The Enhancement of the Thermal Conductivity of Epoxy Resin Reinforced by Bromo-Oxybismuth
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Preparation of the BiOBr
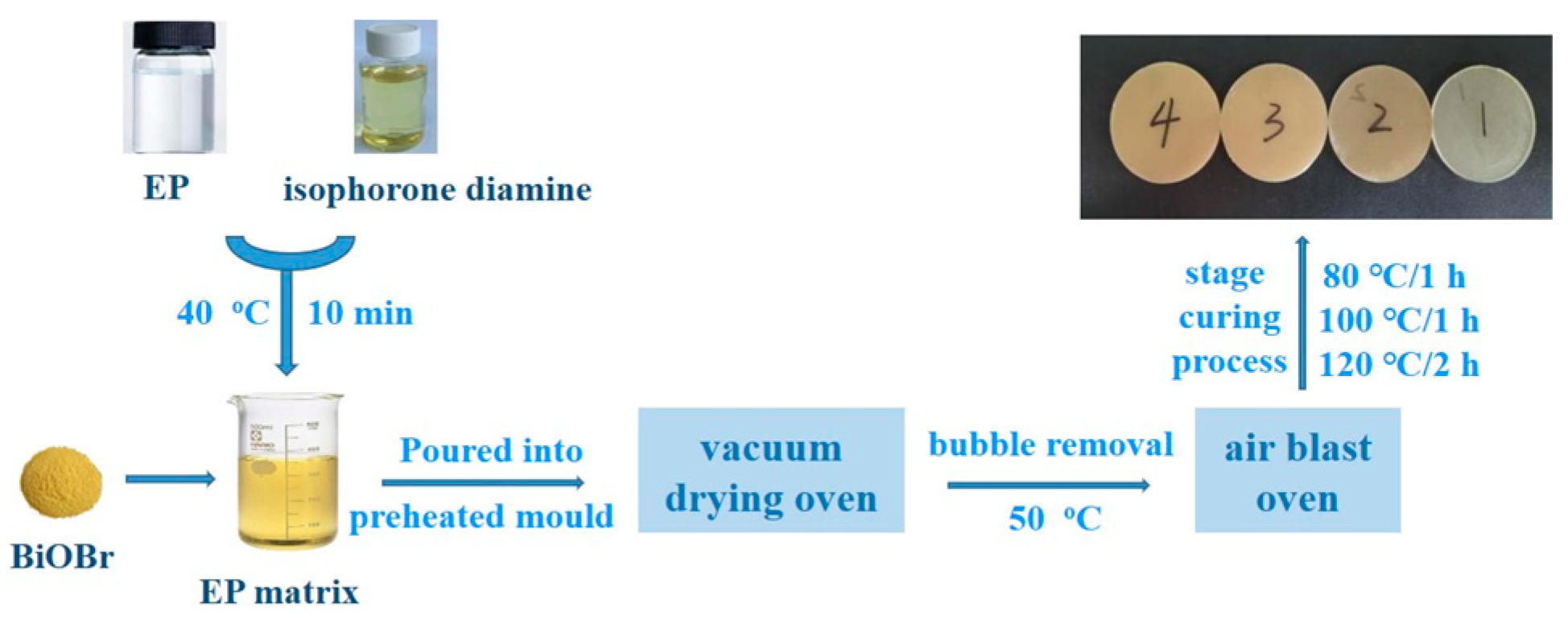
2.3. Preparation of the BiOBr/EP Composites
2.4. Measurements
