Nanosilver-Functionalized Hybrid Hydrogels of Carboxymethyl Cellulose/Poly(Vinyl Alcohol) with Antibacterial Activity for Prevention and Therapy of Infections of Diabetic Chronic Wounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hybrid Polymer Hydrogels CMC/PVA/CA
2.3. Synthesis of Nanosilver-Functionalized Hybrid Polymer Hydrogels
2.4. Characterization of Colloidal AgNPs and Hydrogels
2.5. In Vitro Characterization of CMC–PVA_AgNP Hybrid Membranes
2.5.1. Cytotoxicity
2.5.2. Hemocompatibility
2.6. In Vivo Biocompatibility Test—Mice Model
2.7. Antibacterial Studies
2.7.1. Agar Disk Diffusion Method
2.7.2. Bacteria Growth Inhibition Assay
2.8. Statistical Analysis
3. Results and Discussion
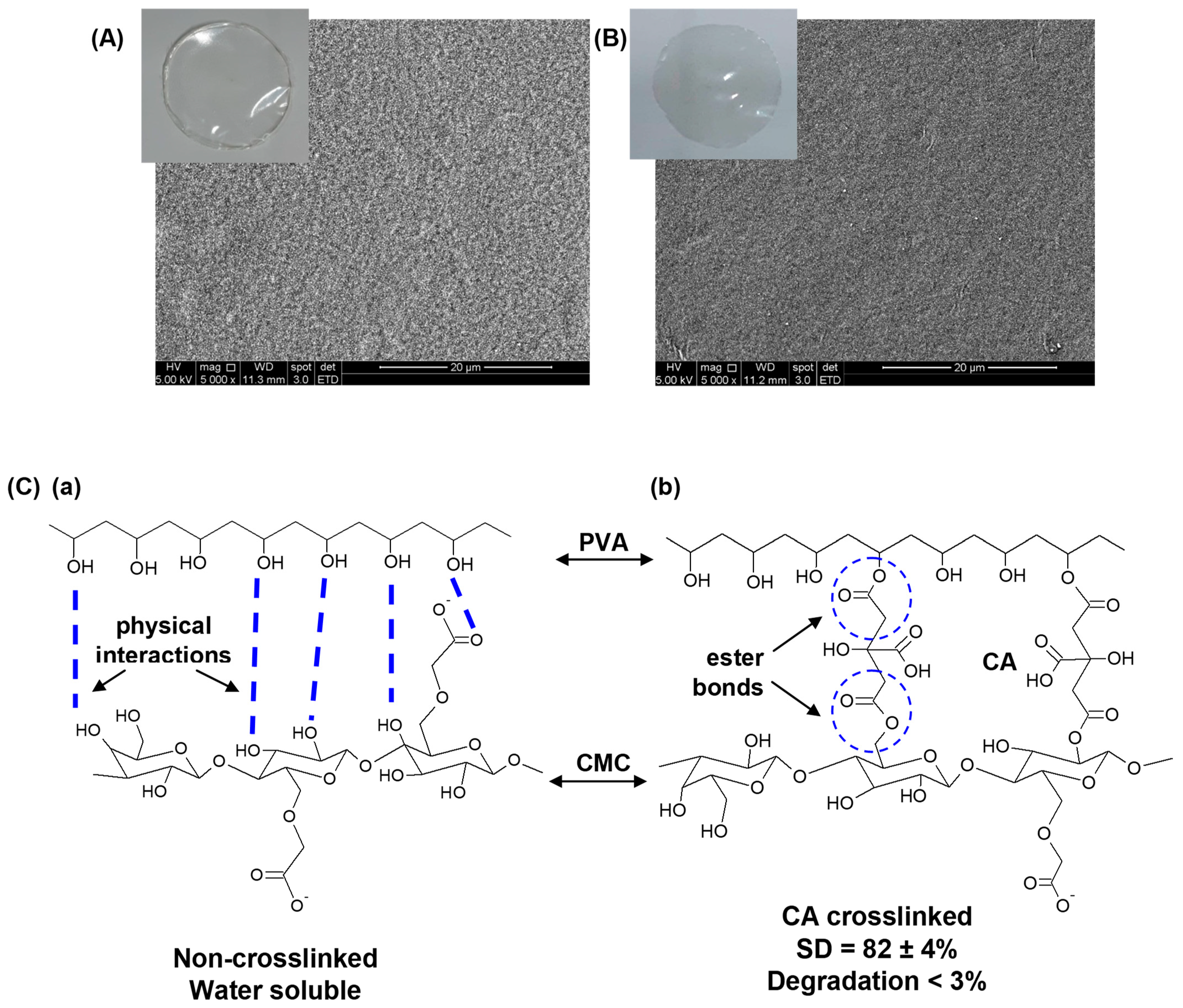
3.1. Characterization of Pristine Hybrid Hydrogel
3.1.1. Physicochemical and Morphological Characterization of Pristine Hybrid Hydrogel
3.1.2. Biological Tests
Cytocompatibility
Hemocompatibility
In Vivo Biocompatibility
3.2. Characterization of Nanosilver and Ag-Functionalized Hybrid Hydrogels
3.2.1. Characterization of Nanosilver
3.2.2. Characterization of Nanosilver-Functionalized Hybrid Hydrogels (CMC/PVA/CA_AgNP)
3.3. Characterization of Nanosilver-Functionalized Hybrid Hydrogels—Biological Assays
3.3.1. Antibacterial Activity
Agar Disk Diffusion Method
Bacteria Growth Inhibition Assay
3.3.2. Cytotoxicity Characterization by MTT Assay
3.3.3. Hemocompatibility Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mariadoss, A.V.A.; Sivakumar, A.S.; Lee, C.-H.; Kim, S.J. Diabetes mellitus and diabetic foot ulcer: Etiology, biochemical and molecular based treatment strategies via gene and nanotherapy. Biomed. Pharmacother. 2022, 151, 113134. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, S.; Ji, W.; Yao, H.; Lin, L.; Cui, H.; Santos, H.A.; Pan, G. Emerging Theranostic Nanomaterials in Diabetes and Its Complications. Adv. Sci. 2022, 9, 2102466. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Stupnitskaia, P.; Matoori, S. Next-Generation Diagnostic Wound Dressings for Diabetic Wounds. ACS Meas. Sci. A 2022, 2, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, N. Mechanism and application of exosomes in the wound healing process in diabetes mellitus. Diabetes Res. Clin. Pract. 2022, 187, 109882. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; de Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Zhao, M.; Liu, G.; Wu, J. Advances of hydrogel dressings in diabetic wounds. Biomater. Sci. 2021, 9, 1530–1546. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; He, D.; Ma, Z.; Xue, K.; Li, H. 45S5 Bioglass® works synergistically with siRNA to downregulate the expression of matrix metalloproteinase-9 in diabetic wounds. Acta Biomater. 2022, 145, 372–389. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.K.; Kiran, V.; Hasnain, M.S.; Nayak, A.K. Alginate-based hydrogel systems for drug releasing in wound healing. Alginates Drug Deliv. 2020, 2020, 323–358. [Google Scholar] [CrossRef]
