Highly Self-Healable Polymeric Coating Materials Based on Charge Transfer Complex Interactions with Outstanding Weatherability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
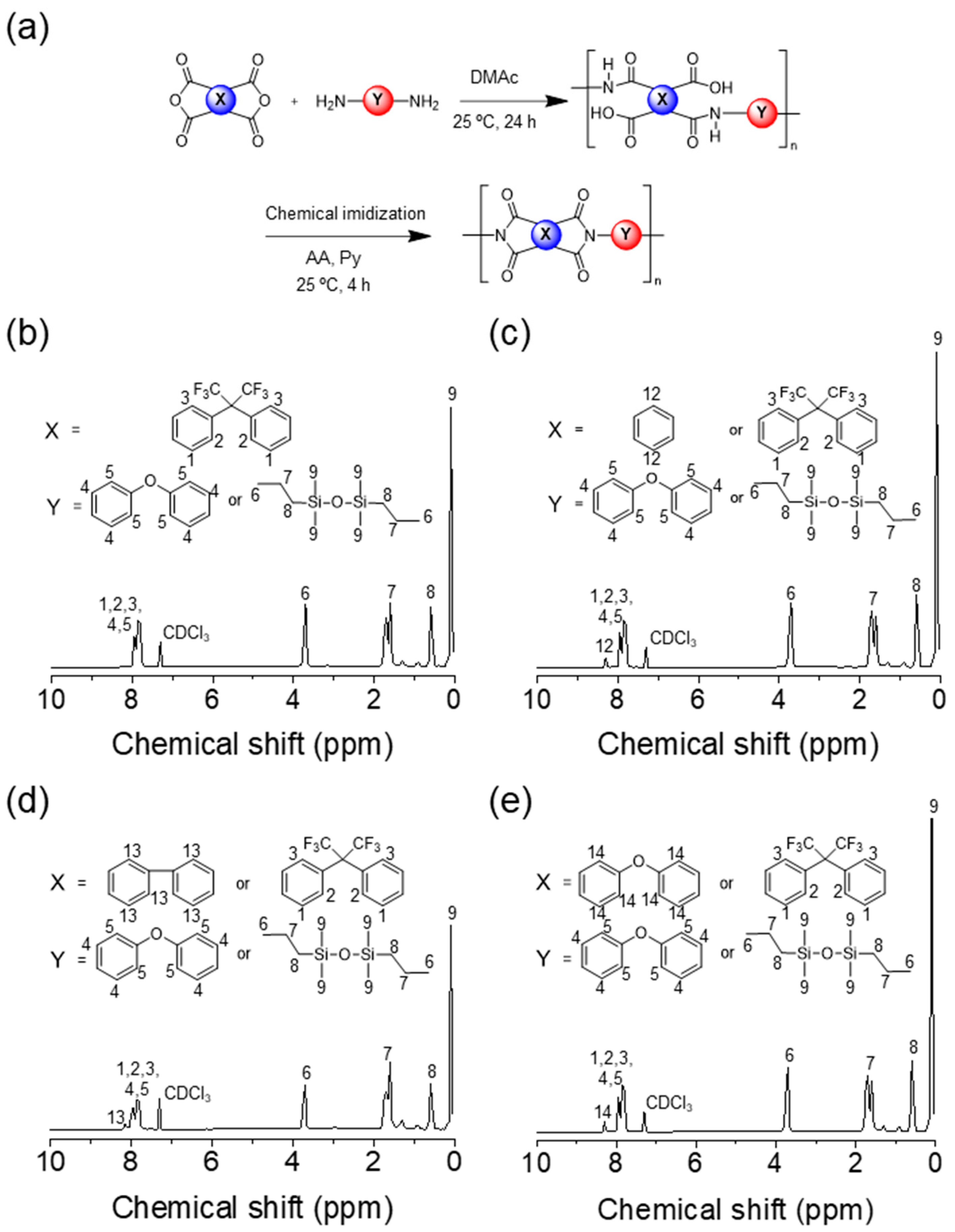
2.2. Synthesis of Self-Healable PIs
2.3. Preparation of Self-Healable PCMs
2.4. Characterization
3. Results and Discussion
3.1. Synthesis and Solubility
3.2. Thermal and Mechanical Surface Properties
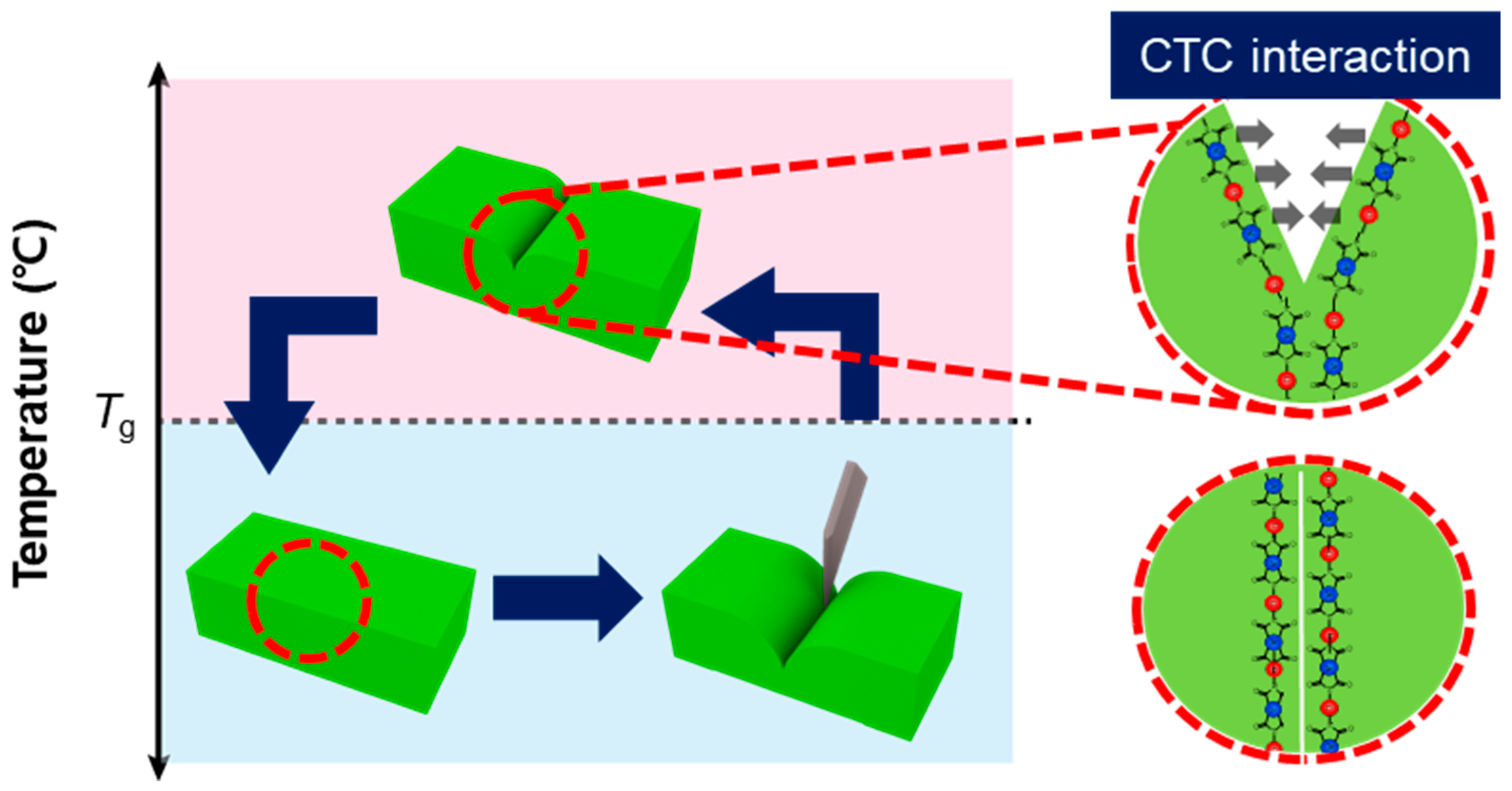
3.3. Self-Healing Properties
3.4. Optical Properties and Weatherability
3.5. Proposed Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wong, W.S.; Salleo, A. Flexible Electronics: Materials and Applications; Springer Science & Business Media: New York, NY, USA, 2009. [Google Scholar]
- Licari, J.J. Coating Materials for Electronic Applications: Polymers, Processing, Reliability, Testing; William Andrew: Norwich, NY, USA, 2003. [Google Scholar]
- Lyu, M.-Y.; Choi, T.G. Research trends in polymer materials for use in lightweight vehicles. Int. J. Precis. Eng. Manuf. 2015, 16, 213–220. [Google Scholar] [CrossRef]
- Andreeva, D.V.; Shchukin, D.G. Smart self-repairing protective coatings. Mater. Today 2008, 11, 24–30. [Google Scholar] [CrossRef]
- Wen, M.; Dušek, K. Protective Coatings; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Harris, K.; Elias, A.; Chung, H.-J. Flexible electronics under strain: A review of mechanical characterization and durability enhancement strategies. J. Mater. Sci. 2016, 51, 2771–2805. [Google Scholar] [CrossRef]
- Domagała, I.; Gil, L.; Firlej, M.; Pieniak, D.; Selech, J.; Romek, D.; Biedziak, B. Statistical Comparison of the Hardness and Scratch-Resistance of the PMMA Polymers Used in Orthodontic Appliances. Adv. Sci. Technol. Res. J. 2020, 14, 250–261. [Google Scholar] [CrossRef]
- Hadal, R.; Misra, R. Scratch deformation behavior of thermoplastic materials with significant differences in ductility. Mater. Sci. Eng. A 2005, 398, 252–261. [Google Scholar] [CrossRef]
- White, J.; Turnbull, A. Weathering of polymers: Mechanisms of degradation and stabilization, testing strategies and modelling. J. Mater. Sci. 1994, 29, 584–613. [Google Scholar] [CrossRef]
- Davis, A.; Sims, D. Weathering of Polymers; Springer Science & Business Media: New York, NY, USA, 1983. [Google Scholar]