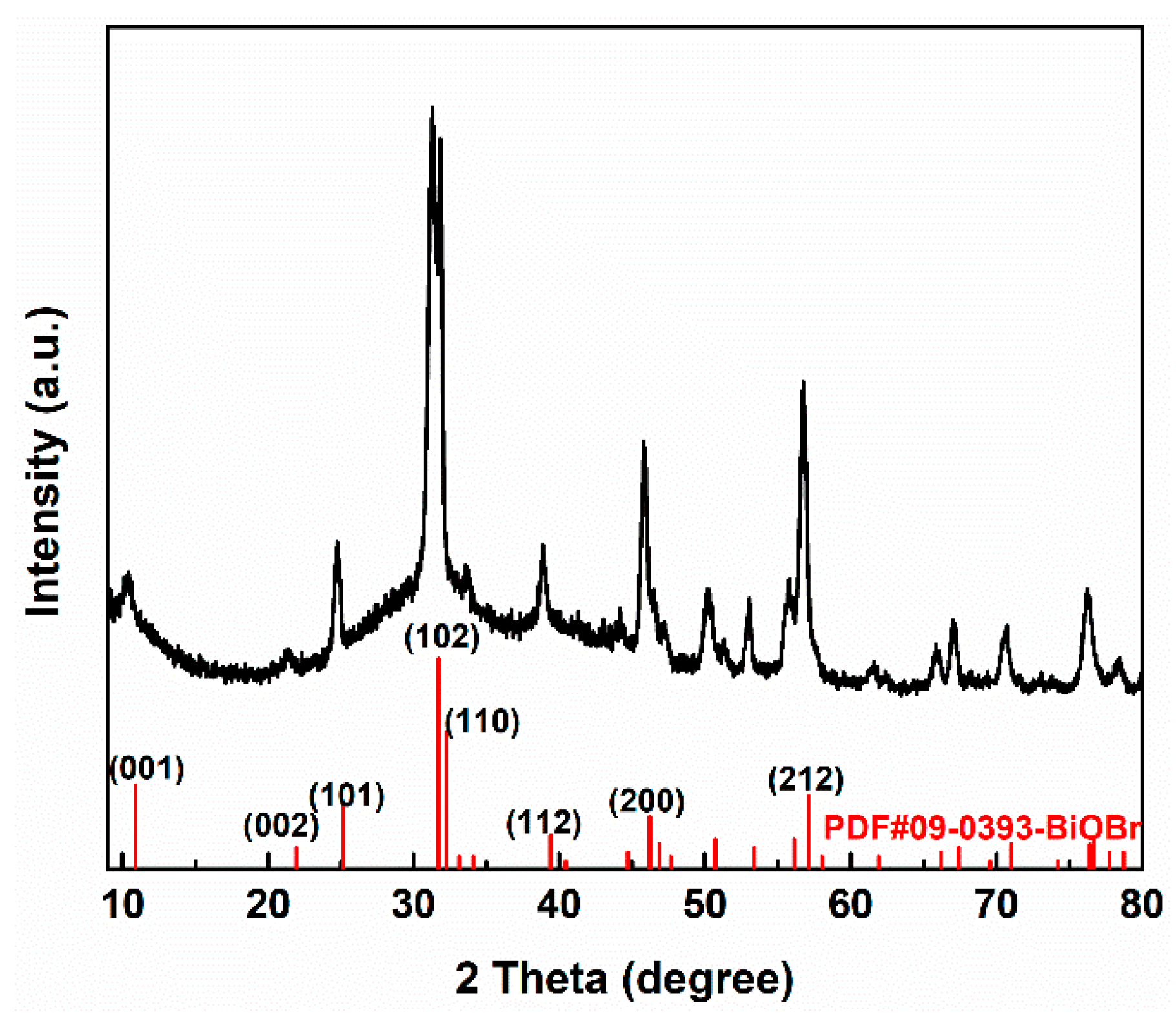
2.4.1. The X-ray Diffraction (XRD)
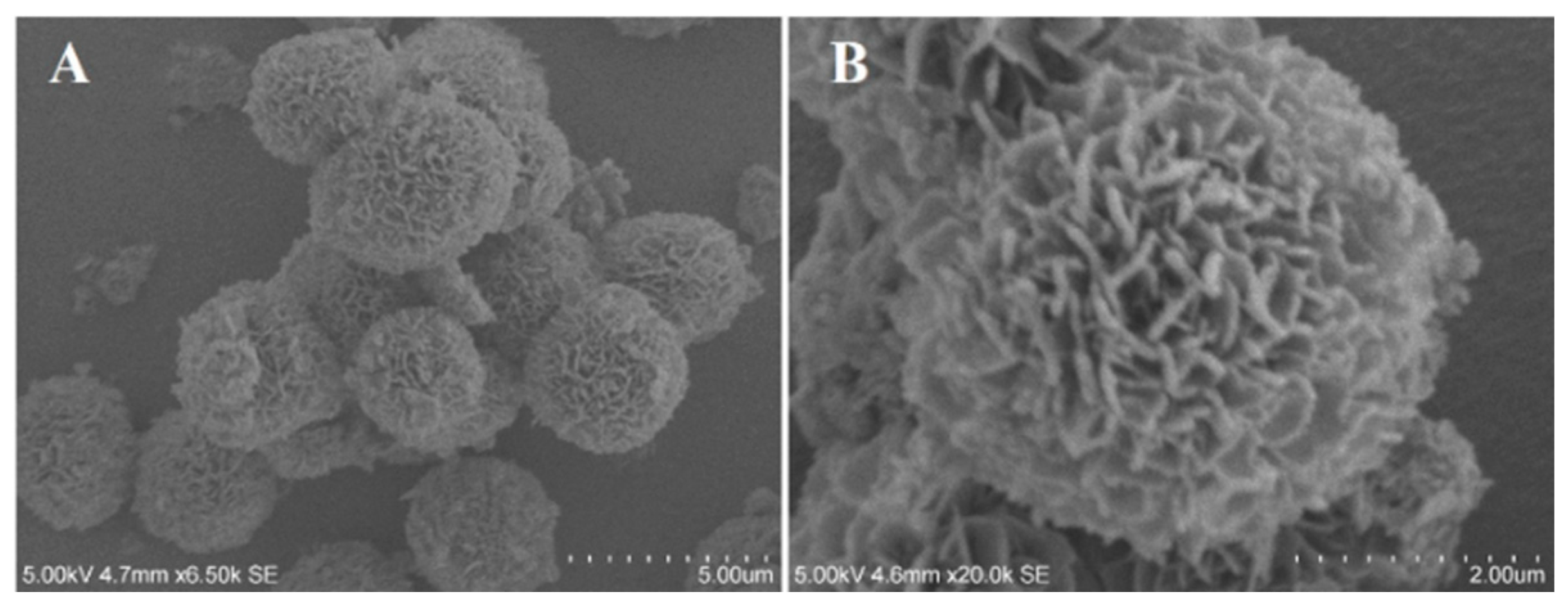
2.4.2. Scanning Electron Microscopy (SEM)
