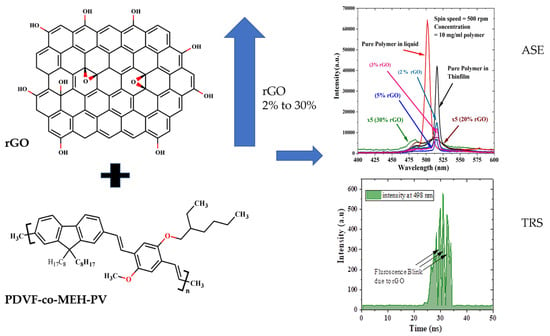
Effects of Graphene Oxide (GO) and Reduced Graphene Oxide (rGO) on Green-Emitting Conjugated Copolymer’s Optical and Laser Properties Using Simulation and Experimental Studies
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
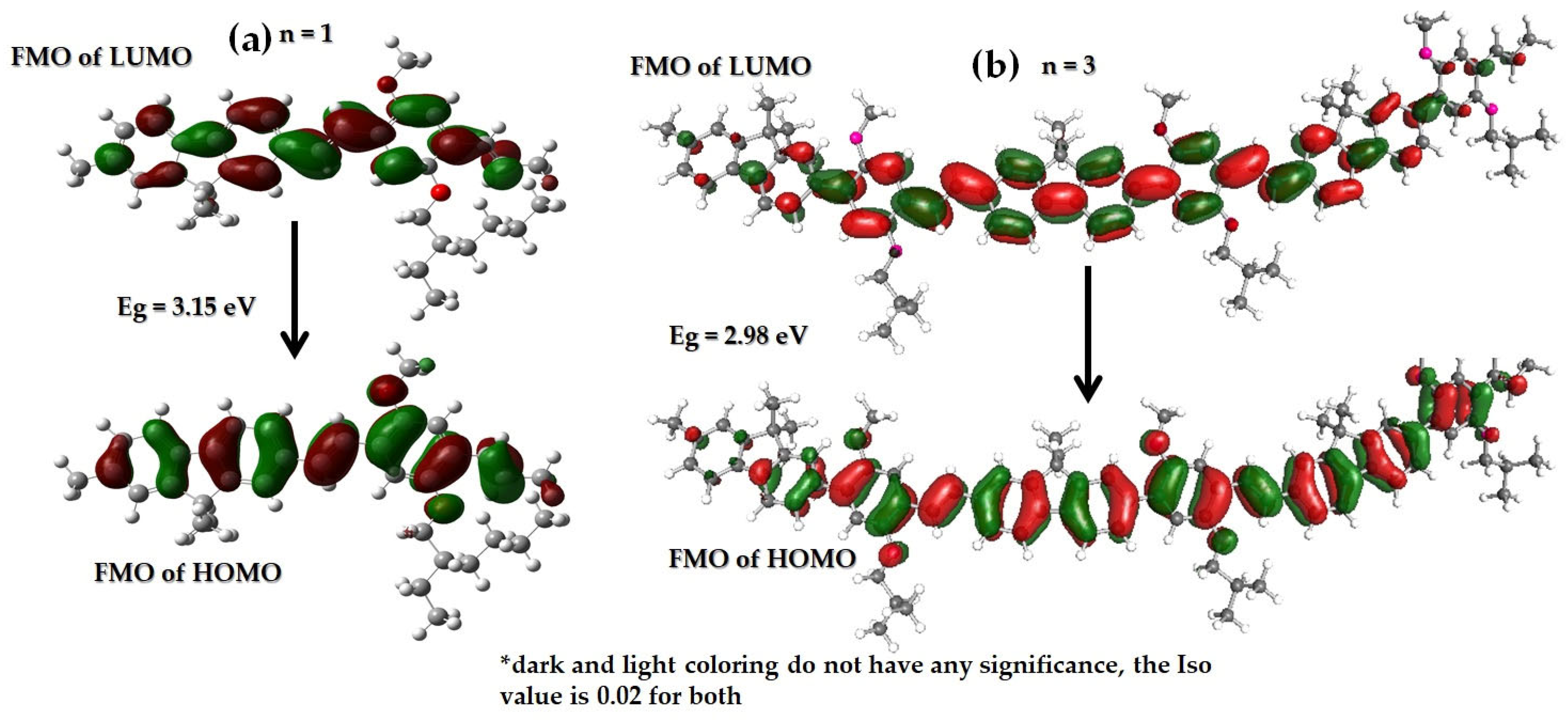
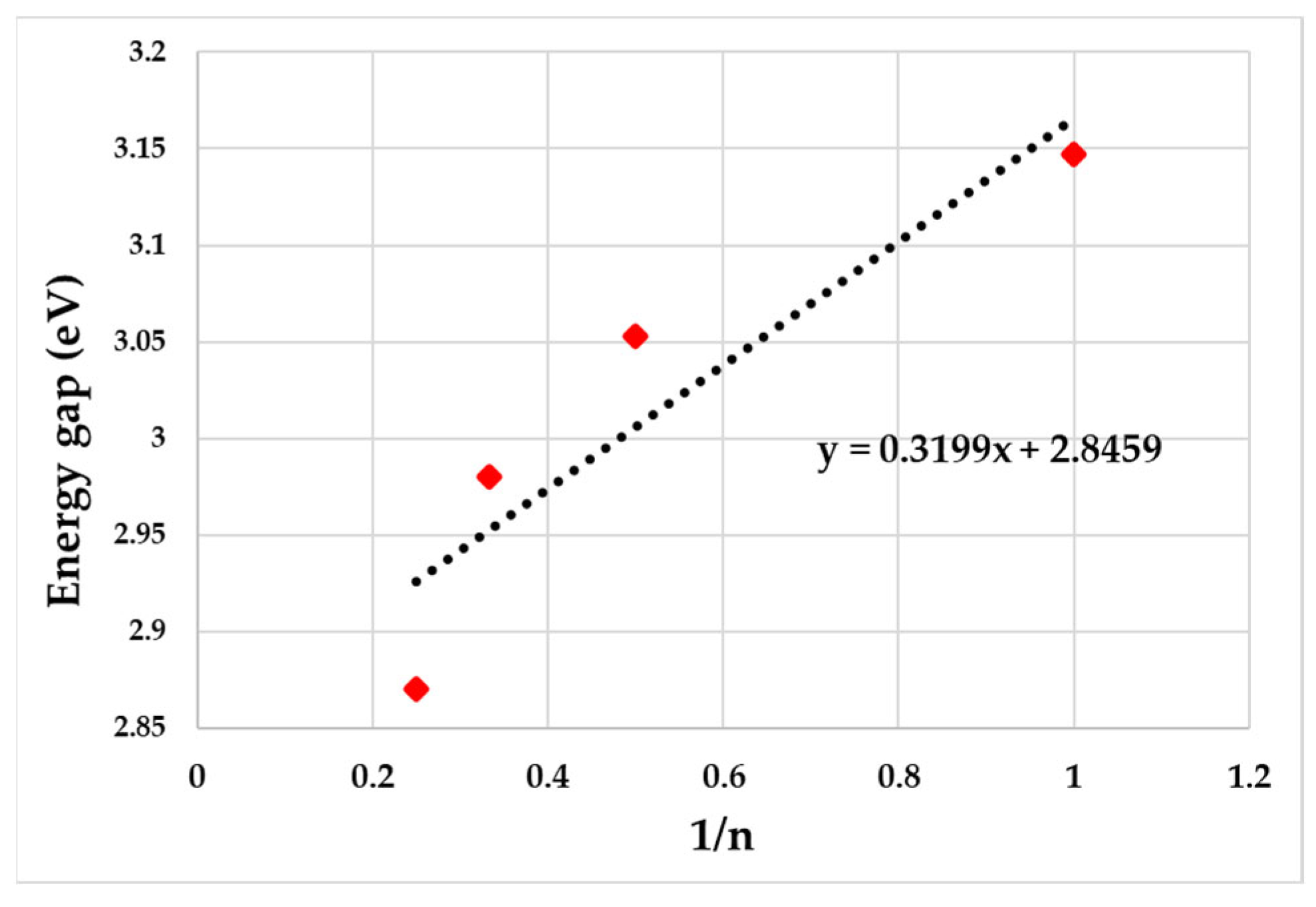
3.1. Computational Studies
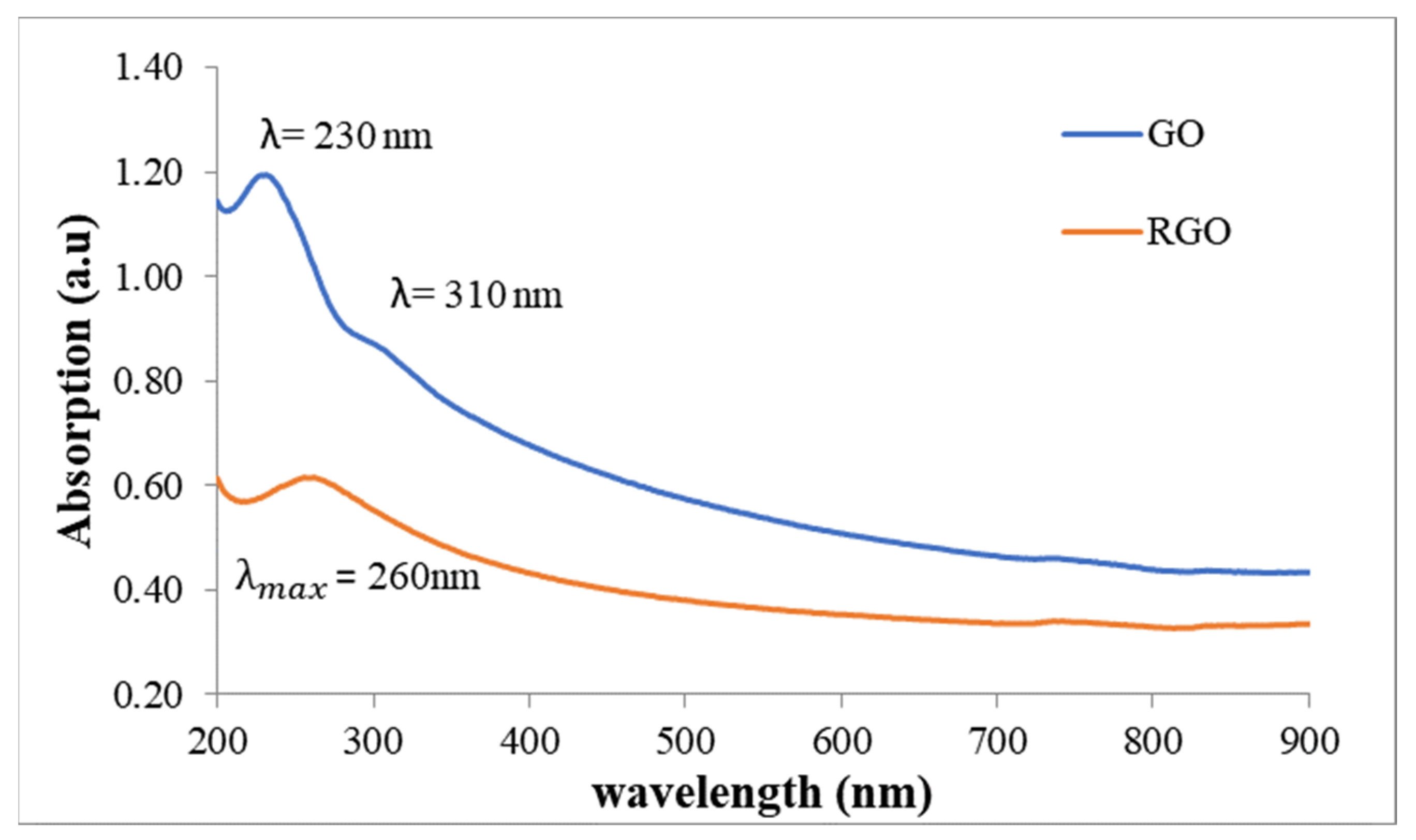
3.2. Absorption of GO and rGO in Deionized Water
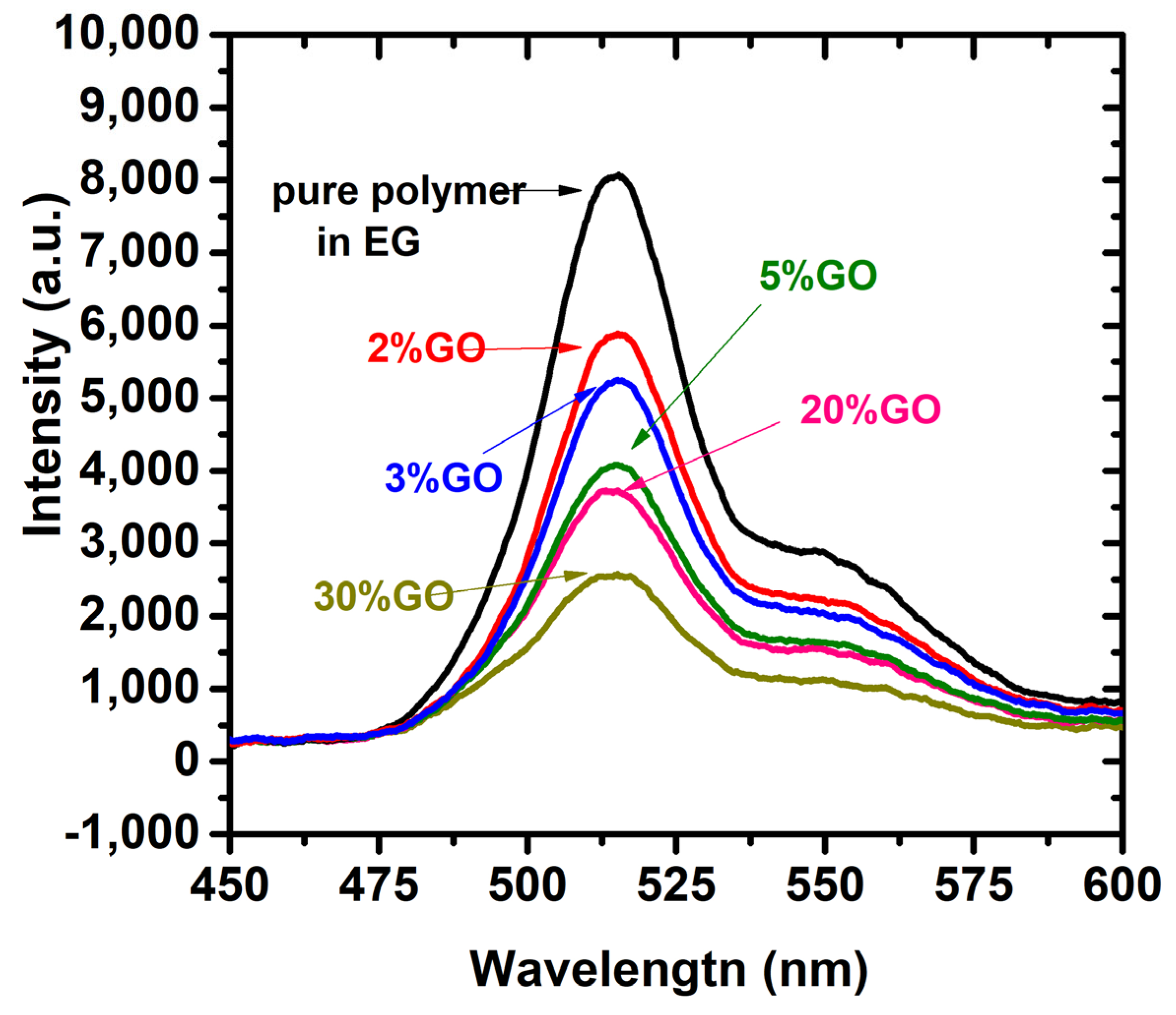
3.3. Absorption of the Polymer under GO and rGO Composites in Chloroform
3.4. Fluorescence of CP/GO and CP/rGO Composite in Chloroform
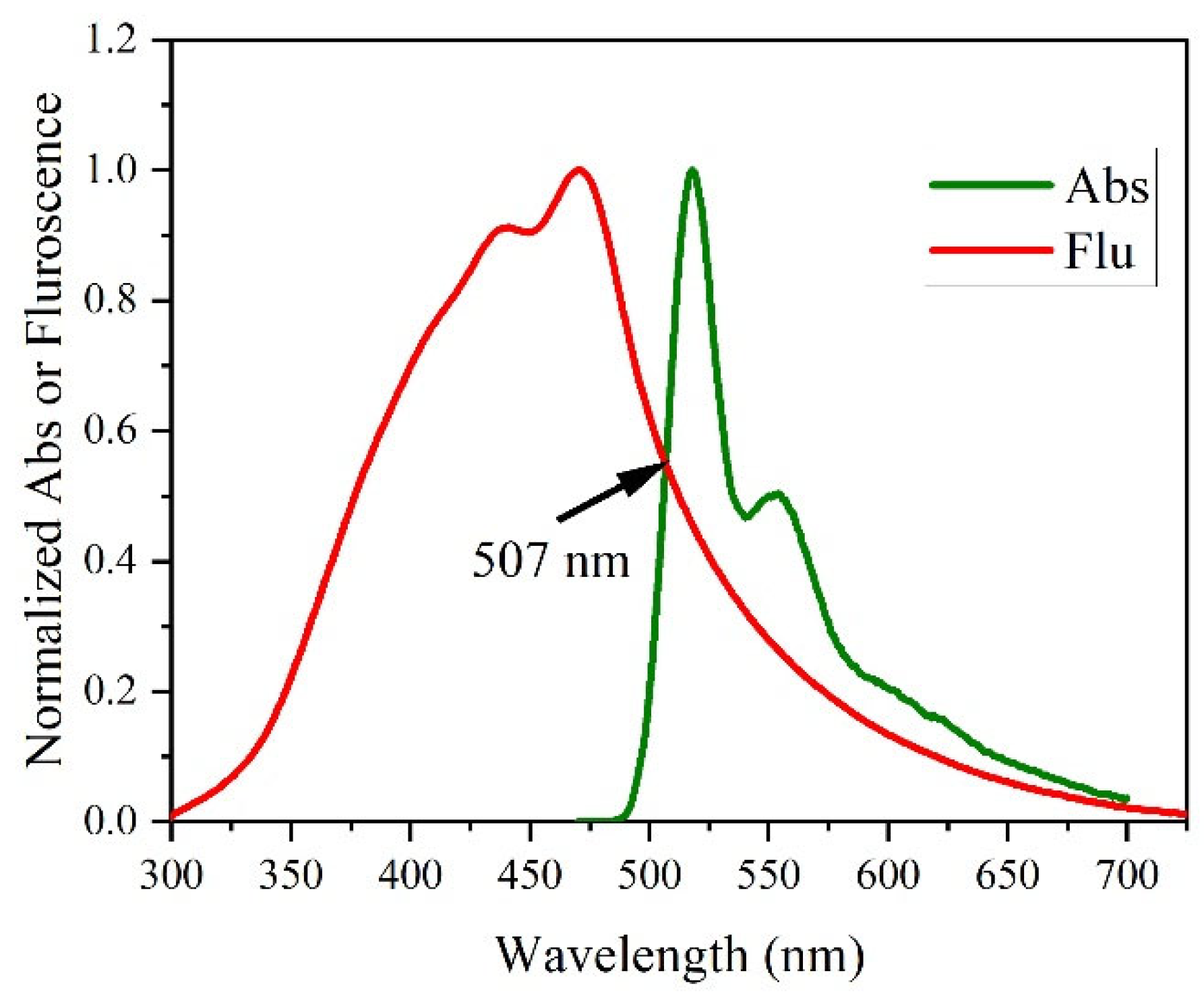
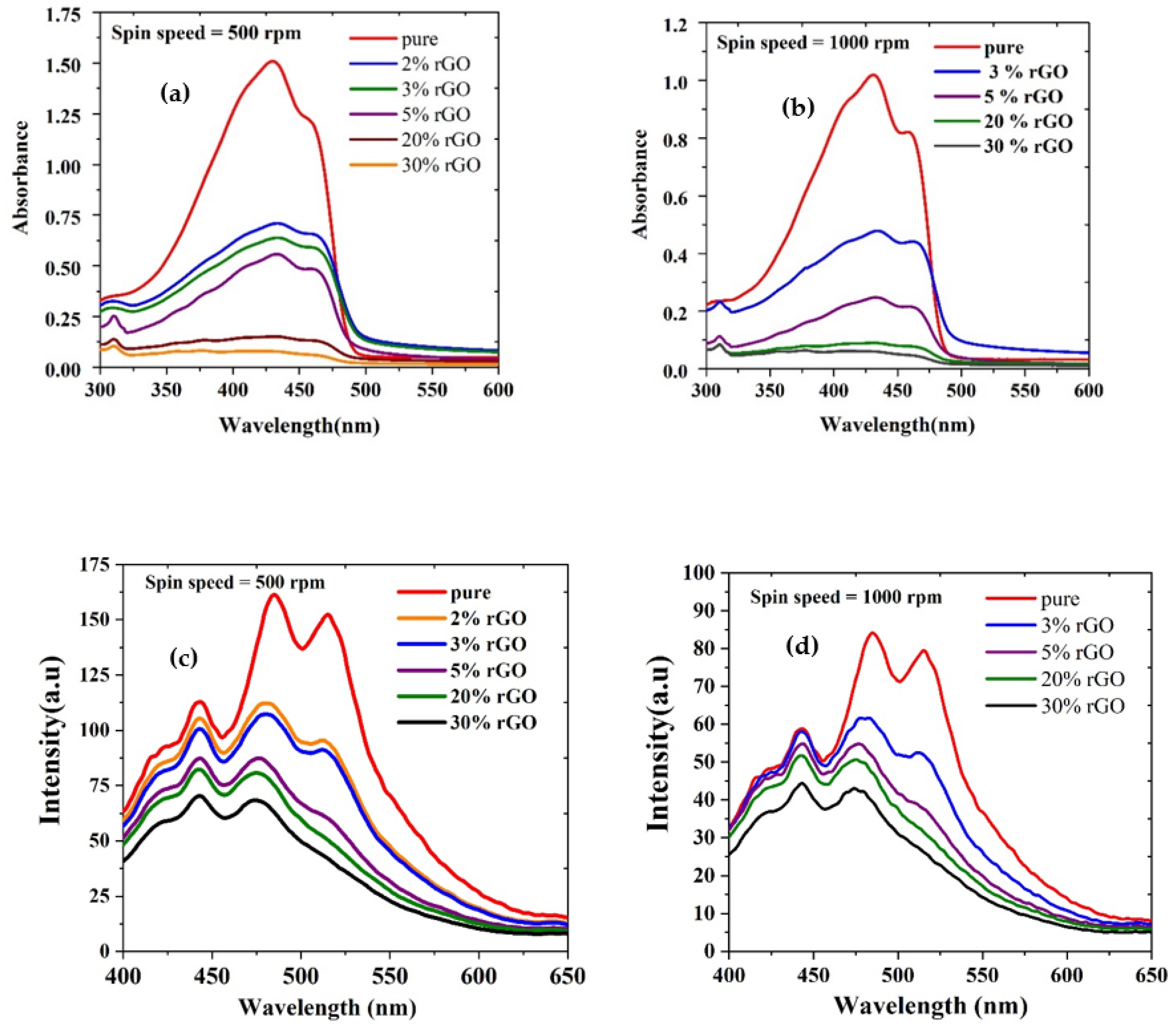
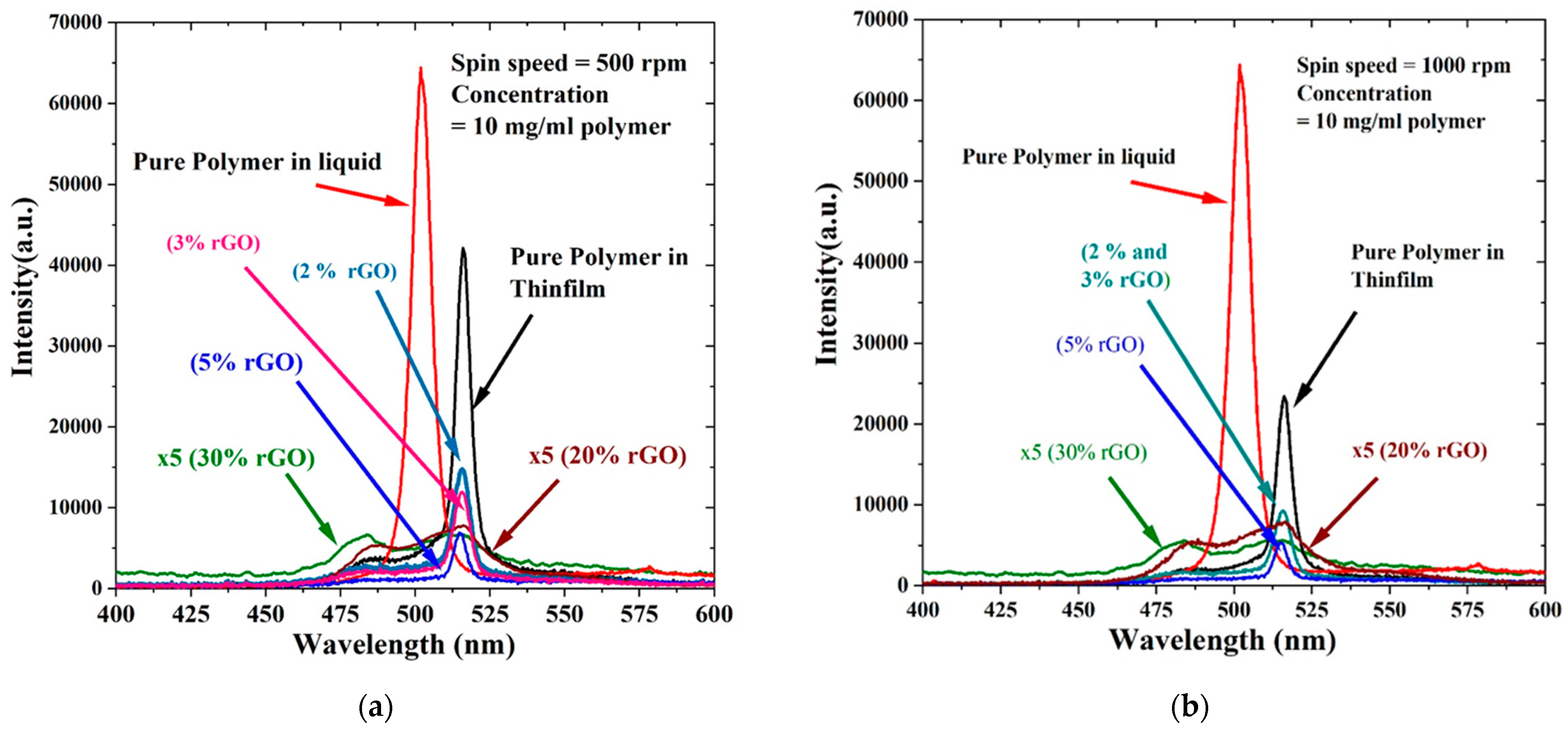
3.5. Absorption of Polymer/rGO Composite in CHF (Thin Films)
3.6. Absorption of Polymer/rGO Composite in CHF (Thin Films)
3.7. Fluorescence of Polymer/rGO Composite in CHF (Thin Films)
4. Laser Properties
4.1. Laser-Induced Fluorescence (LIF) Spectra
4.2. The ASE of CP/rGO in Chloroform and Thin Films
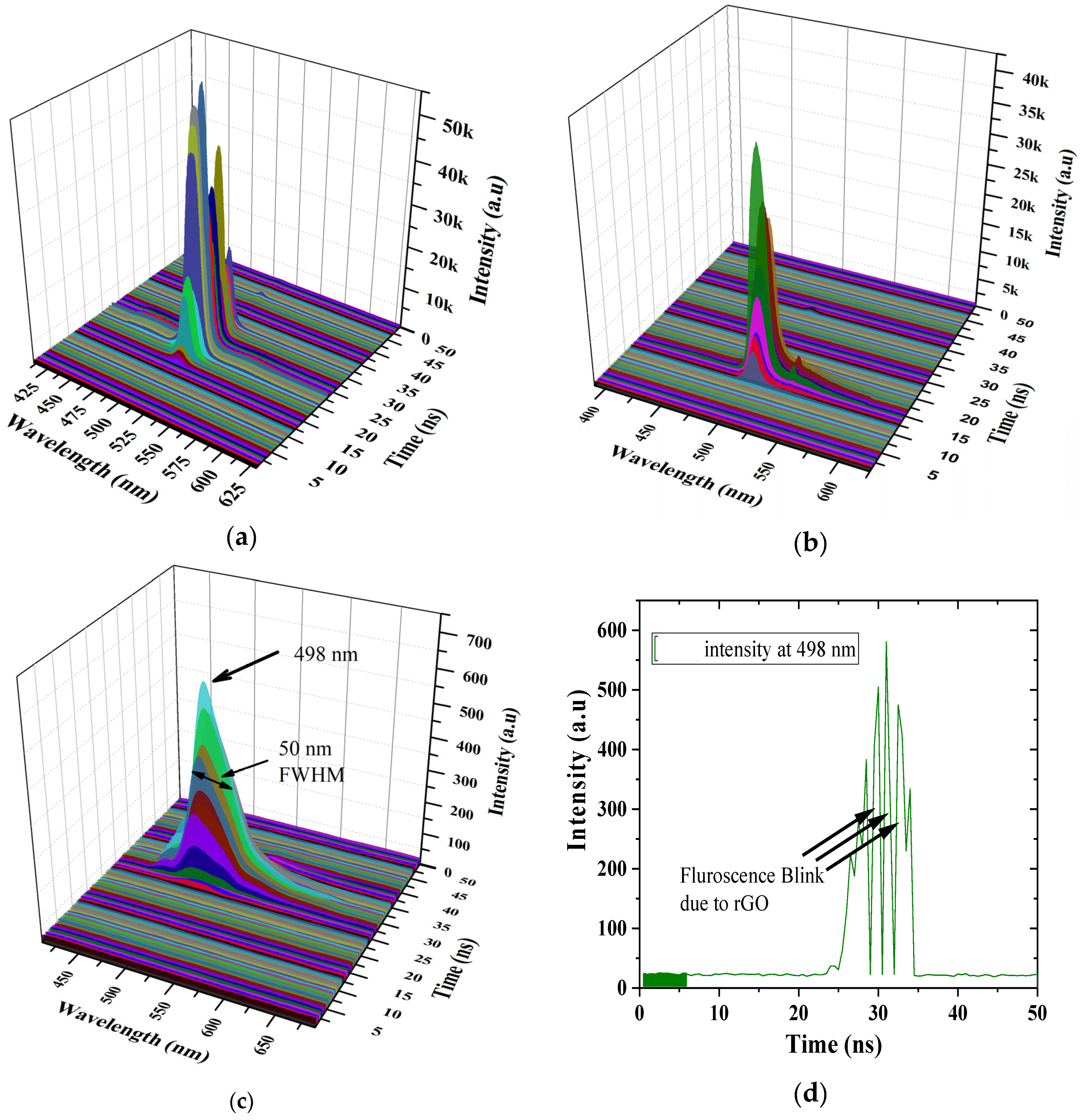
4.3. Time-Resolved Spectroscopy
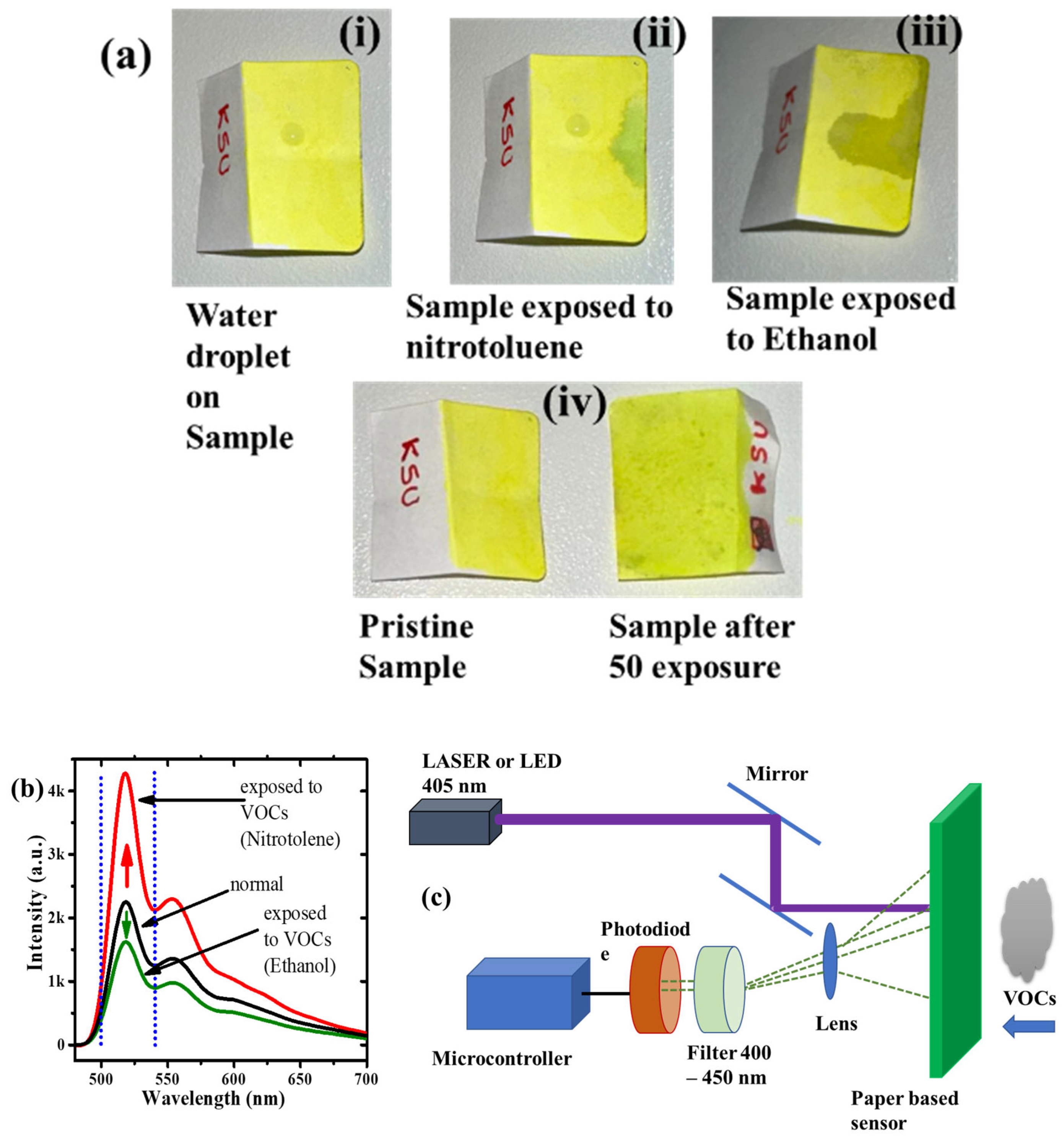
5. Sensor Design
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Zhang, J.; Tang, P.; Yu, G.; Zhang, Z.; Chen, H.; Chen, Y.; Zhao, B.; Tan, S.; Shen, P. Development of a new diindenopyrazine-benzotriazole copolymer for multifunctional application in organic field-effect transistors, polymer solar cells and light-emitting diodes. Org. Electron. 2012, 13, 1671–1679. [Google Scholar] [CrossRef]
