A Comparative Study on Crystallisation for Virgin and Recycled Polyethylene Terephthalate (PET): Multiscale Effects on Physico-Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Processing Protocols
2.2. Melt Flow Index (MFI)
2.3. Size Exclusion Chromatography (SEC)
2.4. Polarised Optical Microscopy
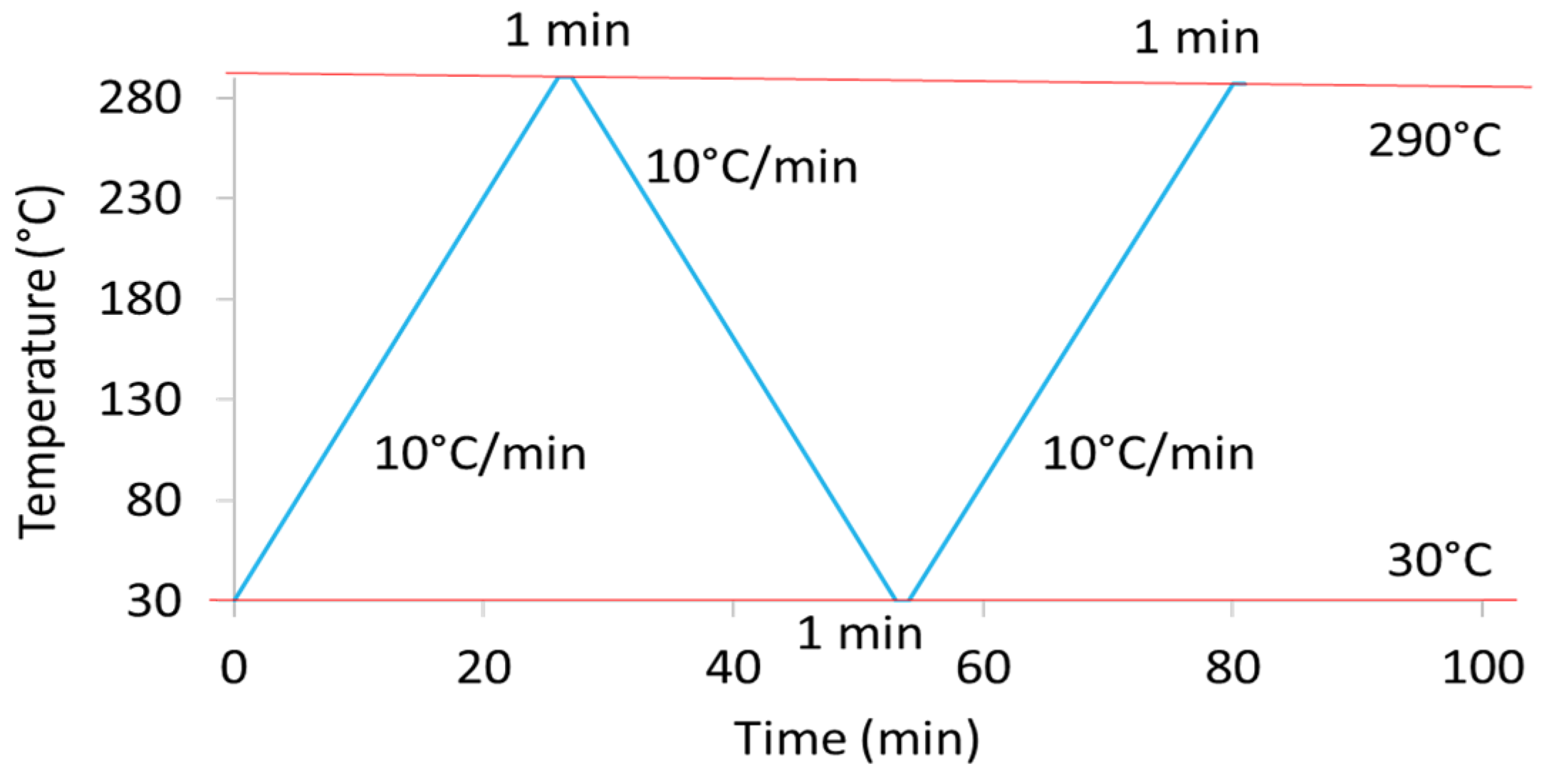
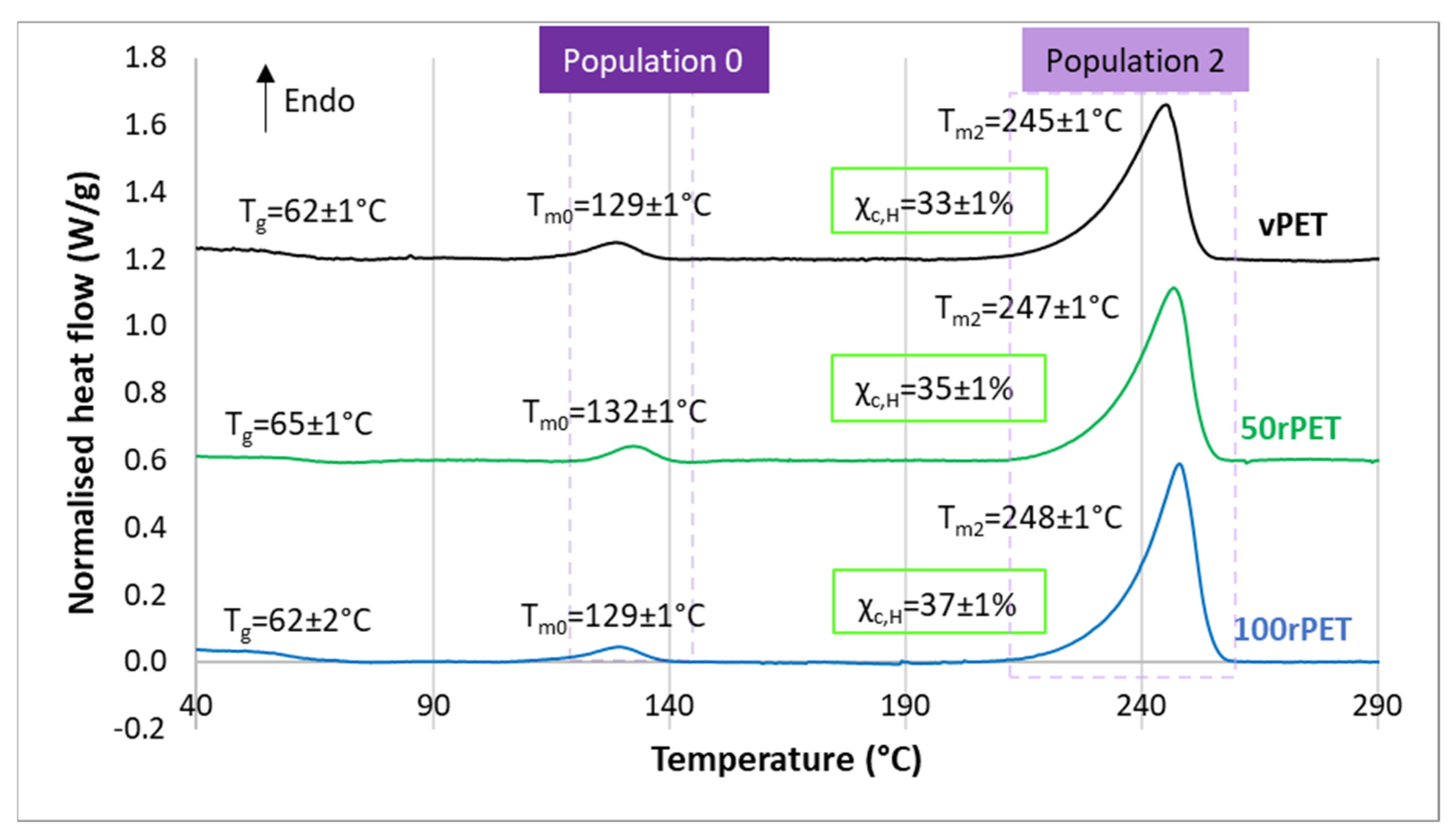
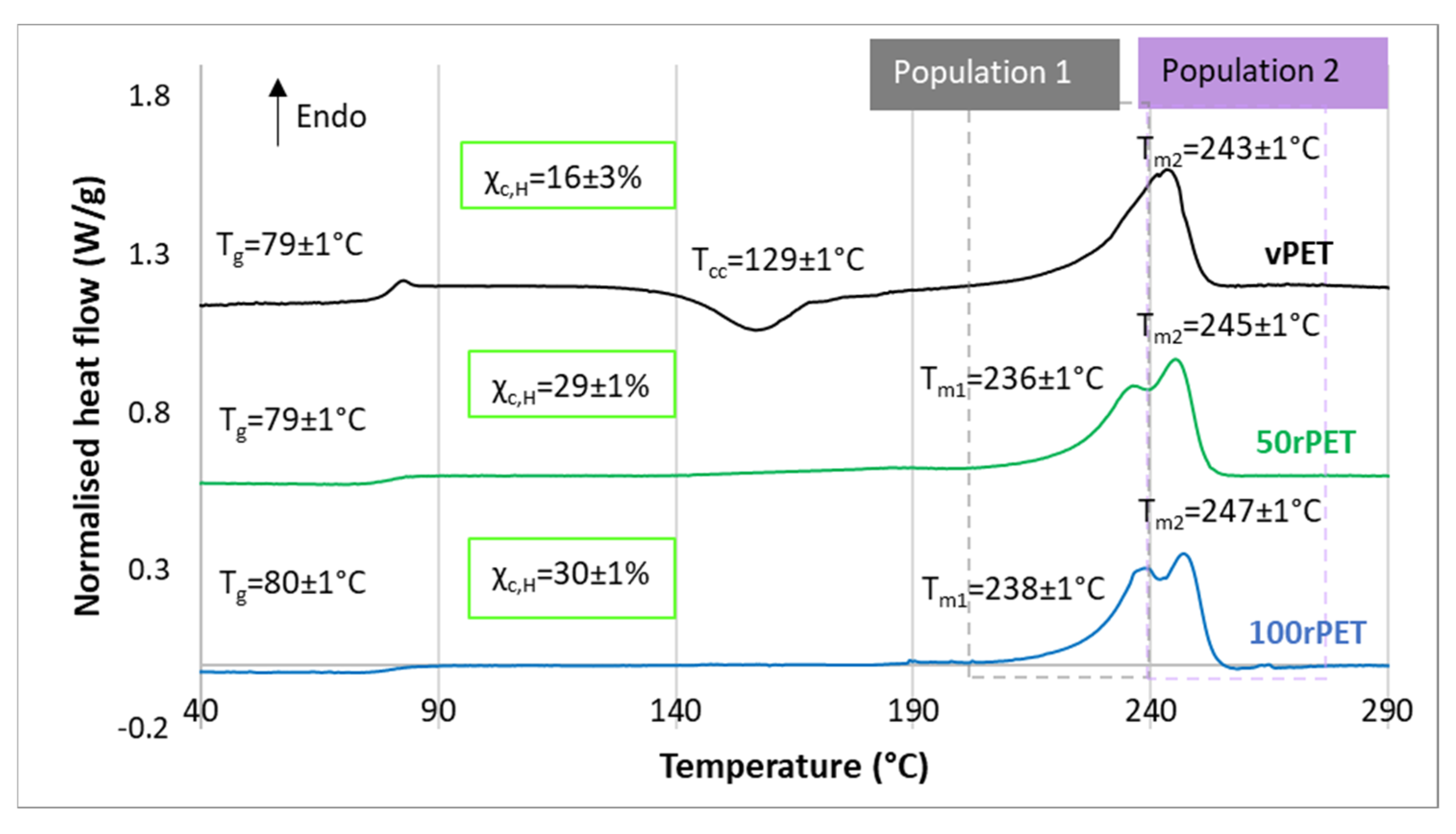
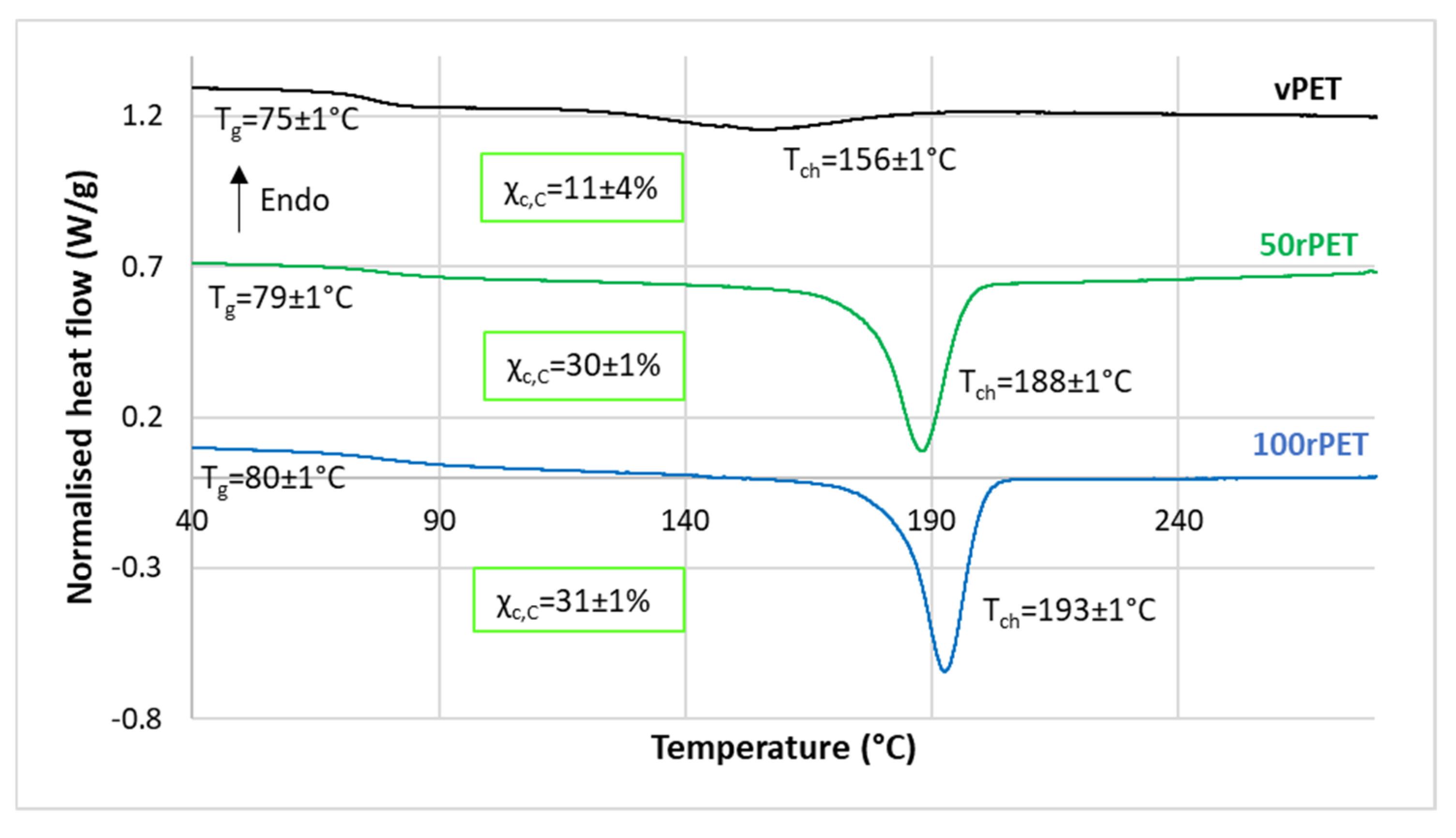