- Capanema, N.S.V.; Mansur, A.A.P.; Carvalho, I.C.; Carvalho, S.M.; Mansur, H.S. Bioengineered Water-Responsive Carboxymethyl Cellulose/Poly(vinyl alcohol) Hydrogel Hybrids for Wound Dressing and Skin Tissue Engineering Applications. Gels 2023, 9, 166. [Google Scholar] [CrossRef]
- Mansur, A.A.P.; Rodrigues, M.A.; Capanema, N.S.V.; Carvalho, S.M.; Gomes, D.A.; Mansur, H.S. Functionalized bioadhesion-enhanced carboxymethyl cellulose/polyvinyl alcohol hybrid hydrogels for chronic wound dressing applications. RSC Adv. 2023, 13, 13156–13168. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; He, J.; Yang, Y.; Qiao, L.; Hu, J.; Zhang, J.; Guo, B. Antibacterial adhesive self-healing hydrogels to promote diabetic wound healing. Acta Biomater. 2022, 146, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Blacklow, S.O.; Li, J.; Freedman, B.R.; Zeidi, M.; Chen, C.; Mooney, D.J. Bioinspired mechanically active adhesive dressings to accelerate wound closure. Sci. Adv. 2019, 5, eaaw3963. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Yin, T.; Jiang, J.; He, Y.; Xiang, T.; Zhou, S. Wound microenvironment self-adaptive hydrogel with efficient angiogenesis for promoting diabetic wound healing. Bioact. Mater. 2022, 20, 561–573. [Google Scholar] [CrossRef]
- Li, C.; Jiang, T.; Zhou, C.; Jiang, A.; Lu, C.; Yang, G.; Nie, J.; Wang, F.; Yang, X.; Chen, Z. Injectable self-healing chitosan-based POSS-PEG hybrid hydrogel as wound dressing to promote diabetic wound healing. Carbohydr. Polym. 2022, 299, 120198. [Google Scholar] [CrossRef] [PubMed]
- Sivaraj, D.; Chen, K.; Chattopadhyay, A.; Henn, D.; Wu, W.; Noishiki, C.; Magbual, N.J.; Mittal, S.; Mermin-Bunnell, A.M.; Bonham, C.A.; et al. Hydrogel Scaffolds to Deliver Cell Therapies for Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 660145. [Google Scholar] [CrossRef]
- Lin, S.; Pei, L.; Zhang, W.; Shu, G.; Lin, J.; Li, H.; Xu, F.; Tang, H.; Peng, G.; Zhao, L.; et al. Chitosan-poloxamer-based thermosensitive hydrogels containing zinc gluconate/recombinant human epidermal growth factor benefit for antibacterial and wound healing. Mater. Sci. Eng. C 2021, 130, 112450. [Google Scholar] [CrossRef]
- Raina, N.; Pahwa, R.; Thakur, V.K.; Gupta, M. Polysaccharide-based hydrogels: New insights and futuristic prospects in wound healing. Int. J. Biol. Macromol. 2022, 223, 1586–1603. [Google Scholar] [CrossRef]
- Guo, B.; Dong, R.; Liang, Y.; Li, M. Haemostatic materials for wound healing applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef]
- Zhou, L.; Min, T.; Bian, X.; Dong, Y.; Zhang, P.; Wen, Y. Rational Design of Intelligent and Multifunctional Dressing to Promote Acute/Chronic Wound Healing. ACS Appl. Bio Mater. 2022, 5, 4055–4085. [Google Scholar] [CrossRef]