- Jin, R.; Zhang, X.; Xiao, W. Theoretical studies of photophysical properties of d–π–a–π–D-type diketopyrrolopyrrole-based molecules for organic light-emitting diodes and organic solar cells. Molecules 2020, 25, 667. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kim, K.; Hong, J.-P.; Lee, W.; Lee, S.; Hong, J.-I. A multifunctional material based on triphenylamine and a naphthyl unit for organic light-emitting diodes, organic solar cells, and organic thin-film transistors. Bull. Korean Chem. Soc. 2013, 34, 1355. [Google Scholar] [CrossRef]
- Xia, H.; Hu, C.; Chen, T.; Hu, D.; Zhang, M.; Xie, K. Advances in conjugated polymer lasers. Polymers 2019, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Tessler, N. Lasers based on semiconducting organic materials. Adv. Mater. 1999, 11, 363–370. [Google Scholar] [CrossRef]
- Kallinger, C.; Hilmer, M.; Haugeneder, A.; Perner, M.; Spirkl, W.; Lemmer, U.; Feldmann, J.; Scherf, U.; Müllen, K.; Gombert, A.; et al. A flexible conjugated polymer laser. Adv. Mater. 1998, 10, 920–923. [Google Scholar] [CrossRef]
- Oyaizu, K.; Materials, H.N.-A. Radical polymers for organic electronic devices: A radical departure from conjugated polymers? Adv. Mater. 2009, 21, 2339–2344. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, F.; Feng, X. Patterning of conjugated polymers for organic optoelectronic devices. Small 2011, 7, 1338–1360. [Google Scholar] [CrossRef]
- Brabec, C.J.; Durrant, J.R. Solution-processed organic solar cells. MRS Bull. 2008, 33, 670–675. [Google Scholar] [CrossRef]
- Sirringhaus, H.; Ando, M. Materials challenges and applications of solution-processed organic field-effect transistors. MRS Bull. 2008, 33, 676–682. [Google Scholar] [CrossRef]