2.5. Differential Scanning Calorimetry (DSC)
2.6. Small-Angle X-ray Scattering (SAXS)
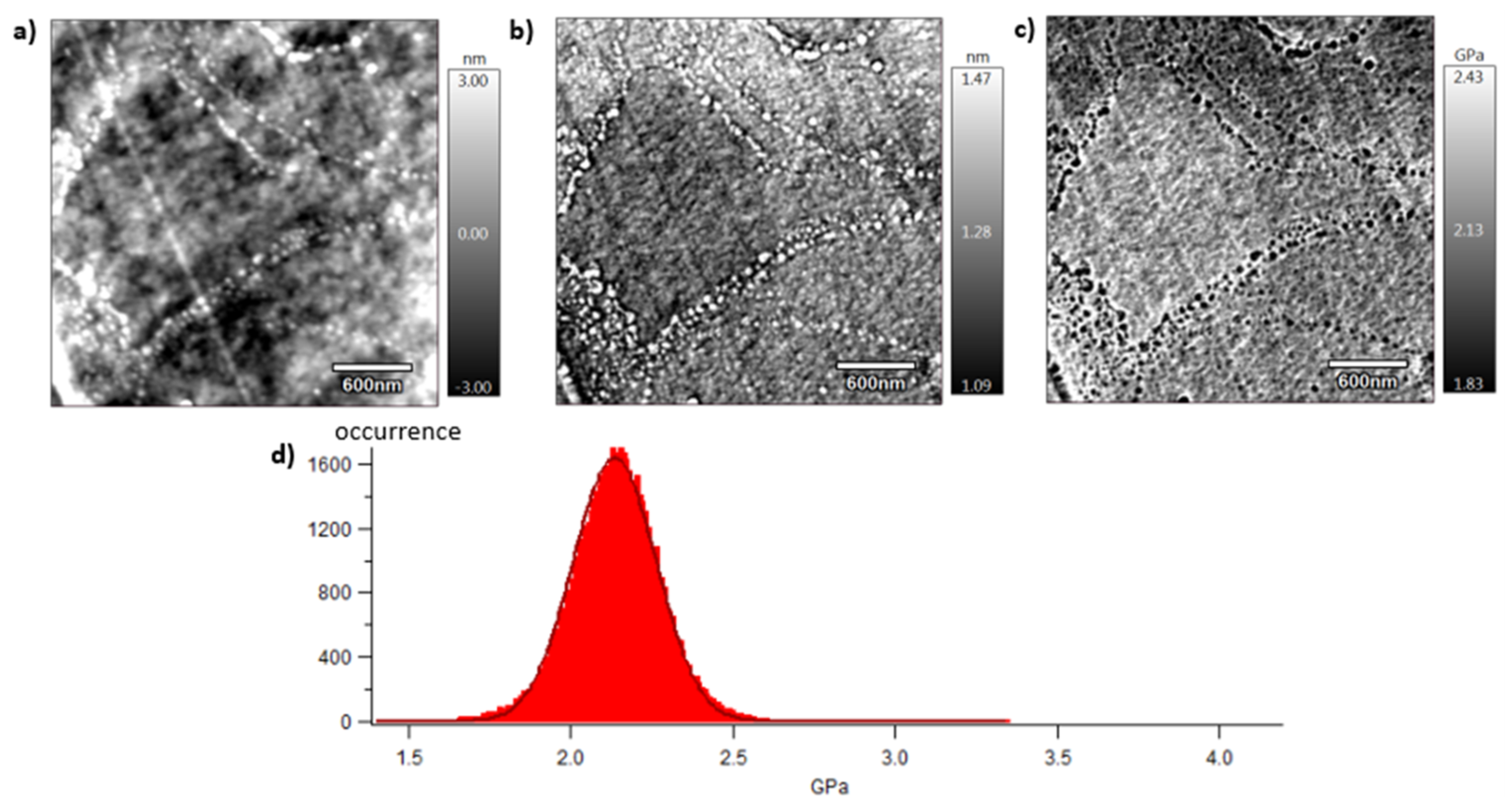
2.7. Atomic Force Microscopy (AFM)
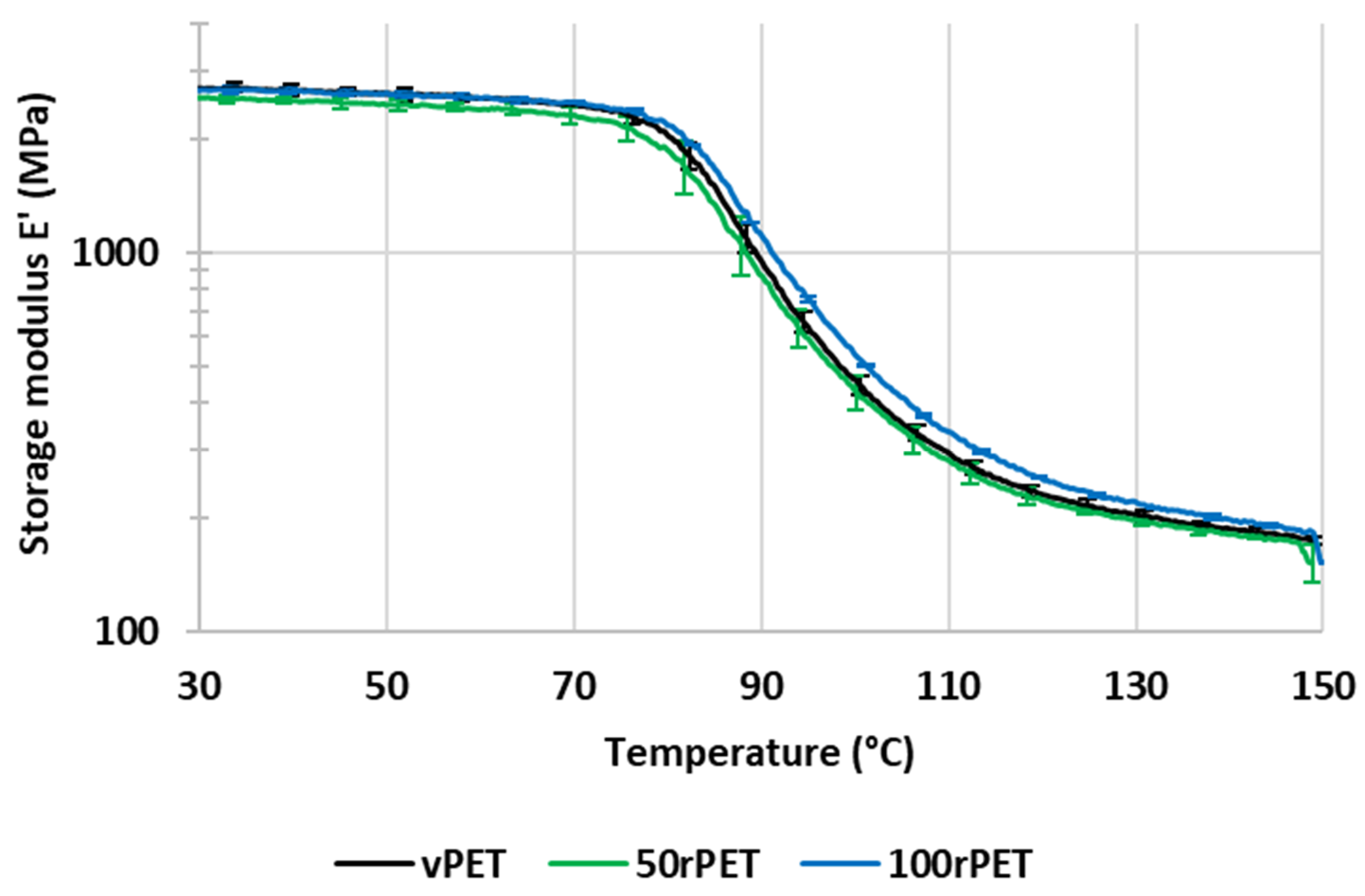
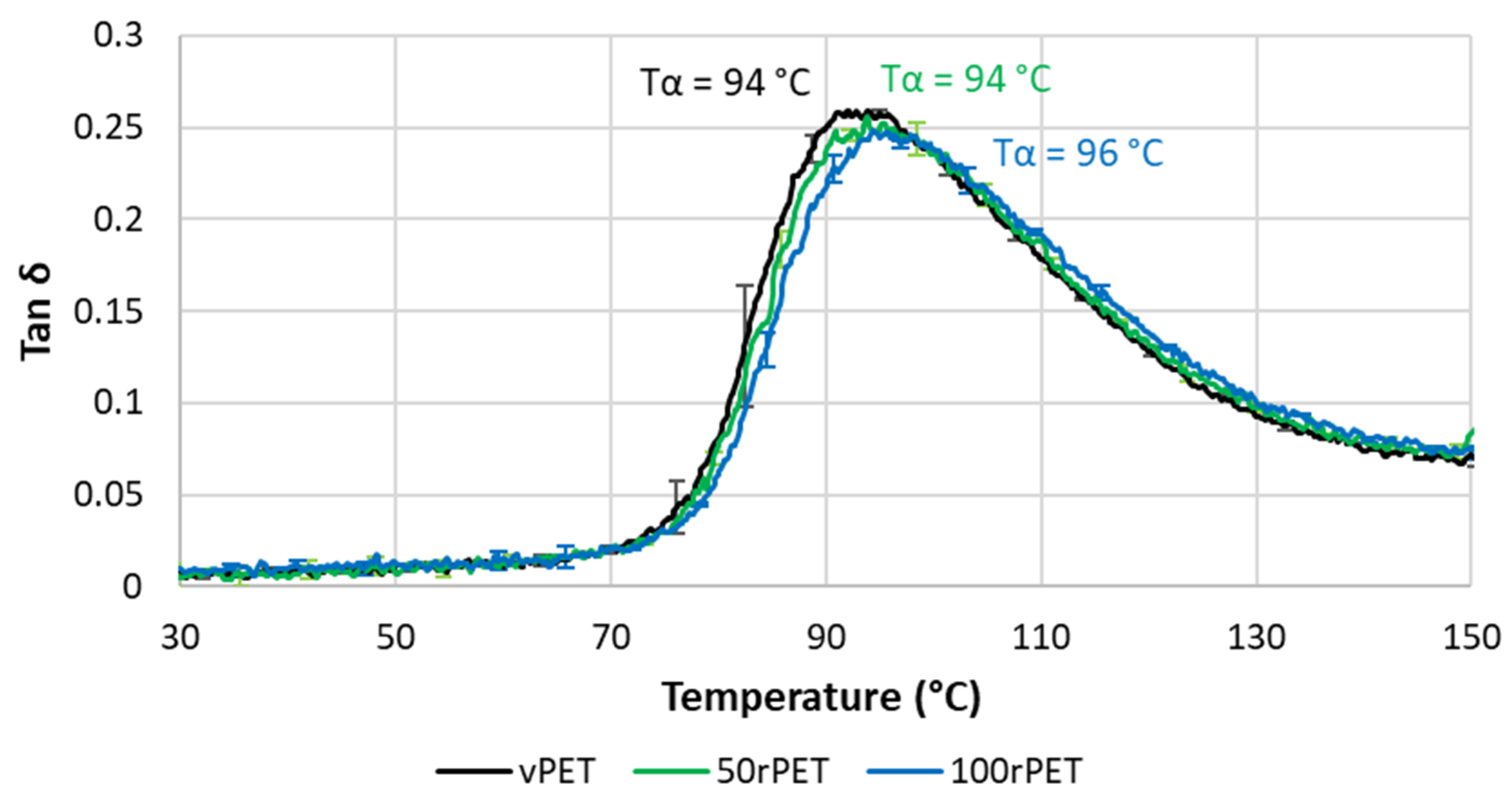
2.8. Dynamic Mechanical Analysis (DMA)
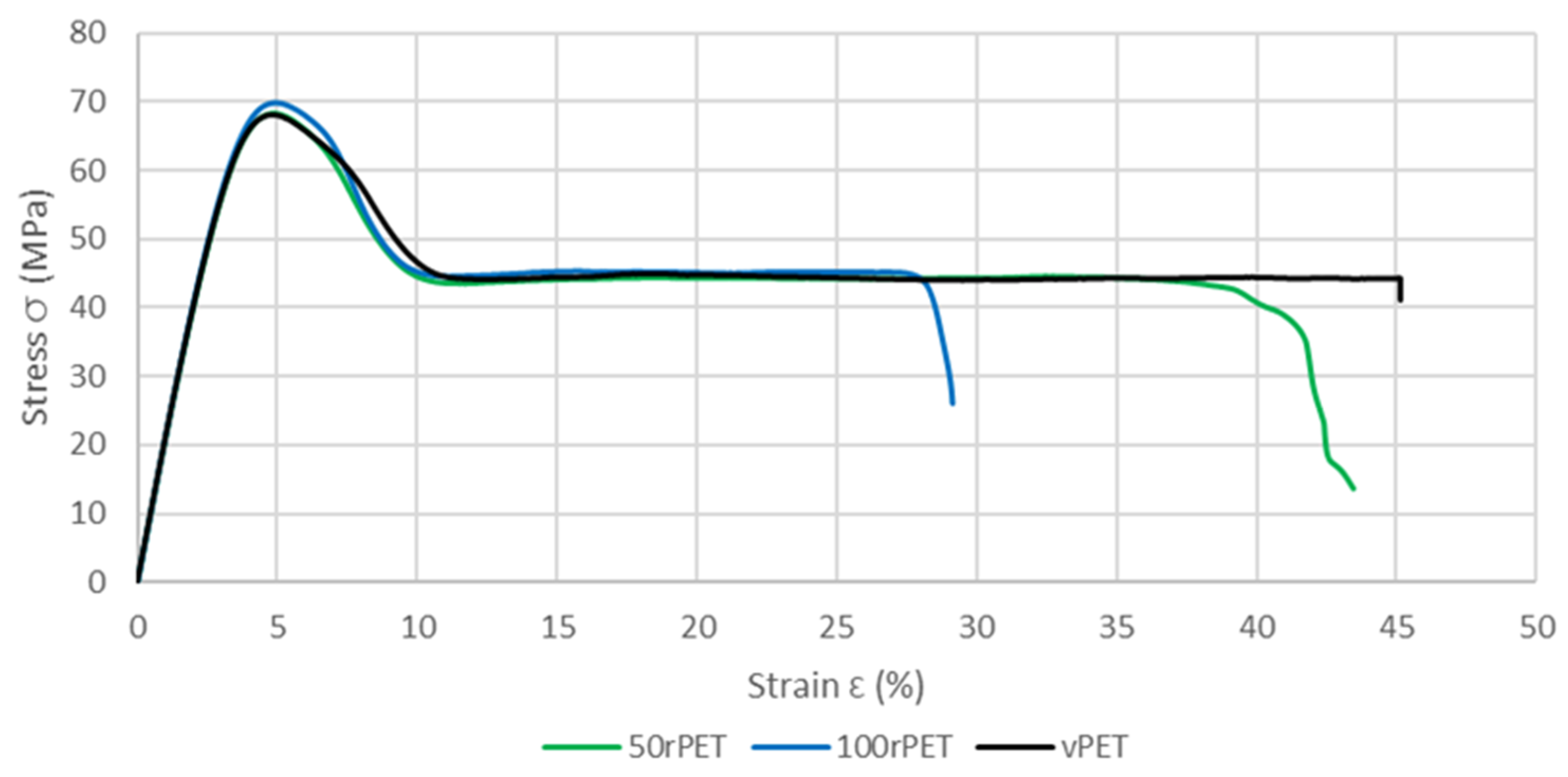
2.9. Uni-Axial Tensile Test
3. Results and Discussion
3.1. Molecular Weight and Rheological Properties