- Hawkins, W.L. Polymer Degradation and Stabilization; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Qin, J.; Jiang, J.; Tao, Y.; Zhao, S.; Zeng, W.; Shi, Y.; Lu, T.; Guo, L.; Wang, S.; Zhang, X. Sunlight tracking and concentrating accelerated weathering test applied in weatherability evaluation and service life prediction of polymeric materials: A review. Polym. Test 2021, 93, 106940. [Google Scholar] [CrossRef]
- Gao, T.; He, Z.; Hihara, L.H.; Mehr, H.S.; Soucek, M.D. Outdoor exposure and accelerated weathering of polyurethane/polysiloxane hybrid coatings. Prog. Org. Coat. 2019, 130, 44–57. [Google Scholar] [CrossRef]
- Caruso, M.M.; Davis, D.A.; Shen, Q.; Odom, S.A.; Sottos, N.R.; White, S.R.; Moore, J. Mechanically-induced chemical changes in polymeric materials. Chem. Rev. 2009, 109, 5755–5798. [Google Scholar] [CrossRef]
- Tjong, S.C. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. R 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.-R.; Lee, J.; Lee, H.; Jeon, Y.; Park, S.; Kim, Y.-S.; Seong, H.-M.; Kwak, G. Highly tough, colorless, transparent polyamide-imide films from one reaction vessel without purification. Macromol. Res. 2023, 31, 213–222. [Google Scholar] [CrossRef]
- Kang, C.; Song, Y.S. Enhancement in surface property via in-mold coating process. Macromol. Res. 2021, 29, 185–190. [Google Scholar] [CrossRef]
- Sangermano, M.; Messori, M. Scratch resistance enhancement of polymer coatings. Macromol. Mater. Eng. 2010, 295, 603–612. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Skowera, E.; Pietniewicz, E.; Zukowska, G.Z.; van der Ven, L.G.; Korczagin, I.; Malanowski, P. UV stability of polymeric binder films used in waterborne facade paints. Prog. Org. Coat. 2014, 77, 298–304. [Google Scholar] [CrossRef]
- López-Rubio, A.; Lagaron, J.M. Improvement of UV stability and mechanical properties of biopolyesters through the addition of β-carotene. Polym. Degrad. Stab. 2010, 95, 2162–2168. [Google Scholar] [CrossRef]
- Hfaiedh, A.; Labiedh, M.; Mabrouk, A.; Braiek, M.; Alimi, K. Synthesis, characterization and structure–property study of new push–pull carbazole materials. Macromol. Res. 2023, 31, 981–999. [Google Scholar] [CrossRef]
- Li, G. Self-Healing Composites: Shape Memory Polymer Based Structures; John Wiley & Sons: Winheim, Germany, 2014. [Google Scholar]
- Binder, W.H. Self-Healing Polymers: From Principles to Applications; John Wiley & Sons: Winheim, Germany, 2013. [Google Scholar]