- Dong, R.; Guo, B. Smart wound dressings for wound healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhu, M.; Chen, L.; Zhang, Y.; Lu, T.; Deng, Y.; Ma, W.; Xu, J.; Huang, C.; Xiong, R. Design the molecule structures to achieve functional advantages of hydrogel wound dressings: Advances and strategies. Compos. Part B Eng. 2022, 247, 110313. [Google Scholar] [CrossRef]
- Capanema, N.S.; Mansur, A.A.; de Jesus, A.C.; Carvalho, S.M.; de Oliveira, L.C.; Mansur, H.S. Superabsorbent crosslinked carboxymethyl cellulose-PEG hydrogels for potential wound dressing applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wu, Y.; Li, W.; Wang, Y.; Kong, Q. Development of a Microenvironment-Responsive Hydrogel Promoting Chronically Infected Diabetic Wound Healing through Sequential Hemostatic, Antibacterial, and Angiogenic Activities. ACS Appl. Mater. Interfaces 2022, 14, 30480–30492. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M. Introduction to cell–hydrogel mechanosensing. Interface Focus 2014, 4, 20130038. [Google Scholar] [CrossRef] [PubMed]
- Smithmyer, M.E.; Sawicki, L.A.; Kloxin, A.M. Hydrogel scaffolds as in vitro models to study fibroblast activation in wound healing and disease. Biomater. Sci. 2014, 2, 634–650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhu, J.; Jin, S.; Zheng, Y.; Gao, W.; Wu, D.; Yu, J.; Dai, Z. Cellulose-nanofibril-reinforced hydrogels with pH sensitivity and mechanical stability for wound healing. Mater. Lett. 2022, 323, 132596. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Karperien, M.; Johnbosco, C.; Mahmood, A.; Kousar, M. Chitosan and carboxymethyl cellulose-based 3D multifunctional bioactive hydrogels loaded with nano-curcumin for synergistic diabetic wound repair. Int. J. Biol. Macromol. 2023, 227, 1203–1220. [Google Scholar] [CrossRef]
- Chen, J.W.; Lim, K.; Bandini, S.B.; Harris, G.M.; Spechler, J.A.; Arnold, C.B.; Fardel, R.; Schwarzbauer, J.E.; Schwartz, J. Controlling the Surface Chemistry of a Hydrogel for Spatially Defined Cell Adhesion. ACS Appl. Mater. Interfaces 2019, 11, 15411–15416. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Tyagi, V.; Thakur, A. Carboxymethyl cellulose-polyvinyl alcohol based materials: A review. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Elangwe, C.N.; Morozkina, S.N.; Olekhnovich, R.O.; Krasichkov, A.; Polyakova, V.O.; Uspenskaya, M.V. A Review on Chitosan and Cellulose Hydrogels for Wound Dressings. Polymers 2022, 14, 5163. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.D.; Banas, D.; Durai, R.D.; Kabanov, D.; Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Ruttkay-Nedecky, B.; Nguyen, H.V.; Farid, A.; et al. Silver Nanomaterials for Wound Dressing Applications. Pharmaceutics 2020, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Sabarees, G.; Velmurugan, V.; Tamilarasi, G.P.; Alagarsamy, V.; Solomon, V.R. Recent Advances in Silver Nanoparticles Containing Nanofibers for Chronic Wound Management. Polymers 2022, 14, 3994. [Google Scholar] [CrossRef] [PubMed]