3.2. Crystalline Morphologies Observation and Thermal Behaviour Analysis
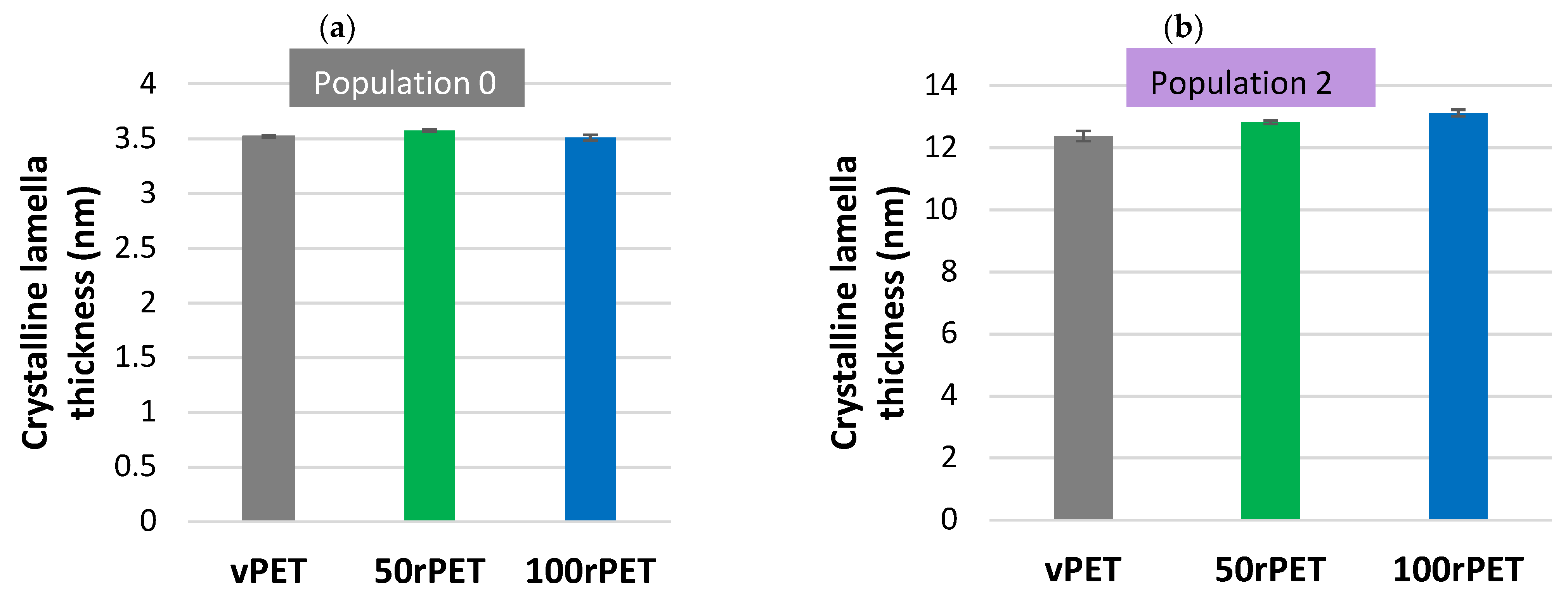
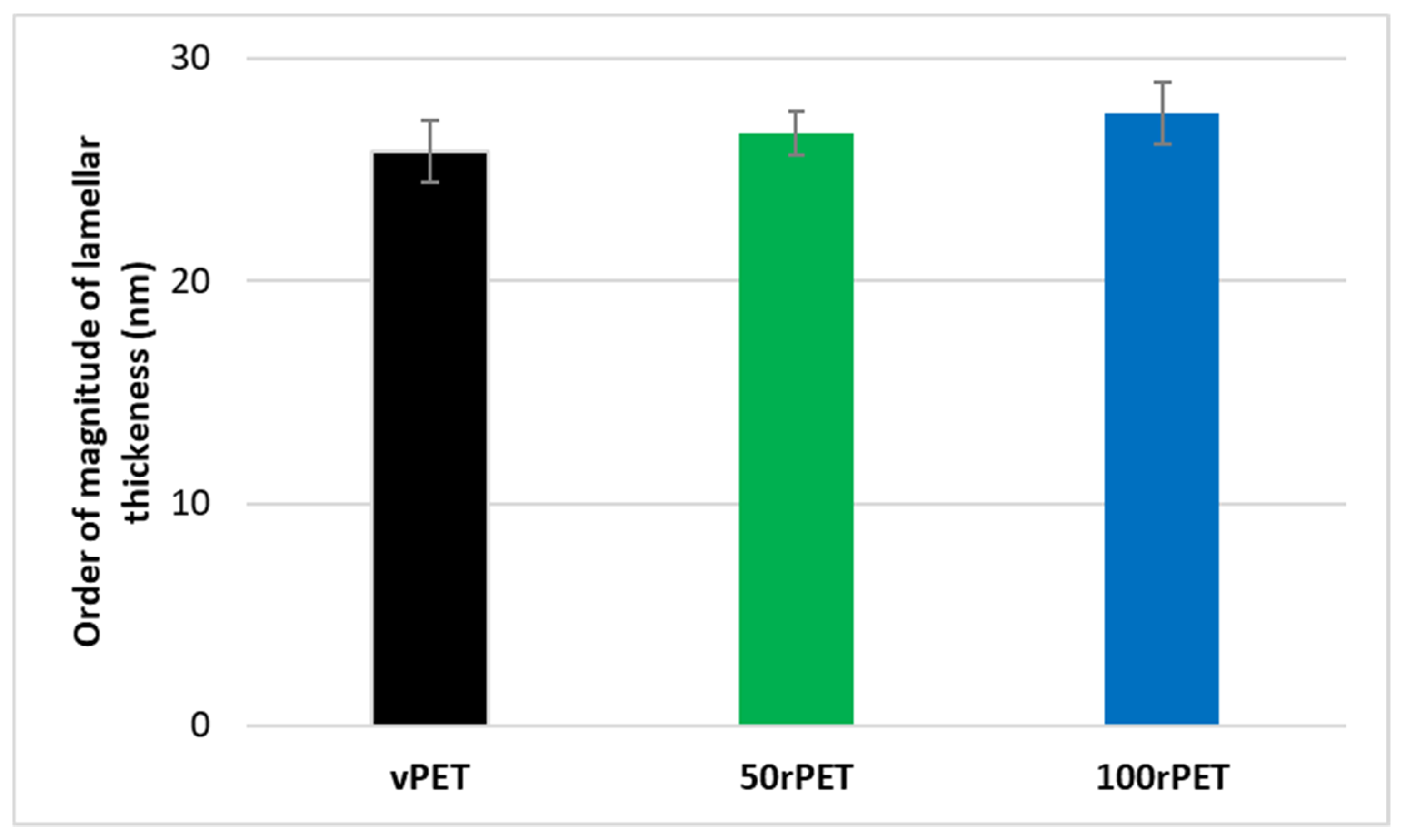
3.3. Crystalline Morphologies Description: Lamella Thickness Measurements
3.4. Mechanical Behaviour at the Mesoscopic Scale
3.5. Mechanical Behaviour at the Macroscopic Scale
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandal, S.; Dey, A. 1—PET Chemistry. In Recycling of Polyethylene Terephthalate Bottles, 1st