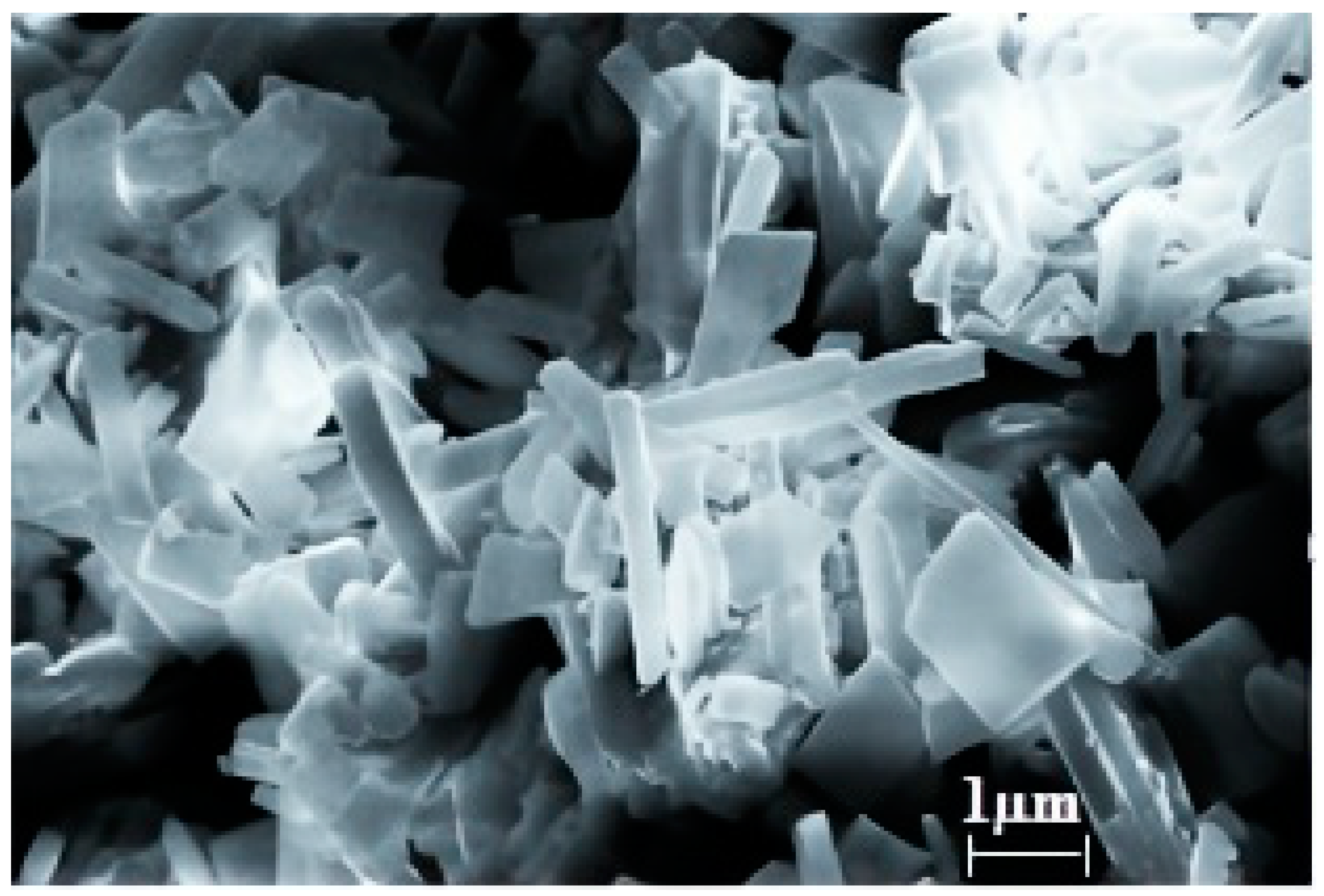
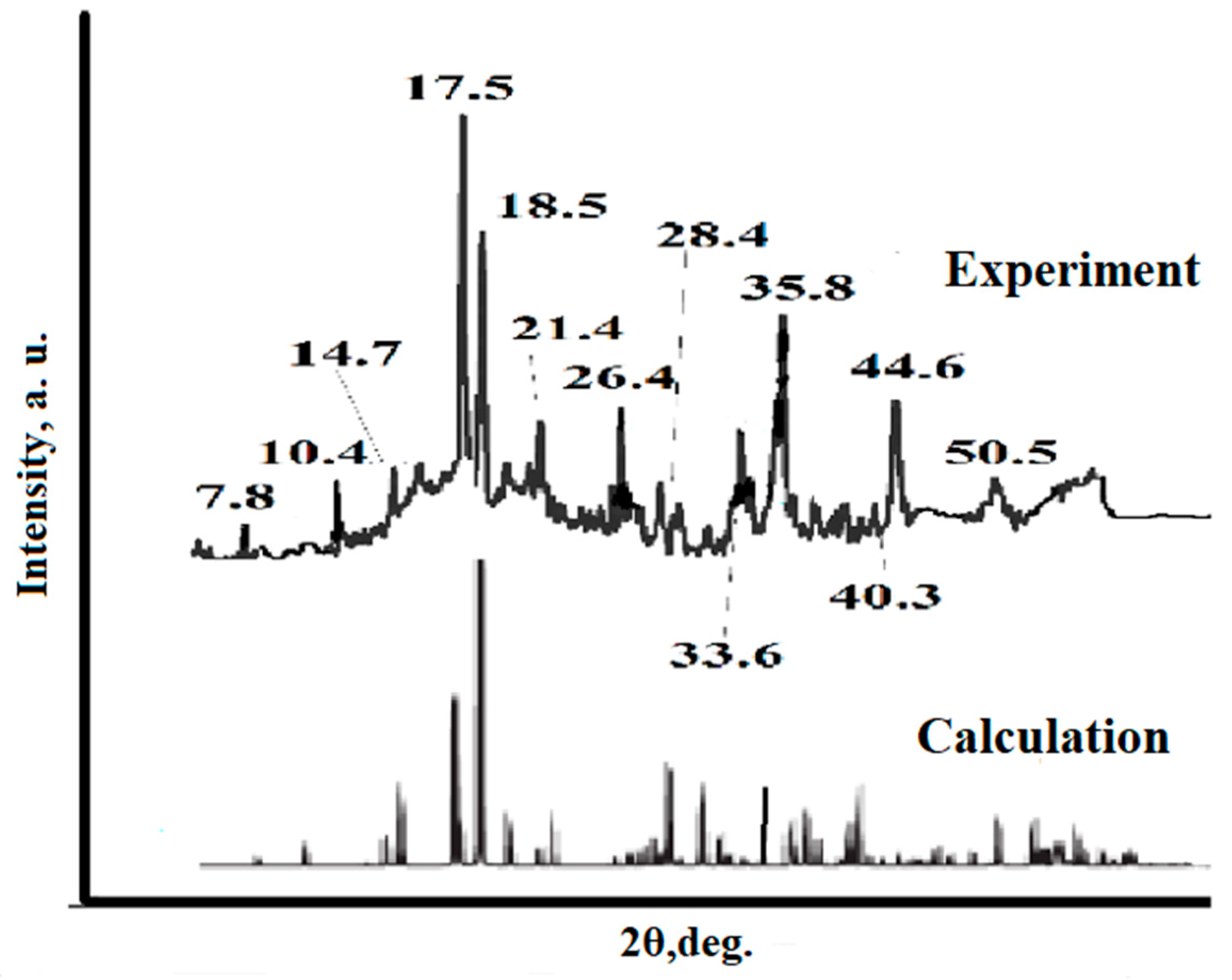
3.1. Synthesis and Study of the Sorbent
3.2. Solid-Phase Extraction of Tetracycline
3.3. Adsorption Isotherms
3.3.1. Langmuir Adsorption Model
3.3.2. Freundlich Adsorption Model
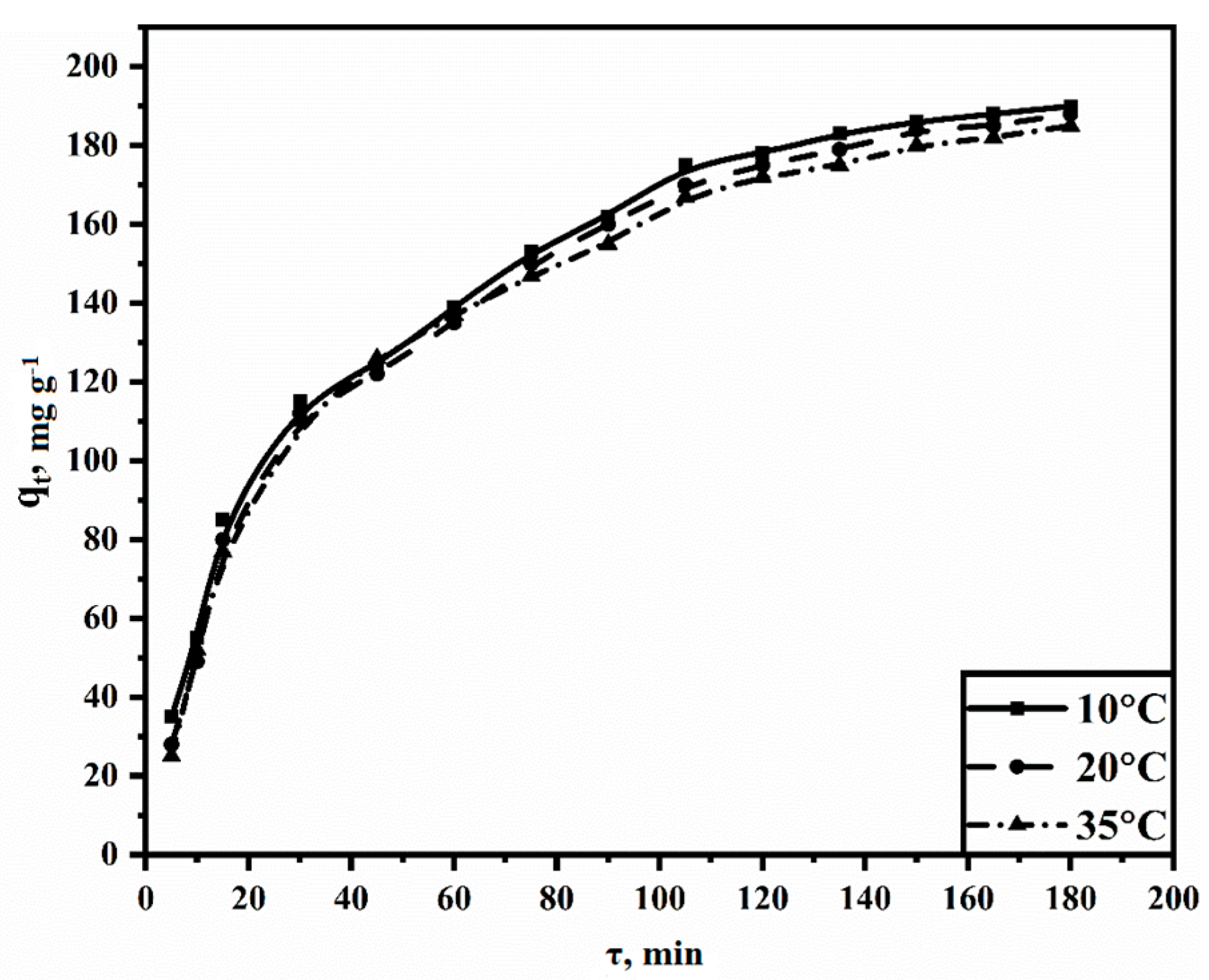
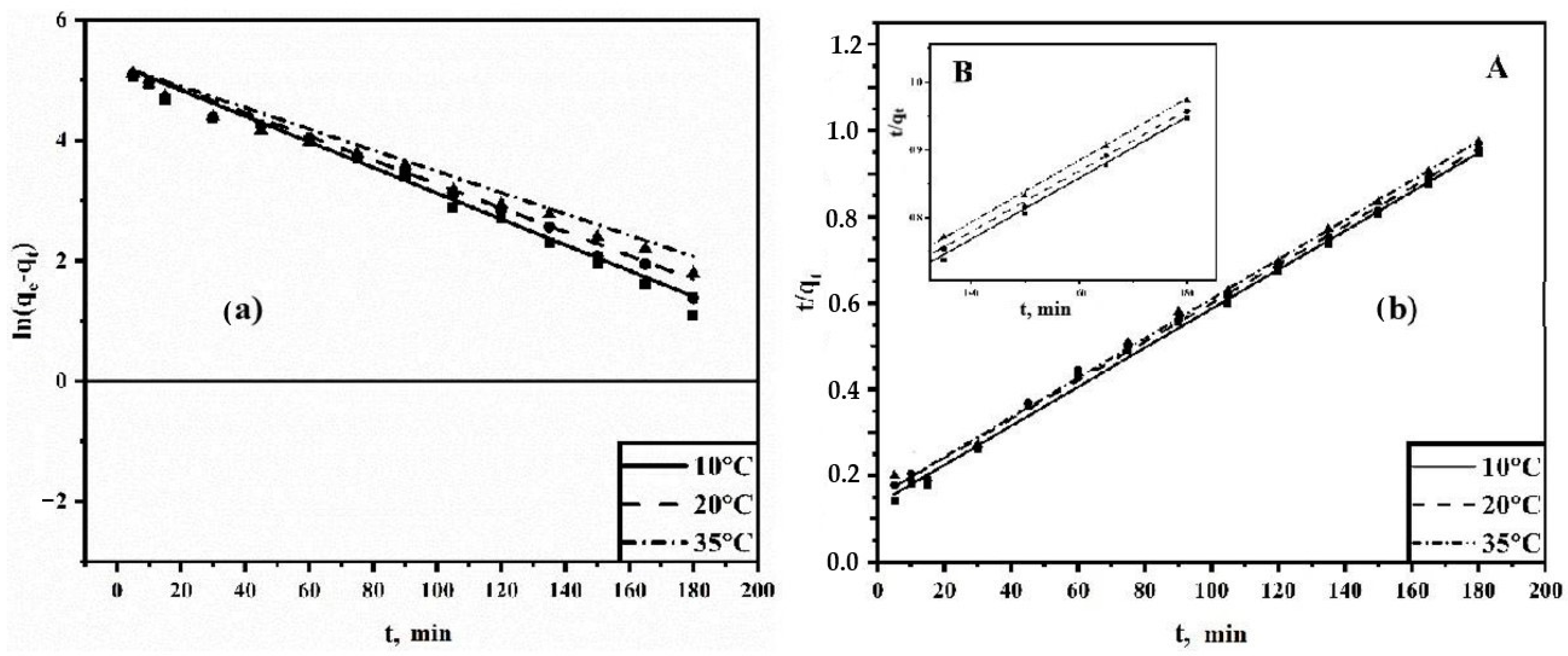
3.3.3. Adsorption Kinetics
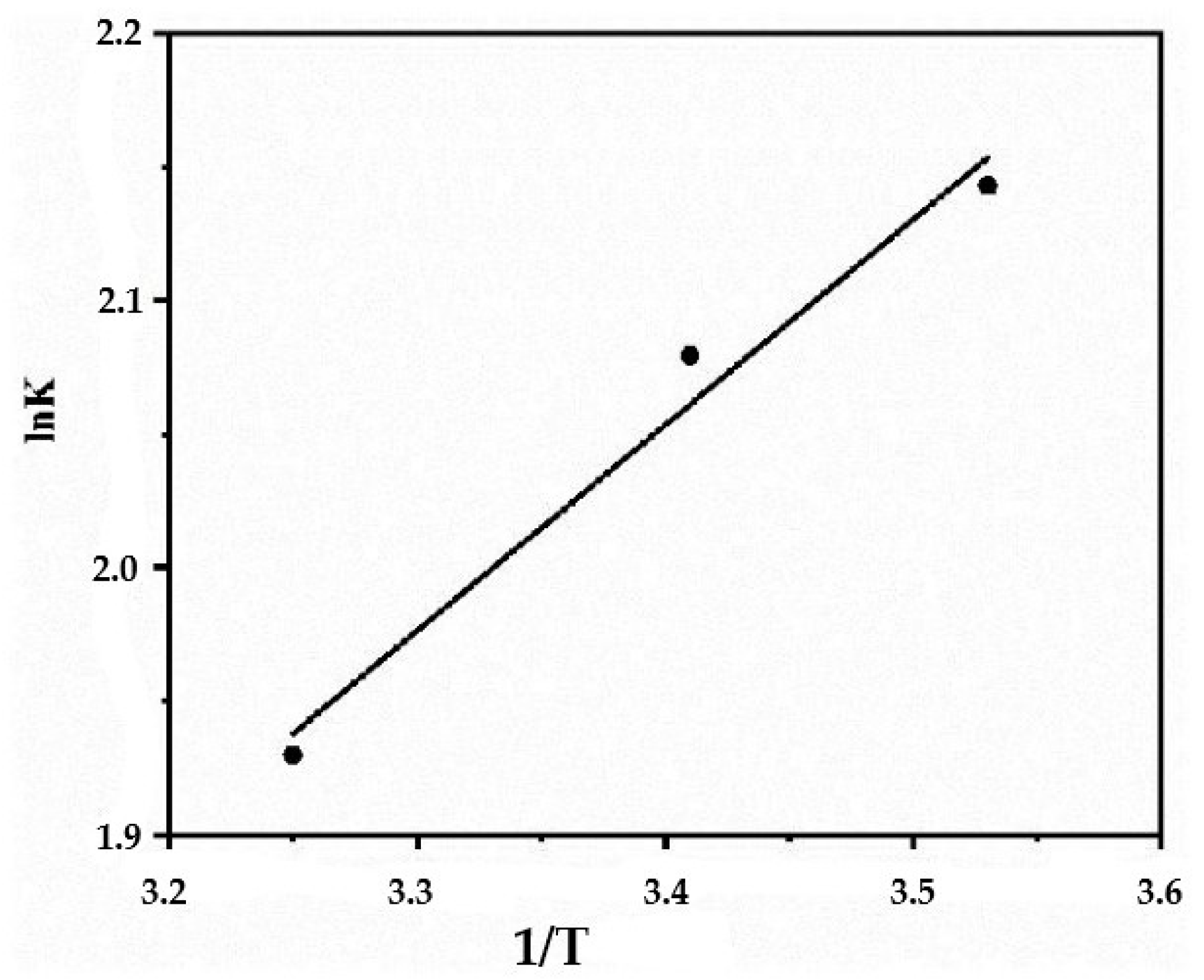
3.4. Thermodynamics of Adsorption
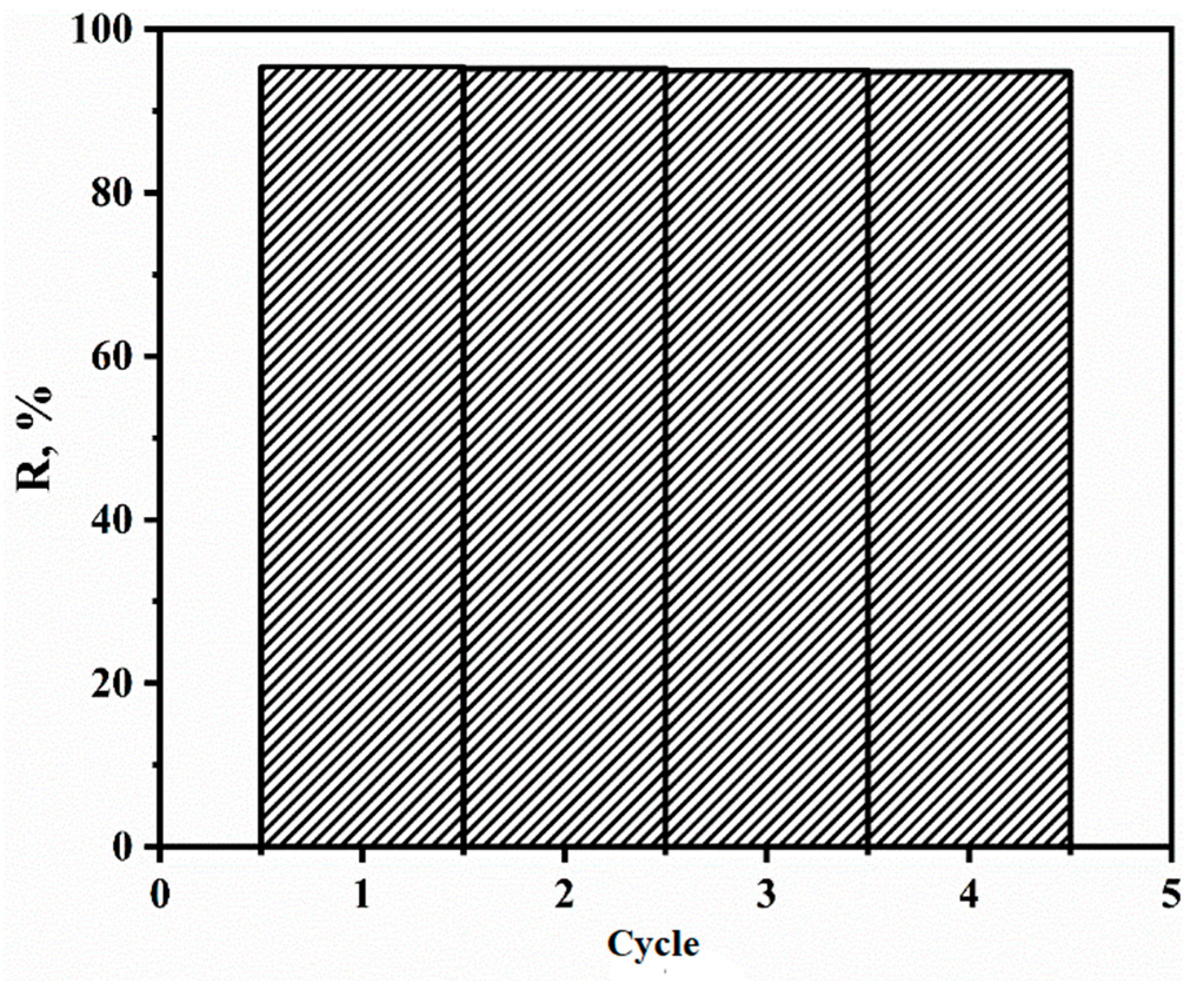
3.5. Number of Extraction Cycles after Regeneration
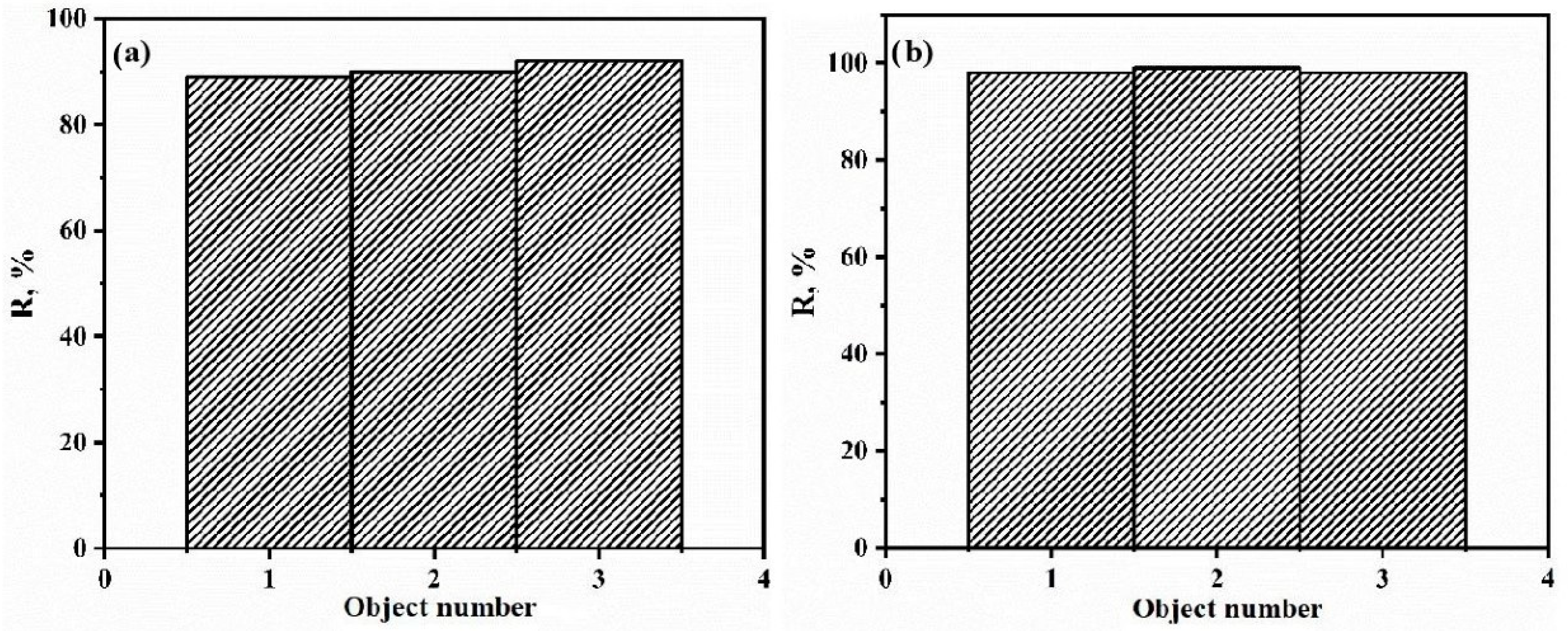
3.6. Determination of the Sorption Activity of CP on Real Objects
- Repeatability index (σr);
- Intra-laboratory reproducibility (σR);
- Accuracy index (±Δc);
- Accuracy rate (±Δ).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, H.; Mi, H.Y.; Guan, M.M.; Shan, H.Y.; Fei, Q.; Huan, Y.F. Residue analysis of tetracyclines in milk by HPLC coupled with hollow fiber membranes-based dynamic liquid-liquid micro-extraction. Food Chem. 2017, 232, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Arabsorkhi, B.; Sereshti, H.; Abbasi, A. Electrospun metal-organic framework/polyacrylonitrile composite nanofibrous mat as a microsorbent for the extraction of tetracycline residue in human blood plasma. J. Sep. Sci. 2019, 42, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Lebedinets, S.; Vakh, C.; Cherkashina, K. Stir membrane liquid phase microextraction of tetracyclines using switchable hydrophilicity solvents followed by high-performance liquid chromatography. J. Chromatogr. A 2019, 1615, 460743. [Google Scholar] [CrossRef] [PubMed]