- Diao, Y.; Shaw, L.; Bao, Z.; Mannsfeld, S.C.B. Morphology control strategies for solution-processed organic semiconductor thin films. Energy Environ. Sci. 2014, 7, 2145–2159. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Fakharuddin, A.; García de Arquer, F.P.; Georgiadou, D.G.; Kim, H.; bin Mohd Yusoff, A.R.; Gao, F.; Nazeeruddin, M.K.; Bolink, H.J.; Sargent, E.H. Advances in solution-processed near-infrared light-emitting diodes. Nat. Photonics 2021, 15, 656–669. [Google Scholar] [CrossRef]
- Doustkhah, E.; Hassandoost, R.; Khataee, A.; Luque, R.; Assadi, M.H.N. Hard-templated metal–organic frameworks for advanced applications. Chem. Soc. Rev. 2021, 50, 2927–2953. [Google Scholar] [CrossRef] [PubMed]
- Doustkhah, E.; Kotb, A.; Tafazoli, S.; Balkan, T.; Kaya, S.; Hanaor, D.A.H.; Assadi, M.H.N. Templated Synthesis of Exfoliated Porous Carbon with Dominant Graphitic Nitrogen. ACS Mater. Au 2023, 3, 231–241. [Google Scholar] [CrossRef]
- Boroumand, F.A.; Fry, P.W.; Lidzey, D.G. Nanoscale conjugated-polymer light-emitting diodes. Nano Lett. 2005, 5, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Palilis, L.C.; Lidzey, D.G.; Redecker, M.; Bradley, D.D.C.; Inbasekaran, M.; Woo, E.P.; Wu, W.W. Bright and efficient blue and green light-emitting diodes based on conjugated polymer blends. Synth. Met. 2000, 111, 159–163. [Google Scholar] [CrossRef]
- van der Zee, B.; Paulus, S.; Png, R.-Q.; Ho, P.K.H.; Chua, L.-L.; Wetzelaer, G.-J.A.H.; Blom, P.W.M.; van der Zee, B.; Paulus, S.; Wetzelaer, G.A.H.; et al. Role of Singlet and Triplet Excitons on the Electrical Stability of Polymer Light-Emitting Diodes. Adv. Electron. Mater. 2020, 6, 2000367. [Google Scholar] [CrossRef]