ed.; Thomas, S., Rane, A., Kanny, K., Abitha, A.K., Thomas, M.G., Eds.; Plastics Design Library; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 1–22. [Google Scholar]
- Nisticò, R. Polyethylene Terephthalate (PET) in the Packaging Industry. Polym. Test. 2020, 90, 106707. [Google Scholar] [CrossRef]
- Antonopoulos, I.; Faraca, G.; Tonini, D. Recycling of Post-Consumer Plastic Packaging Waste in the EU: Recovery Rates, Material Flows, and Barriers. Waste Manag. 2021, 126, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Plastics—The Facts 2020 • Plastics Europe. Plastics Europe. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2020/ (accessed on 18 November 2021).
- Coelho, T.M.; Castro, R.; Gobbo, J.A. PET Containers in Brazil: Opportunities and Challenges of a Logistics Model for Post-Consumer Waste Recycling. Resour. Conserv. Recycl. 2011, 55, 291–299. [Google Scholar] [CrossRef]
- Sang, T.; Wallis, C.J.; Hill, G.; Britovsek, G.J.P. Polyethylene Terephthalate Degradation under Natural and Accelerated Weathering Conditions. Eur. Polym. J. 2020, 136, 109873. [Google Scholar] [CrossRef]
- The Road to 90%. How to Achieve the EU’s PET Bottle Collection Bottle Collection Targets by 2029|petcore-europe.org. Available online: https://www.petcore-europe.org/legislation/334-how-achieve-eu-pet-bottle-collection-bottle-collection-targets-2031.html (accessed on 18 November 2021).