- Udoh, I.I.; Shi, H.; Daniel, E.F.; Li, J.; Gu, S.; Liu, F.; Han, E.-H. Active anticorrosion and self-healing coatings: A review with focus on multi-action smart coating strategies. J. Mater. Sci. Technol. 2022, 116, 224–237. [Google Scholar] [CrossRef]
- Zhang, F.; Ju, P.; Pan, M.; Zhang, D.; Huang, Y.; Li, G.; Li, X. Self-healing mechanisms in smart protective coatings: A review. Corros. Sci. 2018, 144, 74–88. [Google Scholar] [CrossRef]
- Cho, S.H.; White, S.R.; Braun, P.V. Self-healing polymer coatings. Adv. Mater. 2009, 21, 645–649. [Google Scholar] [CrossRef]
- Wool, R.P. Self-healing materials: A review. Soft Matter 2008, 4, 400–418. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Kessler, M.R. Self-healing polymer nanocomposite materials: A review. Polymer 2015, 69, 369–383. [Google Scholar] [CrossRef]
- Oktay, B.; Türkcan, J.H.; Özdemir, O.K.; Kayaman-Apohan, N. Vegetable oil-based epoxy coating materials for self-healing and anticorrosive applications. Macromol. Res. 2023, 31, 1077–1086. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, F.; Cao, X.; Hu, Z.; Cheng, C. Thermally healable polyurethanes based on furfural-derived monomers via Baylis-Hillman reaction. Macromol. Res. 2019, 27, 895–904. [Google Scholar] [CrossRef]
- Kang, J.; Kim, J.; Choi, K.; Hong, P.H.; Park, H.J.; Kim, K.; Kim, Y.K.; Moon, G.; Jeon, H.; Lee, S.; et al. A water-triggered highly self-healable elastomer with enhanced mechanical properties achieved using localized zwitterionic assemblies. Chem. Eng. J. 2021, 420, 127636. [Google Scholar] [CrossRef]
- Cao, Y.; Morrissey, T.G.; Acome, E.; Allec, S.I.; Wong, B.M.; Keplinger, C.; Wang, C. A transparent, self-healing, highly stretchable ionic conductor. Adv. Mater. 2017, 29, 1605099. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, G.; Li, J.; Zeng, S.; Zhang, X.; Zheng, Z.; Ding, X.; Chen, W.; Wang, Q.; Zhang, W. Preparation and self-healing behaviors of poly (acrylic acid)/cerium ions double network hydrogels. Macromol. Res. 2015, 23, 1098–1102. [Google Scholar] [CrossRef]
- Lee, W.-J.; Oh, H.-G.; Cha, S.-H. A brief review of self-healing polyurethane based on dynamic chemistry. Macromol. Res. 2021, 29, 649–664. [Google Scholar] [CrossRef]