- Aljaafreh, M.J.; Prasad, S.; AlSalhi, M.S.; Alhandel, R.H.; Alsaigh, R.A. TD-DFT Simulation and Experimental Studies of a Mirrorless Lasing of Poly[(9,9-dioctylfluorenyl-2,7-diyl)-co-(1,4-diphenylene-vinylene-2-methoxy-5-{2-ethylhexyloxy}-benzene)]. Polymers 2021, 13, 1430. [Google Scholar] [CrossRef]
- Jeong, J.-E.; Uddin, M.A.; Ryu, H.S.; Kim, H.-C.; Kang, M.; Joung, F.; Park, S.; Shim, S.-H.; Woo, H.Y. Green-, red-, and near-infrared-emitting polymer dot probes for simultaneous multicolor cell imaging with a single excitation wavelength. ACS Publ. 2020, 32, 6685–6696. [Google Scholar] [CrossRef]
- Hamilton, I.; Suh, M.; Kim, K.; Jeon, Y.; Bradley, D.; Kim, J.; Seon, C.; Hamilton, I.; Suh, M.; Kim, K.; et al. Organic-inorganic hybrid composites as an electron injection layer in highly efficient inverted green-emitting polymer LEDs. Org. Electron. 2019, 77, 105496. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, J.; Jiang, Y.; Chiu, D.T. Ultrabright Pdots with a Large Absorbance Cross Section and High Quantum Yield. ACS Appl. Mater. Interfaces 2022, 14, 13631–13637. [Google Scholar] [CrossRef]
- Mustapha, N.; AlSalhi, M.S.; Prasad, S. Energy transfer-enhanced external power conversion efficiency in blended polymeric thin film solar devices. J. Mater. Sci. Mater. Electron. 2019, 30, 7840–7849. [Google Scholar] [CrossRef]
- Brega, V.; Thomas, S.W., III. Red-Emitting, Acene-Doped Conjugated Polymer Nanoparticles that Respond Ratiometrically to Photogenerated 1O2. ACS Appl. Mater. Interfaces 2021, 13, 13658–13665. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, M.; Pandey, C.K.; Srivastava, R.; Dhar, R. Electrical transport properties of PFO: MEH-PPV polymer blends. Mater. Lett. 2023, 331, 133400. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A. Dual Förster resonance energy transfer in ternary PFO/MEH-PPV/F7GA hybrid thin films for white organic light-emitting diodes. Dye Pigment. 2021, 186, 109011. [Google Scholar] [CrossRef]