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics Recycling: Challenges and Opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Chilton, T.; Burnley, S.; Nesaratnam, S. A Life Cycle Assessment of the Closed-Loop Recycling and Thermal Recovery of Post-Consumer PET. Resour. Conserv. Recycl. 2010, 54, 1241–1249. [Google Scholar] [CrossRef]
- Awaja, F.; Pavel, D. Recycling of PET. Eur. Polym. J. 2005, 41, 1453–1477. [Google Scholar] [CrossRef]
- Bedell, M.; Brown, M.; Kiziltas, A.; Mielewski, D.; Mukerjee, S.; Tabor, R. A Case for Closed-Loop Recycling of Post-Consumer PET for Automotive Foams. Waste Manag. 2018, 71, 97–108. [Google Scholar] [CrossRef]
- Cakić, S.M.; Ristić, I.S.; M-Cincović, M.; Nikolić, N.Č.; Ilić, O.Z.; Stojiljković, D.T.; B-Simendić, J.K. Glycolyzed Products from PET Waste and Their Application in Synthesis of Polyurethane Dispersions. Prog. Org. Coat. 2012, 74, 115–124. [Google Scholar] [CrossRef]
- Roy, P.K.; Mathur, R.; Kumar, D.; Rajagopal, C. Tertiary Recycling of Poly(Ethylene Terephthalate) Wastes for Production of Polyurethane–Polyisocyanurate Foams. J. Environ. Chem. Eng. 2013, 1, 1062–1069. [Google Scholar] [CrossRef]
- Raheem, A.B.; Noor, Z.Z.; Hassan, A.; Abd Hamid, M.K.; Samsudin, S.A.; Sabeen, A.H. Current Developments in Chemical Recycling of Post-Consumer Polyethylene Terephthalate Wastes for New Materials Production: A Review. J. Clean. Prod. 2019, 225, 1052–1064. [Google Scholar] [CrossRef]
- Kirshanov, K.; Toms, R.; Aliev, G.; Naumova, A.; Melnikov, P.; Gervald, A. Recent Developments and Perspectives of Recycled Poly(Ethylene Terephthalate)-Based Membranes: A Review. Membranes 2022, 12, 1105. [Google Scholar] [CrossRef] [PubMed]
- Damayanti; Wu, H.-S. Strategic Possibility Routes of Recycled PET. Polymers 2021, 13, 1475. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and Chemical Recycling of Solid Plastic Waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Cecon, V.S.; Da Silva, P.F.; Curtzwiler, G.W.; Vorst, K.L. The Challenges in Recycling Post-Consumer Polyolefins for Food Contact Applications: A Review. Resour. Conserv. Recycl. 2021, 167, 105422. [Google Scholar] [CrossRef]
- Singh, A.K.; Bedi, R.; Kaith, B.S. Composite Materials Based on Recycled Polyethylene Terephthalate and Their Properties—A Comprehensive Review. Compos. Part B Eng. 2021, 219, 108928. [Google Scholar] [CrossRef]
- Lonca, G.; Lesage, P.; Majeau-Bettez, G.; Bernard, S.; Margni, M. Assessing Scaling Effects of Circular Economy Strategies: A Case Study on Plastic Bottle Closed-Loop Recycling in the USA PET Market. Resour. Conserv. Recycl. 2020, 162, 105013. [Google Scholar] [CrossRef]
- EUR-Lex—L:2019:155:TOC—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ%3AL%3A2019%3A155%3ATOC (accessed on 16 July 2023).
- Itim, B.; Philip, M. Effect of Multiple Extrusions and Influence of PP Contamination on the Thermal Characteristics of Bottle Grade Recycled PET. Polym. Degrad. Stab. 2015, 117, 84–89. [Google Scholar] [CrossRef]
- Dimitrov, N.; Kratofil Krehula, L.; Ptiček Siročić, A.; Hrnjak-Murgić, Z. Analysis of Recycled PET Bottles Products by Pyrolysis-Gas Chromatography. Polym. Degrad. Stab. 2013, 98, 972–979. [Google Scholar] [CrossRef]
- Franz, R.; Welle, F. Contamination Levels in Recollected PET Bottles from Non-Food Applications and Their Impact on the Safety of Recycled PET for Food Contact. Molecules 2020, 25, 4998. [Google Scholar] [CrossRef]
- Alsewailem, F.D.; Alrefaie, J.K. Effect of Contaminants and Processing Regime on the Mechanical Properties and Moldability of Postconsumer Polyethylene Terephthalate Bottles. Waste Manag. 2018, 81, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Alvarado Chacon, F.; Brouwer, M.T.; van Velzen, E.U.T. Effect of Recycled Content and RPET Quality on the Properties of PET Bottles, Part I: Optical and Mechanical Properties. Packag. Technol. Sci. 2020, 33, 347–357. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Botta, L.; Morreale, M.; Scaffaro, R. Effect of Small Amounts of Poly(Lactic Acid) on the Recycling of Poly(Ethylene Terephthalate) Bottles. Polym. Degrad. Stab. 2012, 97, 21–24. [Google Scholar] [CrossRef]
- Navarro, R.; Ferrándiz, S.; López, J.; Seguí, V.J. The Influence of Polyethylene in the Mechanical Recycling of Polyethylene Terephtalate. J. Mater. Process. Technol. 2008, 195, 110–116. [Google Scholar] [CrossRef]
- López, M.D.M.C.; Ares Pernas, A.I.; Abad López, M.J.; Latorre, A.L.; López Vilariño, J.M.; González Rodríguez, M.V. Assessing Changes on Poly(Ethylene Terephthalate) Properties after Recycling: Mechanical Recycling in Laboratory versus Postconsumer Recycled Material. Mater. Chem. Phys. 2014, 147, 884–894. [Google Scholar] [CrossRef]
- Nait-Ali, K.L.; Bergeret, A.; Ferry, L.; Colin, X. Chain Branching Detection by Cole–Cole Modeling of Rheological Properties Changes during PET Mechanical Recycling. Polym. Test. 2012, 31, 500–504. [Google Scholar] [CrossRef]
- Wu, H.; Lv, S.; He, Y.; Qu, J.-P. The Study of the Thermomechanical Degradation and Mechanical Properties of PET Recycled by Industrial-Scale Elongational Processing. Polym. Test. 2019, 77, 105882. [Google Scholar] [CrossRef]
- Nait-Ali, L.K.; Colin, X.; Bergeret, A. Kinetic Analysis and Modelling of PET Macromolecular Changes during Its Mechanical Recycling by Extrusion. Polym. Degrad. Stab. 2011, 96, 236–246. [Google Scholar] [CrossRef]
- Awaja, F.; Pavel, D. Injection Stretch Blow Moulding Process of Reactive Extruded Recycled PET and Virgin PET Blends. Eur. Polym. J. 2005, 41, 2614–2634. [Google Scholar] [CrossRef]
- Curtzwiler, G.W.; Schweitzer, M.; Li, Y.; Jiang, S.; Vorst, K.L. Mixed Post-Consumer Recycled Polyolefins as a Property Tuning Material for Virgin Polypropylene. J. Clean. Prod. 2019, 239, 117978. [Google Scholar] [CrossRef]