- Yudaev, P.; Mezhuev, Y.; Chistyakov, E. Nanoparticle-Containing Wound Dressing: Antimicrobial and Healing Effects. Gels 2022, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Capanema, N.S.V.; Mansur, A.A.P.; Carvalho, S.M.; Mansur, L.L.; Ramos, C.P.; Lage, A.P.; Mansur, H.S. Physicochemical properties and antimicrobial activity of biocompatible carboxymethylcellulose-silver nanoparticle hybrids for wound dressing and epidermal repair. J. Appl. Polym. Sci. 2018, 135, 45812. [Google Scholar] [CrossRef]
- Dumont, V.C.; Mansur, H.S.; Mansur, A.A.; Carvalho, S.M.; Capanema, N.S.; Barrioni, B.R. Glycol chitosan/nanohydroxyapatite biocomposites for potential bone tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016, 93, 1465–1478. [Google Scholar] [CrossRef]
- Boonkaew, B.; Kempf, M.; Kimble, R.; Supaphol, P.; Cuttle, L. Antimicrobial efficacy of a novel silver hydrogel dressing compared to two common silver burn wound dressings: Acticoat™ and PolyMem Silver®. Burns 2014, 40, 89–96. [Google Scholar] [CrossRef]
- Sacco, P.; Travan, A.; Borgogna, M.; Paoletti, S.; Marsich, E. Silver-containing antimicrobial membrane based on chitosan-TPP hydrogel for the treatment of wounds. J. Mater. Sci. Mater. Med. 2015, 26, 128. [Google Scholar] [CrossRef]
- Catanzano, O.; D’esposito, V.; Pulcrano, G.; Maiolino, S.; Ambrosio, M.R.; Esposito, M.; Miro, A.; Ungaro, F.; Formisano, P.; Catania, M.R.; et al. Ultrasmall silver nanoparticles loaded in alginate–hyaluronic acid hybrid hydrogels for treating infected wounds. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 626–634. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Ghorpade, V.S.; Dias, R.J.; Mali, K.K.; Mulla, S.I. Citric acid crosslinked carboxymethylcellulose-polyvinyl alcohol hydrogel films for extended release of water soluble basic drugs. J. Drug Deliv. Sci. Technol. 2019, 52, 421–430. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 5th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Xie, Y.; Liao, X.; Zhang, J.; Yang, F.; Fan, Z. Novel chitosan hydrogels reinforced by silver nanoparticles with ultrahigh mechanical and high antibacterial properties for accelerating wound healing. Int. J. Biol. Macromol. 2018, 119, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Son, D.; Cho, S.; Nam, J.; Lee, H.; Kim, M. X-ray-Based Spectroscopic Techniques for Characterization of Polymer Nanocomposite Materials at a Molecular Level. Polymers 2020, 12, 1053. [Google Scholar] [CrossRef]
- Demeter, M.; Scărișoreanu, A.; Călina, I. State of the Art of Hydrogel Wound Dressings Developed by Ionizing Radiation. Gels 2023, 9, 55. [Google Scholar] [CrossRef]
- Chang, G.; Dang, Q.; Liu, C.; Wang, X.; Song, H.; Gao, H.; Sun, H.; Zhang, B.; Cha, D. Carboxymethyl chitosan and carboxymethyl cellulose based self-healing hydrogel for accelerating diabetic wound healing. Carbohydr. Polym. 2022, 292, 119687. [Google Scholar] [CrossRef]