- Castillo-García, M.L.; Aguilar-Caballos, M.P.; Gómez-Hens, A. A europium- and terbium-coated magnetic nanocomposite as sorbent in dispersive solid phase extraction coupled with ultra-high performance liquid chromatography for antibiotic determination in meat samples. J. Chromatogr. A 2015, 1425, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lin, K.; Huang, X.; Chen, M. A Simple and Fast Extraction Method for the Determination of Multiclass Antibiotics in Eggs Using LC-MS/MS. J. Agric. Food Chem. 2017, 65, 5064–5073. [Google Scholar] [CrossRef]
- Gaudin, V.; Rault, A.; Verdon, E. Validation of a commercial receptor kit for tetracycline residues in honey according to the European guideline for screening methods. Food Agric. Immunol. 2013, 24, 111–128. [Google Scholar] [CrossRef]
- Cháfer-Pericás, C.; Maquieira, Á.; Puchades, R.; Miralles, J.; Moreno, A.; Pastor-Navarro, N. Immunochemical determination of oxytetracycline in fish: Comparison between enzymatic and time-resolved fluorometric assays. Anal. Chim. Acta 2010, 662, 177–185. [Google Scholar] [CrossRef]
- Yakout, A.A.; El-Hady, D.A. A combination of β-cyclodextrin functionalized magnetic graphene oxide nanoparticles with β-cyclodextrin-based sensor for highly sensitive and selective voltammetric determination of tetracycline and doxycycline in milk samples. RSC Adv. 2016, 6, 41675–41686. [Google Scholar] [CrossRef]
- Ni, Y.; Li, S.; Kokot, S. Simultaneous voltammetric analysis of tetracycline antibiotics in foods. Food Chem. 2011, 124, 1157–1163. [Google Scholar] [CrossRef]
- Islas, G.; Rodriguez, J.A.; Perez-Silva, I.; Miranda, J.M. Solid-phase extraction and large-volume sample stacking-capillary electrophoresis for determination of tetracycline residues in milk. J. Anal. Methods Chem. 2018, 2018, 5394527. [Google Scholar] [CrossRef]