- Cheon, H.J.; Shin, S.Y.; Van Tran, V.; Park, B.; Yoon, H.; Chang, M. Preparation of conjugated polymer/reduced graphene oxide nanocomposites for high-performance volatile organic compound sensors. Chem. Eng. J. 2021, 425, 131424. [Google Scholar] [CrossRef]
- da Silva Gascho, J.L.; da Costa, S.F.; Morcelles, K.F.; Filho, P.B.; Recco, A.A.C.; Pezzin, S.H. The effect of silver nanowires on the formation of aggregates of poly(3-hexylthiophene) in films deposited on reduced graphene oxide. J. Nanoparticle Res. 2021, 23, 153. [Google Scholar] [CrossRef]
- Ran, C.; Wang, M.; Gao, W.; Ding, J.; Shi, Y.; Song, X.; Chen, H.; Ren, Z. Study on photoluminescence quenching and photostability enhancement of MEH-PPV by reduced graphene oxide. J. Phys. Chem. C 2012, 116, 23053–23060. [Google Scholar] [CrossRef]
- Agbolaghi, S.; Ebrahimi, S.; Massoumi, B.; Abbaspoor, S.; Sarvari, R.; Abbasi, F. Enhanced properties of photovoltaic devices tailored with novel supramolecular structures based on reduced graphene oxide nanosheets grafted/functionalized with thiophenic materials. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 1877–1889. [Google Scholar] [CrossRef]
- Kausar, A. Conjugated polymer/graphene oxide nanocomposites—State-of-the-art. J. Compos. Sci. 2021, 5, 292. [Google Scholar] [CrossRef]
- Compagnini, G.; Russo, P.; Tomarchio, F.; Puglisi, O.; D’Urso, L.; Scalese, S. Laser assisted green synthesis of free standing reduced graphene oxides at the water-air interface. Nanotechnology 2012, 23, 505601. [Google Scholar] [CrossRef]