- Cecon, V.S.; Da Silva, P.F.; Vorst, K.L.; Curtzwiler, G.W. The Effect of Post-Consumer Recycled Polyethylene (PCRPE) on the Properties of Polyethylene Blends of Different Densities. Polym. Degrad. Stab. 2021, 190, 109627. [Google Scholar] [CrossRef]
- Nofar, M.; Oguz, H.; Ovalı, D. Effects of the Matrix Crystallinity, Dispersed Phase, and Processing Type on the Morphological, Thermal, and Mechanical Properties of Polylactide-Based Binary Blends with Poly[(Butylene Adipate)-Co-Terephthalate] and Poly[(Butylene Succinate)-Co-Adipate]. J. Appl. Polym. Sci. 2019, 136, 47636. [Google Scholar] [CrossRef]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. The Role of Crystalline, Mobile Amorphous and Rigid Amorphous Fractions in the Performance of Recycled Poly (Ethylene Terephthalate) (PET). Polym. Degrad. Stab. 2012, 97, 98–107. [Google Scholar] [CrossRef]
- Asensio, M.; Nuñez, K.; Guerrero, J.; Herrero, M.; Merino, J.C.; Pastor, J.M. Rheological Modification of Recycled Poly(Ethylene Terephthalate): Blending and Reactive Extrusion. Polym. Degrad. Stab. 2020, 179, 109258. [Google Scholar] [CrossRef]
- Cser, F. About the Lorentz Correction Used in the Interpretation of Small Angle X-Ray Scattering Data of Semicrystalline Polymers. J. Appl. Polym. Sci. 2001, 80, 2300–2308. [Google Scholar] [CrossRef]
- Hsiao, B.S.; Verma, R.K. A Novel Approach to Extract Morphological Variables in Crystalline Polymers from Time-Resolved Synchrotron SAXS Data. J. Synchrotron. Rad. 1998, 5, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Herruzo, E.T.; Perrino, A.P.; Garcia, R. Fast Nanomechanical Spectroscopy of Soft Matter. Nat. Commun. 2014, 5, 3126. [Google Scholar] [CrossRef]
- Benaglia, S.; Álvarez Amo, C.; Garcia, R. Fast, Quantitative and High Resolution Mapping of Viscoelastic Properties with Bimodal AFM. Nanoscale 2019, 11, 15289–15297. [Google Scholar] [CrossRef]
- Welle, F. Twenty Years of PET Bottle to Bottle Recycling—An Overview. Resour. Conserv. Recycl. 2011, 55, 865–875. [Google Scholar] [CrossRef]
- Kim, H.G.; Robertson, R.E. Multiple Melting Endotherms in Isothermally Melt-Crystallized Poly(Butylene Terephthalate). J. Polym. Sci. Part B Polym. Phys. 1998, 36, 1757–1767. [Google Scholar] [CrossRef]
- Rohart, V.; Combeaud, C. Stretchability above Glass Transition of Mechanically Recycled Poly(Ethylene Terephtalate), PET. Polymer 2022, 257, 125218. [Google Scholar] [CrossRef]
- Celik, Y.; Shamsuyeva, M.; Endres, H.J. Thermal and Mechanical Properties of the Recycled and Virgin PET—Part I. Polymers 2022, 14, 1326. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Wang, X.; Yang, W.; Zheng, K. Non-Isothermal Crystallization Kinetics of Recycled PET-Si3N4 Nanocomposites. Polym. Test. 2012, 31, 110–116. [Google Scholar] [CrossRef]
- Lu, X.F.; Hay, J.N. Isothermal Crystallization Kinetics and Melting Behaviour of Poly(Ethylene Terephthalate). Polymer 2001, 42, 9423–9431. [Google Scholar] [CrossRef]
- Badía, J.D.; Vilaplana, F.; Karlsson, S.; Ribes-Greus, A. Thermal Analysis as a Quality Tool for Assessing the Influence of Thermo-Mechanical Degradation on Recycled Poly(Ethylene Terephthalate). Polym. Test. 2009, 28, 169–175. [Google Scholar] [CrossRef]
- Jonas, A.M.; Russell, T.P.; Yoon, D.Y. Time-Resolved SAXS Studies of Morphological Changes in Cold Crystallized Poly(Ethylene Terephthalate) during Annealing and Heating. Colloid Polym. Sci. 1994, 272, 1344–1351. [Google Scholar] [CrossRef]
- Gere, D.; Czigany, T. Future Trends of Plastic Bottle Recycling: Compatibilization of PET and PLA. Polym. Test. 2020, 81, 106160. [Google Scholar] [CrossRef]
- Zander, N.E.; Gillan, M.; Lambeth, R.H. Recycled Polyethylene Terephthalate as a New FFF Feedstock Material. Addit. Manuf. 2018, 21, 174–182. [Google Scholar] [CrossRef]





| Polymer | Mn (kg·mol−1) | Mw (kg·mol−1) | PDI |
|---|---|---|---|
| vPET | 31.5 ± 0.1 | 53.8 ± 1.1 | 1.71 ± 0.04 |
| 100rPET | 31.2 ± 1.6 | 44.9 ± 0.1 | 1.54 ± 0.08 |
| Polymer | MFI (g/10 min) |
|---|---|
| vPET | 20.7 ± 0.3 |
| 100rPET | 36.5 ± 0.4 |
| Material | Max Modulus AFM (GPa) | Elastic Modulus (GPa) | Young’s Modulus (GPa) |
|---|---|---|---|
| vPET | 2.13 ± 0.12 | 2.72 ± 0.09 | 2.83 ± 0.01 |
| 50rPET | 3.03 ± 0.29 | 2.56 ± 0.06 | 2.79 ± 0.03 |
| 100rPET | 2.13 ± 0.13 | 2.69 ± 0.03 | 2.78 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viora, L.; Combeau, M.; Pucci, M.F.; Perrin, D.; Liotier, P.-J.; Bouvard, J.-L.; Combeaud, C. A Comparative Study on Crystallisation for Virgin and Recycled Polyethylene Terephthalate (PET): Multiscale Effects on Physico-Mechanical Properties. Polymers 2023, 15, 4613. https://doi.org/10.3390/polym15234613
Viora L, Combeau M, Pucci MF, Perrin D, Liotier P-J, Bouvard J-L, Combeaud C. A Comparative Study on Crystallisation for Virgin and Recycled Polyethylene Terephthalate (PET): Multiscale Effects on Physico-Mechanical Properties. Polymers. 2023; 15(23):4613. https://doi.org/10.3390/polym15234613
Chicago/Turabian StyleViora, Laurianne, Marie Combeau, Monica Francesca Pucci, Didier Perrin, Pierre-Jacques Liotier, Jean-Luc Bouvard, and Christelle Combeaud. 2023. "A Comparative Study on Crystallisation for Virgin and Recycled Polyethylene Terephthalate (PET): Multiscale Effects on Physico-Mechanical Properties" Polymers 15, no. 23: 4613. https://doi.org/10.3390/polym15234613
APA StyleViora, L., Combeau, M., Pucci, M. F., Perrin, D., Liotier, P.-J., Bouvard, J.-L., & Combeaud, C. (2023). A Comparative Study on Crystallisation for Virgin and Recycled Polyethylene Terephthalate (PET): Multiscale Effects on Physico-Mechanical Properties. Polymers, 15(23), 4613. https://doi.org/10.3390/polym15234613