- Kim, J.; Hong, P.H.; Choi, K.; Moon, G.; Kang, J.; Lee, S.; Lee, S.; Jung, H.W.; Ko, M.J.; Hong, S.W. A heterocyclic polyurethane with enhanced self-healing efficiency and outstanding recovery of mechanical properties. Polymers 2020, 12, 968. [Google Scholar] [CrossRef]
- Lee, S.; Hong, P.H.; Kim, J.; Choi, K.; Moon, G.; Kang, J.; Lee, S.; Ahn, J.B.; Eom, W.; Ko, M.J.; et al. Highly self-healable polymeric blend synthesized using polymeric glue with outstanding mechanical properties. Macromolecules 2020, 53, 2279–2286. [Google Scholar] [CrossRef]
- Deng, Z.; Guo, Y.; Zhao, X.; Ma, P.X.; Guo, B. Multifunctional stimuli-responsive hydrogels with self-healing, high conductivity, and rapid recovery through host–guest interactions. Chem. Mater. 2018, 30, 1729–1742. [Google Scholar] [CrossRef]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host–guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.; Jeon, S.I.; Chung, I.J.; Ahn, C.-H. Self-healable dielectric polydimethylsiloxane composite based on zinc-imidazole coordination bond. Macromol. Res. 2019, 27, 435–443. [Google Scholar] [CrossRef]
- Li, C.H.; Zuo, J.L. Self-healing polymers based on coordination bonds. Adv. Mater. 2020, 32, 1903762. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ding, P.; Wang, Y.; Zhang, Y.; Ossipov, D.; Hilborn, J. Self-healing polymeric hydrogel formed by metal–ligand coordination assembly: Design, fabrication, and biomedical applications. Macromol. Rapid Commun. 2019, 40, 1800837. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, C.; Wei, C.; Huang, J.; Zou, X.; Feng, Y.; Ang, S.; Guo, S.; Qu, J.; Qing, N. Charge-Transfer Polymeric Hydrogels with Self-Healing, Injectable, Thermosensitive, Adhesive, and Antibacterial Properties for Diabetic Wound Healing. Adv. Mater. Technol. 2023, 8, 2201527. [Google Scholar] [CrossRef]
- Ahn, C.; Hong, P.H.; Lee, J.; Kim, J.; Moon, G.; Lee, S.; Park, I.; Han, H.; Hong, S.W. Highly Self-Healable Polymeric Coating Materials with Enhanced Mechanical Properties Based on the Charge Transfer Complex. Polymers 2022, 14, 5181. [Google Scholar] [CrossRef]
- Sung, S.; Kim, S.Y.; Lee, T.H.; Favaro, G.; Park, Y.I.; Lee, S.-H.; Ahn, J.B.; Noh, S.M.; Kim, J.C. Thermally reversible polymer networks for scratch resistance and scratch healing in automotive clear coats. Prog. Org. Coat. 2019, 127, 37–44. [Google Scholar] [CrossRef]