- Pang, Y.Q.; Cui, H.; Zheng, H.S.; Wan, G.H.; Liu, L.J. Flow injection analysis of tetracyclines using inhibited Ru(bpy)32+/tripropylamine electrochemiluminescence system. Luminescence 2005, 20, 8–15. [Google Scholar] [CrossRef]
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. (Eds.) Principles of Instrumental Analysis; Crouch Cengage Learning: Boston, MA, USA, 2017; 992p. [Google Scholar]
- Pastor-Navarro, N.; Maquieira, Á.R.; Puchades, R. Review on immunoanalytical determination of tetracycline and sulfonamide residues in edible products. Anal. Bioanal. Chem. 2009, 395, 907–920. [Google Scholar] [CrossRef]
- Erkes, D.A.; Selvan, S.R. Hapten-induced contact hypersensitivity, autoimmune reactions, and tumor regression: Plausibility of mediating antitumor immunity. J. Immunol. Res. 2014, 2014, 175265. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Rupp, H.S.; Wu, W.H. Complexities in tetracycline analysis—Chemistry, matrix extraction, cleanup, and liquid chromatography. J. Chromatogr. A 2005, 1075, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Oka, H.; Ito, Y.; Matsumoto, H. Chromatographic analysis of tetracycline antibiotics in foods. J. Chromatogr. A 2000, 882, 109–133. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, V.N.; Zui, M.F. Preconcentration by solid-phase microextraction. J. Anal. Chem. 2014, 69, 715–727. [Google Scholar] [CrossRef]
- Wardencki, W.; Michulec, M.; Curyło, J. A review of theoretical and practical aspects of solid-phase microextraction in food analysis. Int. J. Food Sci. Technol. 2004, 39, 703–717. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Liu, X.; Zhao, Y.; Wang, P.; Wang, Z.; Sun, W. Novel cadmium(II) frameworks with mixed carboxylate and imidazole-containing ligands for selective detection of antibiotics. Polyhedron 2018, 154, 350–356. [Google Scholar] [CrossRef]