- Minkin, V.I. Dipole Moments in Organic Chemistry; Springer Science & Business Media: Berlin, Germany, 2012; ISBN 1468417703. [Google Scholar]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef]
- Tadyszak, K.; Wychowaniec, J.; Litowczenko, J. Biomedical applications of graphene-based structures. Nanomaterials 2018, 8, 944. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.G.; Muthoosamy, K.; Manickam, S.; Hilal-Alnaqbi, A. Graphene-based 3D scaffolds in tissue engineering: Fabrication, applications, and future scope in liver tissue engineering. Int. J. Nanomed. 2019, 14, 5753–5783. [Google Scholar] [CrossRef]
- Prasad, S.; Aljaafreh, M.J.; AlSalhi, M.S. Time-resolved spectroscopy of radiative energy transfer between a conjugated oligomer and polymer in solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 232, 118151. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian 16; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lazare, J.; Daggag, D.; Dinadayalane, T. DFT study on binding of single and double methane with aromatic hydrocarbons and graphene: Stabilizing CH…HC interactions between two methane molecules. Struct. Chem. 2021, 32, 591–605. [Google Scholar] [CrossRef]
- Al-Bagawi, A.H.; Bayoumy, A.M.; Ibrahim, M.A. Molecular modeling analyses for graphene functionalized with Fe3O4 and NiO. Heliyon 2020, 6, E04456. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Soltanabadi, A.; Abdouss, M.; Mazinani, S. Investigating the structure of the product of graphene oxide reaction with folic acid and chitosan: Density functional theory calculations. J. Biomol. Struct. Dyn. 2021, 40, 14146–14159. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, Y.; Zhou, L.; Li, B.; Gu, L. Photoluminescence and fluorescence quenching of graphene oxide: A review. Nanomaterials 2022, 12, 2444. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Gao, W.; Yan, F.; Ji, H.; Ju, H. Fluorescence resonance energy transfer between quantum dots and graphene oxide for sensing biomolecules. Anal. Chem. 2010, 82, 5511–5517. [Google Scholar] [CrossRef]
- Lai, Q.; Zhu, S.; Luo, X.; Zou, M.; Huang, S. Ultraviolet-visible spectroscopy of graphene oxides. AIP Adv. 2012, 2, 032146. [Google Scholar] [CrossRef]
- Saxena, S.; Tyson, T.; Shukla, S.; Negusse, E.; Chen, H.; Bai, J. Investigation of structural and electronic properties of graphene oxide. Appl. Phys. Lett. 2011, 99, 013104. [Google Scholar] [CrossRef]
- Verástegui-Domínguez, L.H.; Elizondo-Villarreal, N.; Irma Martínez-Delgado, D.; Ángel Gracia-Pinilla, M. Eco-Friendly Reduction of Graphene Oxide by Aqueous Extracts for Photocatalysis Applications. Nanomaterials 2022, 12, 3882. [Google Scholar] [CrossRef]
- Bozkurt, E.; Acar, M.; Onganer, Y.; Meral, K. Rhodamine 101-graphene oxide composites in aqueous solution: The fluorescence quenching process of rhodamine 101. Phys. Chem. Chem. Phys. 2014, 16, 18276–18281. [Google Scholar] [CrossRef] [PubMed]
- AlSalhi, M.S.; Aljaafreh, M.J.; Prasad, S. Narrowband spontaneous emission amplification from a conjugated oligomer thin film. Polymers 2020, 12, 232. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Mármol, R.; Bonal, V.; Paternò, G.M.; Ross, A.M.; Boj, P.G.; Villalvilla, J.M.; Quintana, J.A.; Scotognella, F.; D’andrea, C.; Sardar, S.; et al. Dual amplified spontaneous emission and lasing from nanographene films. Nanomaterials 2020, 10, 1525. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Aljaafreh, M.J.; Masilamani, V.; AlSalhi, M.S.; Mujamammi, W.M. Time-resolved excited state dynamics of super-exciplex in the coumarin dye laser. J. Mol. Liq. 2020, 315, 113814. [Google Scholar] [CrossRef]




| Film Name | Speed (rpm) | Concentration Ratio (%) | Thickness (nm) |
|---|---|---|---|
| F1 | 500 | 2% | 360 nm |
| F2 | 500 | 3% | 357 nm |
| F3 | 500 | 5% | 358 nm |
| F4 | 500 | 20% | 342 nm |
| F5 | 500 | 30% | 320 nm |
| F6 | 1000 | 2% | 208 nm |
| F7 | 1000 | 3% | 210 nm |
| F8 | 1000 | 5% | 195 nm |
| F9 | 1000 | 20% | 185 nm |
| F10 | 1000 | 30% | 181 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasad, S.; Alhandel, R.H.; Asemi, N.N.; AlSalhi, M.S. Effects of Graphene Oxide (GO) and Reduced Graphene Oxide (rGO) on Green-Emitting Conjugated Copolymer’s Optical and Laser Properties Using Simulation and Experimental Studies. Polymers 2023, 15, 4572. https://doi.org/10.3390/polym15234572
Prasad S, Alhandel RH, Asemi NN, AlSalhi MS. Effects of Graphene Oxide (GO) and Reduced Graphene Oxide (rGO) on Green-Emitting Conjugated Copolymer’s Optical and Laser Properties Using Simulation and Experimental Studies. Polymers. 2023; 15(23):4572. https://doi.org/10.3390/polym15234572
Chicago/Turabian StylePrasad, Saradh, Raya H. Alhandel, Nassar N. Asemi, and Mohamad S. AlSalhi. 2023. "Effects of Graphene Oxide (GO) and Reduced Graphene Oxide (rGO) on Green-Emitting Conjugated Copolymer’s Optical and Laser Properties Using Simulation and Experimental Studies" Polymers 15, no. 23: 4572. https://doi.org/10.3390/polym15234572