- Duceac, I.A.; Verestiuc, L.; Dimitriu, C.D.; Maier, V.; Coseri, S. Design and Preparation of New Multifunctional Hydrogels Based on Chitosan/Acrylic Polymers for Drug Delivery and Wound Dressing Applications. Polymers 2020, 12, 1473. [Google Scholar] [CrossRef]
- Alves, P.; Santos, M.; Mendes, S.; Miguel, S.P.; de Sá, K.D.; Cabral, C.S.D.; Correia, I.J.; Ferreira, P. Photocrosslinkable Nanofibrous Asymmetric Membrane Designed for Wound Dressing. Polymers 2019, 11, 653. [Google Scholar] [CrossRef]
- Barrioni, B.R.; de Laia, A.G.S.; Valverde, T.M.; Martins, T.M.d.M.; Caliari, M.V.; de Sá, M.A.; de Goes, A.M.; Pereira, M.d.M. Evaluation of in vitro and in vivo biocompatibility and structure of cobalt-releasing sol-gel bioactive glass. Ceram. Int. 2018, 44, 20337–20347. [Google Scholar] [CrossRef]
- Yang, X.; Jia, M.; Li, Z.; Ma, Z.; Lv, J.; Jia, D.; He, D.; Zeng, R.; Luo, G.; Yu, Y. In-situ synthesis silver nanoparticles in chitosan/Bletilla striata polysaccharide composited microneedles for infected and susceptible wound healing. Int. J. Biol. Macromol. 2022, 215, 550–559. [Google Scholar] [CrossRef]
- Song, S.; Liu, Z.; Abubaker, M.A.; Ding, L.; Zhang, J.; Yang, S.; Fan, Z. Antibacterial polyvinyl alcohol/bacterial cellulose/nano-silver hydrogels that effectively promote wound healing. Mater. Sci. Eng. C 2021, 126, 112171. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Hutter, E.; Fendler, J.H. Exploitation of Localized Surface Plasmon Resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Scholl, J.A.; Koh, A.L.; Dionne, J.A. Quantum plasmon resonances of individual metallic nanoparticles. Nature 2012, 483, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Pauly, N.; Yubero, F.; Tougaard, S. Quantitative analysis of satellite structures in XPS spectra of gold and silver. Appl. Surf. Sci. 2016, 383, 317–323. [Google Scholar] [CrossRef]
- Macdonald, K.E.; Boeckh, S.; Stacey, H.J.; Jones, J.D. The microbiology of diabetic foot infections: A meta-analysis. BMC Infect. Dis. 2021, 21, 770. [Google Scholar] [CrossRef]
- Kalantari, K.; Mostafavi, E.; Afifi, A.M.; Izadiyan, Z.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Wound dressings functionalized with silver nanoparticles: Promises and pitfalls. Nanoscale 2019, 12, 2268–2291. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, H.-S.; Ryu, D.-S.; Choi, S.-J.; Lee, D.-S. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Karnwal, A.; Kumar, G.; Pant, G.; Hossain, K.; Ahmad, A.; Alshammari, M.B. Perspectives on Usage of Functional Nanomaterials in Antimicrobial Therapy for Antibiotic-Resistant Bacterial Infections. ACS Omega 2023, 8, 13492–13508. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [PubMed]
- ASTM 756-00; Standard Practice for Assessment of Hemolytic Properties of Materials. American Society for Testing and Materials: Philadelphia, PA, USA, 2000.