- Fedotov, P.S.; Malofeeva, G.I.; Savonina, E.Y.; Spivakov, B.Y. Solid-Phase Extraction of Organic Substances: Unconventional Methods and Approaches. J. Anal. Chem. 2019, 74, 205–212. [Google Scholar] [CrossRef]
- Du, F.; Sun, L.; Tan, W.; Wei, Z.; Nie, H.; Huang, Z. Magnetic stir cake sorptive extraction of trace tetracycline antibiotics in food samples: Preparation of metal–organic framework-embedded polyHIPE monolithic composites, validation and application. Anal. Bioanal. Chem. 2019, 411, 2239–2248. [Google Scholar] [CrossRef]
- Grazhulene, S.S.; Zolotareva, N.I.; Redkin, A.N. Magnetic nanocomposites based on carbon nanotubes: Promising sorbents for analytical and technological purposes. Lab. Prod. 2019, 2019, 108–112. [Google Scholar] [CrossRef]
- Khan, M.S.; Li, Y.; Li, D.-S.; Qiu, J.; Xu, X.; Yang, H.Y. A review of metal–organic framework (MOF) materials as an effective photocatalyst for degradation of organic pollutants. Nanoscale Adv. 2023, 5, 6318–6348. [Google Scholar] [CrossRef]
- Jin, J.; Yang, Z.; Xiong, W.; Zhou, Y.; Xu, R.; Zhang, Y. Cu and Co nanoparticles co-doped MIL-101 as a novel adsorbent for efficient removal of tetracycline from aqueous solutions. Sci. Total Environ. 2019, 650, 408–418. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Fan, X.; Quan, X.; Tan, F.; Zhang, Y. Adsorption of ciprofloxacin, bisphenol and 2-chlorophenol on electrospun carbon nanofibers: In comparison with powder activated carbon. J. Colloid Interface Sci. 2015, 447, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, B.; Li, H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J. Hazard. Mater. 