- Araujo-Morera, J.; Utrera-Barrios, S.; Doral Olivares, R.; Reyes Verdugo Manzanares, M.d.l.; López-Manchado, M.Á.; Verdejo, R.; Hernandez Santana, M. Solving the Dichotomy between Self-Healing and Mechanical Properties in Rubber Composites by Combining Reinforcing and Sustainable Fillers. Macromol. Mater. Eng. 2022, 307, 2200261. [Google Scholar] [CrossRef]
- Santana, M.H.; Huete, M.; Lameda, P.; Araujo, J.; Verdejo, R.; López-Manchado, M.A. Design of a new generation of sustainable SBR compounds with good trade-off between mechanical properties and self-healing ability. Eur. Polym. J. 2018, 106, 273–283. [Google Scholar] [CrossRef]
- Sperling, L.H. Introduction to Physical Polymer Science; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Park, S.-A.; Jeon, H.; Kim, H.; Shin, S.-H.; Choy, S.; Hwang, D.S.; Koo, J.M.; Jegal, J.; Hwang, S.Y.; Park, J. Sustainable and recyclable super engineering thermoplastic from biorenewable monomer. Nat. Commun. 2019, 10, 2601. [Google Scholar] [CrossRef] [PubMed]
- Kemmish, D.J. High Performance Engineering Plastics; Rapra Technology LTD.: Shawbury, UK, 1995. [Google Scholar]
- Sezer Hicyilmaz, A.; Celik Bedeloglu, A. Applications of polyimide coatings: A review. SN Appl. Sci. 2021, 3, 363. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Ghosh, M. Polyimides: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Feger, C. Advances in Polyimide: Science and Technology; CRC Press: Lancaster, PA, USA, 1993. [Google Scholar]
- Jiao, L.; Luo, F.; Du, Z.; Dai, X.; Mu, J.; Wang, H.; Dong, Z.; Qiu, X. Ultra-high Tg and ultra-low CTE polyimide films based on tunable interchain crosslinking. React. Funct. Polym. 2022, 181, 105449. [Google Scholar] [CrossRef]
- Yokota, R.; Yamamoto, S.; Yano, S.; Sawaguchi, T.; Hasegawa, M.; Yamaguchi, H.; Ozawa, H.; Sato, R. Molecular design of heat resistant polyimides having excellent processability and high glass transition temperature. High Perform. Polym. 2001, 13, S61–S72. [Google Scholar] [CrossRef]
- Ekeocha, J.; Ellingford, C.; Pan, M.; Wemyss, A.M.; Bowen, C.; Wan, C. Challenges and opportunities of self-healing polymers and devices for extreme and hostile environments. Adv. Mater. 2021, 33, 2008052. [Google Scholar] [CrossRef] [PubMed]
- Meyersohn, M.S.; Haque, F.M.; Hillmyer, M.A. Dynamic Aliphatic Polyester Elastomers Crosslinked with Aliphatic Dianhydrides. ACS Polym. Au 2023, 3, 365–375. [Google Scholar] [CrossRef]