| Silver Nanocomposites Identification | CMC (m/v %) | PVA (m/v %) | [Ag]/ [CMC + PVA] m/m% | [CA]/ [CMC + PVA] m/m% | Mass of Ag/Nanocomposite Area (μg/cm2) |
|---|---|---|---|---|---|
| CMC/PVA/CA or CMC/PVA/CA_Ag0 | 80 | 20 | 0.00 | 25 | 0.0 |
| CMC/PVA/CA_Ag015 | 80 | 20 | 0.15 | 25 | 7.5 |
| CMC/PVA/CA_Ag030 | 80 | 20 | 0.30 | 25 | 15.0 |
| CMC/PVA/CA_Ag060 | 80 | 20 | 0.60 | 25 | 30.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capanema, N.S.V.; Mansur, A.A.P.; Carvalho, S.M.; Martins, T.; Gonçalves, M.S.; Andrade, R.S.; Dorneles, E.M.S.; Lima, L.C.D.; de Alvarenga, É.L.F.C.; da Fonseca, E.V.B.; et al. Nanosilver-Functionalized Hybrid Hydrogels of Carboxymethyl Cellulose/Poly(Vinyl Alcohol) with Antibacterial Activity for Prevention and Therapy of Infections of Diabetic Chronic Wounds. Polymers 2023, 15, 4542. https://doi.org/10.3390/polym15234542
Capanema NSV, Mansur AAP, Carvalho SM, Martins T, Gonçalves MS, Andrade RS, Dorneles EMS, Lima LCD, de Alvarenga ÉLFC, da Fonseca EVB, et al. Nanosilver-Functionalized Hybrid Hydrogels of Carboxymethyl Cellulose/Poly(Vinyl Alcohol) with Antibacterial Activity for Prevention and Therapy of Infections of Diabetic Chronic Wounds. Polymers. 2023; 15(23):4542. https://doi.org/10.3390/polym15234542
Chicago/Turabian StyleCapanema, Nádia S. V., Alexandra A. P. Mansur, Sandhra M. Carvalho, Talita Martins, Maysa S. Gonçalves, Rafaella S. Andrade, Elaine M. S. Dorneles, Letícia C. D. Lima, Érika L. F. C. de Alvarenga, Emanuel V. B. da Fonseca, and et al. 2023. "Nanosilver-Functionalized Hybrid Hydrogels of Carboxymethyl Cellulose/Poly(Vinyl Alcohol) with Antibacterial Activity for Prevention and Therapy of Infections of Diabetic Chronic Wounds" Polymers 15, no. 23: 4542. https://doi.org/10.3390/polym15234542
APA StyleCapanema, N. S. V., Mansur, A. A. P., Carvalho, S. M., Martins, T., Gonçalves, M. S., Andrade, R. S., Dorneles, E. M. S., Lima, L. C. D., de Alvarenga, É. L. F. C., da Fonseca, E. V. B., Sá, M. A. d., Lage, A. P., Lobato, Z. I. P., & Mansur, H. S. (2023). Nanosilver-Functionalized Hybrid Hydrogels of Carboxymethyl Cellulose/Poly(Vinyl Alcohol) with Antibacterial Activity for Prevention and Therapy of Infections of Diabetic Chronic Wounds. Polymers, 15(23), 4542. https://doi.org/10.3390/polym15234542