2015, 282, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Huang, W.; Chen, B.; Zhao, Y.; Liu, D.; Sun, Y. Removal of tetracycline from aqueous solution by MCM-41-zeolite A loaded nano zero valent iron: Synthesis, characteristic, adsorption performance and mechanism. J. Hazard. Mater. 2017, 339, 22–32. [Google Scholar] [CrossRef]
- Xiong, W.; Zeng, G.; Yang, Z.; Zhou, Y.; Zhang, C. Adsorption of tetracycline antibiotics from aqueous solutions on nanocomposite multi-walled carbon nanotube functionalized MIL-53 (Fe) as new adsorbent. Sci. Total Environ. 2018, 627, 235–244. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Zhinzhilo, V.A.; Bryantseva, J.D.; Uflyand, I.E.; Kharisov, B.I. ZrIV metal-organic framework based on terephthalic acid and 1,10-phenanthroline as adsorbent for solid-phase extraction of tetracycline antibiotics. Mendeleev Commun. 2022, 32, 661–663. [Google Scholar] [CrossRef]
- Neff, H.; Laborde, H.M.; Lima, A.M.N. Periodic protein adsorption at the gold/biotin aqueous solution interface: Evidence of kinetics with time delay. Sci. Rep. 2016, 6, 36232. [Google Scholar] [CrossRef]
- Dybtsev, D.N.; Yutkin, M.P.; Peresypkina, E.V.; Virovets, A.V.; Hasegawa, Y.; Nishihara, H.; Fedin, V.P. Synthesis, structure, and magnetic properties of the cobalt(II) 1,3,5-benzenetricarboxylate layered coordination polymer. Russ. Chem. Bull. 2007, 56, 1782–1786. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H.; Groy, T.L. Construction of porous solids from hydrogen-bonded metal complexes of 1,3,5-benzenetricarboxylic acid. J. Am. Chem. Soc. 1996, 118, 9096–9101. [Google Scholar] [CrossRef]