- Volksen, W. Polyimides; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Harris, F.W. Synthesis of Aromatic Polyimides from Dianhydrides and Diamines; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Sato, Y.; Hayami, R.; Gunji, T. Characterization of NMR, IR, and Raman spectra for siloxanes and silsesquioxanes: A mini review. J. Sol-Gel Sci. Technol. 2022, 104, 36–52. [Google Scholar] [CrossRef]
- Dankert, F.; von Hänisch, C. Siloxane coordination revisited: Si–O bond character, reactivity and magnificent molecular shapes. Eur. J. Inorg. Chem. 2021, 2021, 2907–2927. [Google Scholar] [CrossRef]
- Voronkov, M.; Deich, A.Y. The donor-acceptor properties of the siloxane bond. J. Struct. Chem. 1964, 5, 443–448. [Google Scholar] [CrossRef]
- Yilgör, İ.; McGrath, J.E. Polysiloxane Containing Copolymers: A Survey of Recent Developments; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Bott, R.; Summers, J.; Arnold, C.; Taylor, L.; Ward, T.; McGrath, J. Synthesis and characteristics of novel poly (imide siloxane) segmented copolymers. J. Adhes. 1987, 23, 67–82. [Google Scholar] [CrossRef]
- De Abajo, J.; De la Campa, J. Processable Aromatic Polyimides; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A.; Abe, A.; Bloch, D.R. Polymer Handbook; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Jin, H.-S.; Chang, J.-H.; Kim, J.-C. Synthesis and characterization of colorless polyimide nanocomposite films containing pendant trifluoromethyl groups. Macromol. Res. 2008, 16, 503–509. [Google Scholar] [CrossRef]
- Hill, D.J.; Rasoul, F.A.; Forsythe, J.S.; O’Donnell, J.H.; Pomery, P.J.; George, G.A.; Young, P.R.; Connell, J.W. Effect of simulated low earth orbit radiation on polyimides (UV degradation study). J. Appl. Polym. Sci. 1995, 58, 1847–1856. [Google Scholar] [CrossRef]
- Hasegawa, M.; Shindo, Y.; Sugimura, T.; Ohshima, S.; Horie, K.; Kochi, M.; Yokota, R.; Mita, I. Photophysical processes in aromatic polyimides. Studies with model compounds. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 1617–1625. [Google Scholar] [CrossRef]
- Wakita, J.; Sekino, H.; Sakai, K.; Urano, Y.; Ando, S. Molecular design, synthesis, and properties of highly fluorescent polyimides. J. Phys. Chem. B 2009, 113, 15212–15224. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-healing polymeric materials. Chem. Soc. Rev. 2013, 42, 7446–7467. [Google Scholar] [CrossRef]