- Wang, S.; Boyjoo, Y.; Choueib, A. A comparative study of dye removal using fly ash treated by different methods. Chemosphere 2005, 60, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H.; Krishni, R.R.; Sata, S.A. A novel agricultural waste adsorbent for the removal of cationic dye from aqueous solutions. J. Hazard. Mater. 2009, 162, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Halsey, G.D. The role of surface heterogeneity in adsorption. Adv. Catal. 1952, 4, 259–269. [Google Scholar]
- Wang, S.; Ma, R.; Mazzu, Y.; Zhang, J.; Li, W.; Tan, L.; Zhou, L.; Xia, Z.; Zhang, Q.; Yuan, C. Specific adsorption of tetracycline from milk by using biocompatible magnetic molecular imprinting material and evaluation by ECD. Food Chem. 2020, 326, 126969. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, C.; Li, Y.; Ke, H.; Cheng, G. Efficient removal of tetracycline hydrochloride from aqueous solution by mesoporous cage MOF-818. SN Appl. Sci. 2020, 2, 669. [Google Scholar] [CrossRef]
- Saremi, F.; Miroliaei, M.; Nejad, M.S.; Sheibani, H. Adsorption of tetracycline antibiotic from aqueous solutions onto vitamin B6-upgraded biochar derived from date palm leaves. J. Mol. Liq. 2020, 318, 114126. [Google Scholar] [CrossRef]
- Yu, F.; Ma, J.; Han, S. Adsorption of tetracycline from aqueous solutions onto multi-walled carbon nanotubes with different oxygen contents. Sci. Rep. 2014, 4, 5326. [Google Scholar] [CrossRef]
- Simonin, J.P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]





| Element | Found, % | Calculated for Co3(L)2, % |
|---|---|---|
| C | 36.8 | 36.56 |
| H | 0.99 | 1.01 |
| Co | 30.2 | 29.92 |
| Model | T, °C | ||
|---|---|---|---|
| 10 | 20 | 35 | |
| Langmuir | qmax = 400.25 mg g−1 KL = 0.25 L mg−1 R2 = 0.956 | qmax = 391.53 mg g−1 KL = 0.1562 L mg−1 R2 = 0.978 | qmax = 386.92 mg g−1 KL = 0.1184 L mg−1 R2 = 0.946 |
| Freundlich | 1/n = 0.811 KF = 62.74 R2 = 0.995 | 1/n = 0.813 KF = 48.47 R2 = 0.996 | 1/n = 0.804 KF = 38.37 R2 = 0.997 |
| Adsorbent | qmax, mg g−1 | References |
|---|---|---|
| cobalt trimesinate | 400.25 | this work |
| zirconium terephthalate | 139.40 | [27] |
| BMMIPs * based on Fe3O4 | 236.40 | [36] |
| MOF 818 | 442.5 | [37] |
| palm-based biochar | 76.92 | [38] |
| carbon nanotubes | 269.25 | [38] |
| carbon nanotubes | 210.43 | [39] |
| Model | T, °C | ||
|---|---|---|---|
| 10 | 20 | 35 | |
| qe = 193.10 mg g−1 k1 = 0.02128 min−1 k2 = 0.00020 | qe = 192.80 mg g−1 k1 = 0.01907 min−1 k2 = 0.00018 | qe = 191.20 mg g−1 k1 = 0.01753 min−1 k2 = 0.00018 | |
| Pseudo-first order | R2 = 0.9633 | R2 = 0.9604 | R2 = 0.9589 |
| Pseudo-second order | R2 = 0.9901 | R2 = 0.9973 | R2 = 0.9976 |
| T, °C | Kc | ΔG0, kJ mol−1 | ΔH0, kJ mol−1 | ΔS0, J mol−1 K−1 |
|---|---|---|---|---|
| 283 | 8.53 | −5.042 | −6.41 | 4.72 |
| 293 | 8.08 | −5.065 | ||
| 308 | 6.89 | −4.941 |
| Metrological Characteristics | Determination with Whey Evaporation | Determination Using SPE |
|---|---|---|
| LOD | 0.3 mg kg−1 | 0.1 mg kg−1 |
| R2 | 0.917 | 0.985 |
| Sample variance of the results of parallel determinations, S2 | 4·10–7 | 1.1·10–6 |
| Repeatability index σr, % | 91 ± 4% | 92.4 ± 2% |
| Intra-laboratory reproducibility σR, mg kg−1 | 0.0074 ± 0.00578 | 0.0062 ± 0.00274 |
| Accuracy index ±Δc, % (systematic error) | 3.4 ± 5% | 3.1 ± 2% |
| Accuracy rate ±Δ, % (error characteristic) | 5.2 ± 3% | 4.7 ± 2% |
| Object of Study | TC Concentration before SPE, mg kg−1 | TC Concentration after SPE, mg kg−1 | Degree of Extraction of TC, % |