- Garcia, S.J. Effect of polymer architecture on the intrinsic self-healing character of polymers. Eur. Polym. J. 2014, 53, 118–125. [Google Scholar] [CrossRef]
- Utrera-Barrios, S.; Verdejo, R.; López-Manchado, M.A.; Santana, M.H. Evolution of self-healing elastomers, from extrinsic to combined intrinsic mechanisms: A review. Mater. Horiz. 2020, 7, 2882–2902. [Google Scholar] [CrossRef]
- Pielichowski, K.; Njuguna, J.; Majka, T.M. Thermal Degradation of Polymeric Materials; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]



| Sample Designations | Compositions | Thickness | Thermal Properties | Mechanical Surface Properties a | Optical Properties b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dianhydrides | Diamines | (Diamines) /(Dianhydrides) | Td c | Tg d | HIT e | EIT f | Transmittance g | YIi h | ΔYI i | ||||||
| 6FDA | PMDA | BPDA | ODPA | TBDS | ODA | ||||||||||
| (Molar Ratio) | - | (μm) | (°C) | (°C) | (MPa) | (GPa) | (%) | (a.u.) | (a.u.) | ||||||
| 6F | 100 | - | - | - | 99.9 | 0.1 | 1.01 | 2.6 | 422.8 | 89.5 | 205.33 ±1.24 | 3.39 ±0.02 | 91.1 ±0.10 | 2.02 ±0.10 | 2.51 ±0.20 |
| P20 | 80 | 20 | - | - | 1.01 | 2.8 | 424.0 | 84.4 | 194.77 ±1.04 | 3.31 ±0.02 | 91.3 ±0.12 | 2.28 ±0.10 | 3.48 ±0.22 | ||
| B20 | 80 | - | 20 | - | 1.01 | 2.0 | 425.5 | 85.5 | 212.23 ±0.57 | 3.42 ±0.05 | 91.6 ±0.11 | 2.23 ±0.08 | 0.55 ±0.25 | ||
| O20 | 80 | - | - | 20 | 1.01 | 3.8 | 425.7 | 84.9 | 212.3 ±0.95 | 3.50 ±0.04 | 91.3 ±0.10 | 2.22 ±0.10 | 1.16 ±0.15 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, P.H.; Moon, G.; Kim, J.; Choi, K.; Ko, M.J.; Yoon, H.G.; Hong, S.W. Highly Self-Healable Polymeric Coating Materials Based on Charge Transfer Complex Interactions with Outstanding Weatherability. Polymers 2023, 15, 4544. https://doi.org/10.3390/polym15234544
Hong PH, Moon G, Kim J, Choi K, Ko MJ, Yoon HG, Hong SW. Highly Self-Healable Polymeric Coating Materials Based on Charge Transfer Complex Interactions with Outstanding Weatherability. Polymers. 2023; 15(23):4544. https://doi.org/10.3390/polym15234544
Chicago/Turabian StyleHong, Pyong Hwa, Gyeongmin Moon, Jinsil Kim, Kiwon Choi, Min Jae Ko, Ho Gyu Yoon, and Sung Woo Hong. 2023. "Highly Self-Healable Polymeric Coating Materials Based on Charge Transfer Complex Interactions with Outstanding Weatherability" Polymers 15, no. 23: 4544. https://doi.org/10.3390/polym15234544