|---|---|---|---|
| Farm (sample 1) | 0.0467 | 0.00051 | 92 |
| Ultra-pasteurized (sample 2) | 0.0305 | 0.00035 | 90 |
| Pasteurized (sample 3) | 0.0237 | 0.00089 | 89 |
| Object of Study | TC Concentration before SPE, mg kg−1 | TC Concentration after SPE, mg kg−1 | Degree of Extraction of TC, % |
|---|---|---|---|
| Farm (sample 1) | 0.0467 | 0.00093 | 98 |
| Ultra-pasteurized (sample 2) | 0.0305 | 0.00031 | 99 |
| Pasteurized (sample 3) | 0.0237 | 0.00047 | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naumkina, V.N.; Lyamina, V.M.; Zhinzhilo, V.A.; Uflyand, I.E. Determination of Tetracycline Antibiotics in Milk by Solid-Phase Extraction Using a Coordination Polymer Based on Cobalt Trimesinate as a Sorbent. Polymers 2023, 15, 4539. https://doi.org/10.3390/polym15234539
Naumkina VN, Lyamina VM, Zhinzhilo VA, Uflyand IE. Determination of Tetracycline Antibiotics in Milk by Solid-Phase Extraction Using a Coordination Polymer Based on Cobalt Trimesinate as a Sorbent. Polymers. 2023; 15(23):4539. https://doi.org/10.3390/polym15234539
Chicago/Turabian StyleNaumkina, Victoria N., Veronika M. Lyamina, Vladimir A. Zhinzhilo, and Igor E. Uflyand. 2023. "Determination of Tetracycline Antibiotics in Milk by Solid-Phase Extraction Using a Coordination Polymer Based on Cobalt Trimesinate as a Sorbent" Polymers 15, no. 23: 4539. https://doi.org/10.3390/polym15234539
APA StyleNaumkina, V. N., Lyamina, V. M., Zhinzhilo, V. A., & Uflyand, I. E. (2023). Determination of Tetracycline Antibiotics in Milk by Solid-Phase Extraction Using a Coordination Polymer Based on Cobalt Trimesinate as a Sorbent. Polymers, 15(23), 4539. https://doi.org/10.3390/polym15234539