2.4.3. Dielectric Properties
2.4.4. Thermal Conductivity
2.4.5. Thermal Resistant Properties
3. Results and Discussion
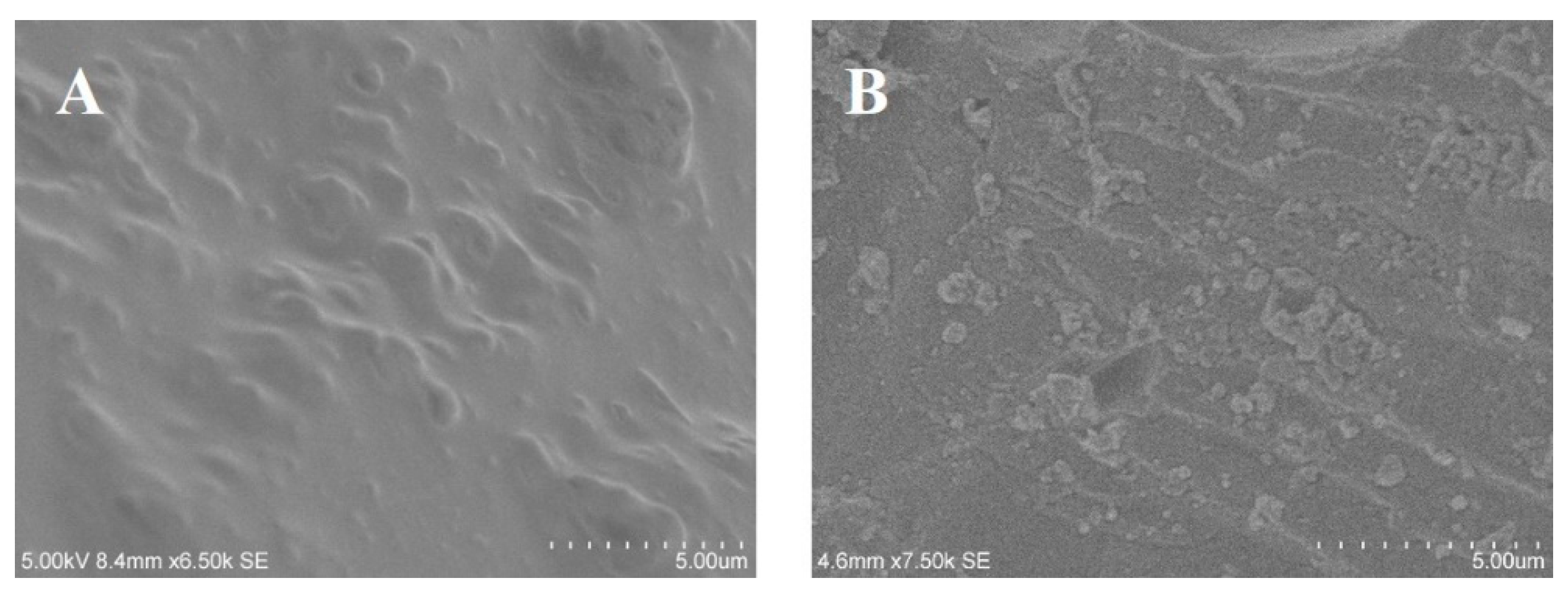
3.1. Morphology Structure of the BiOBr
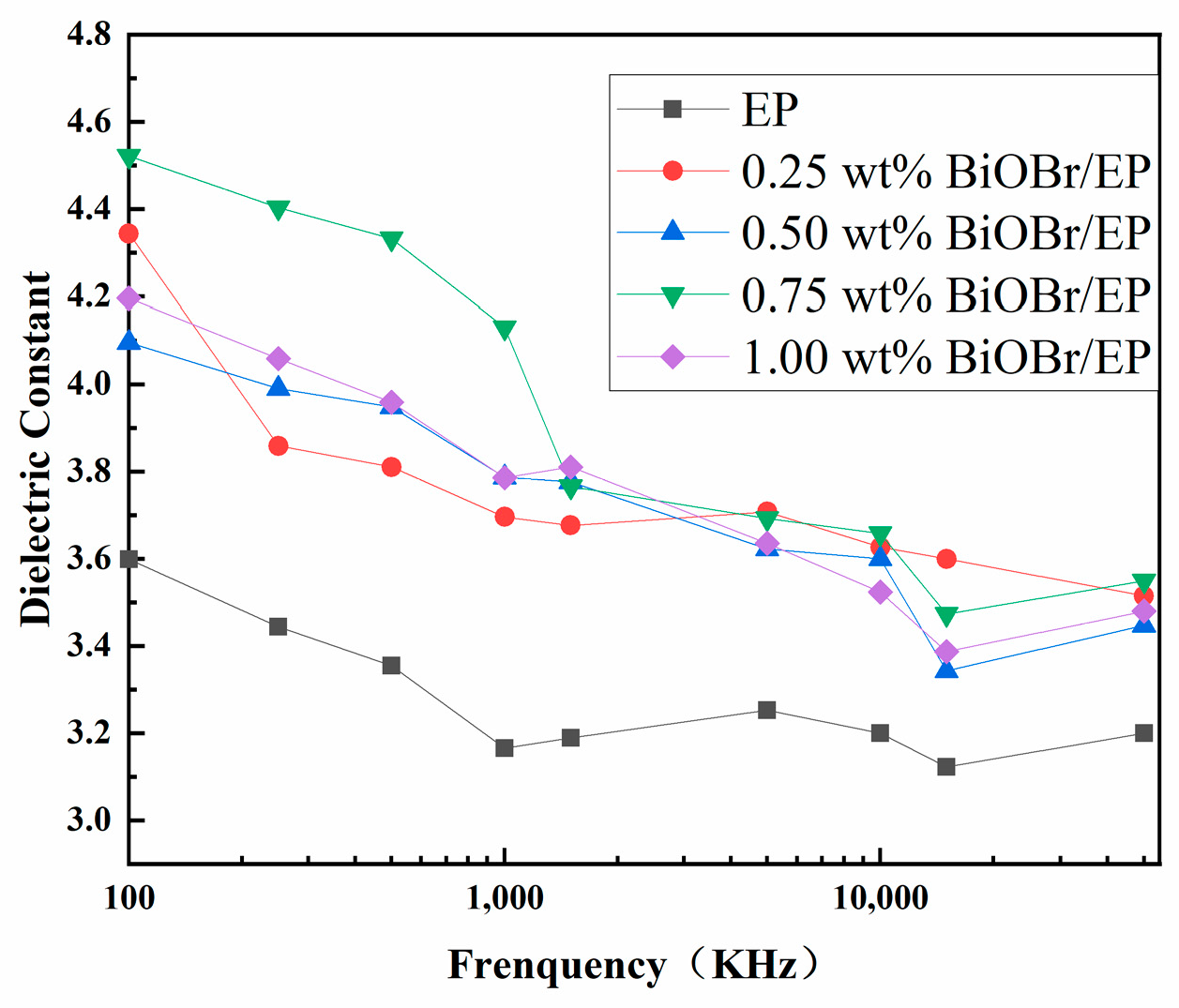
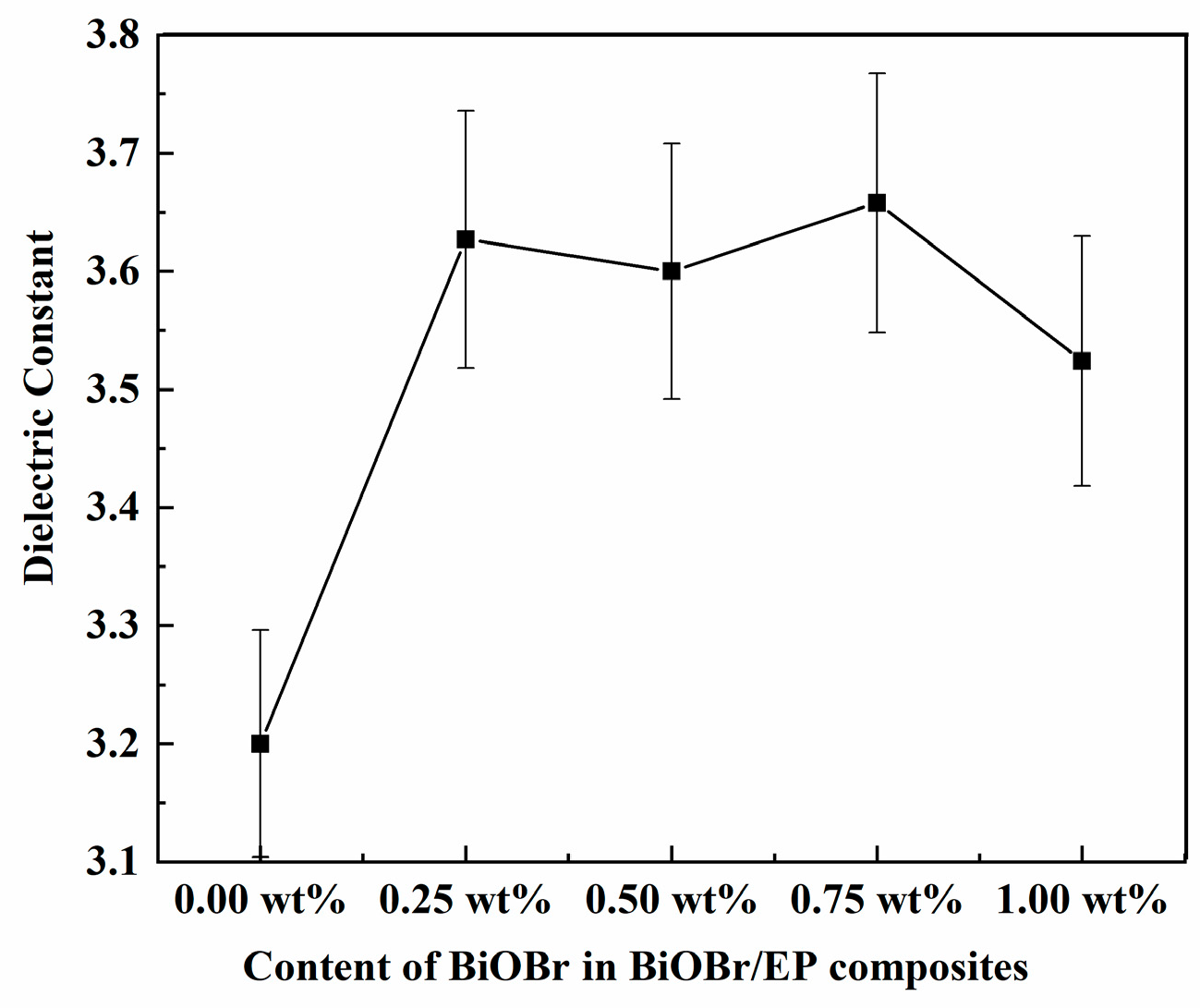
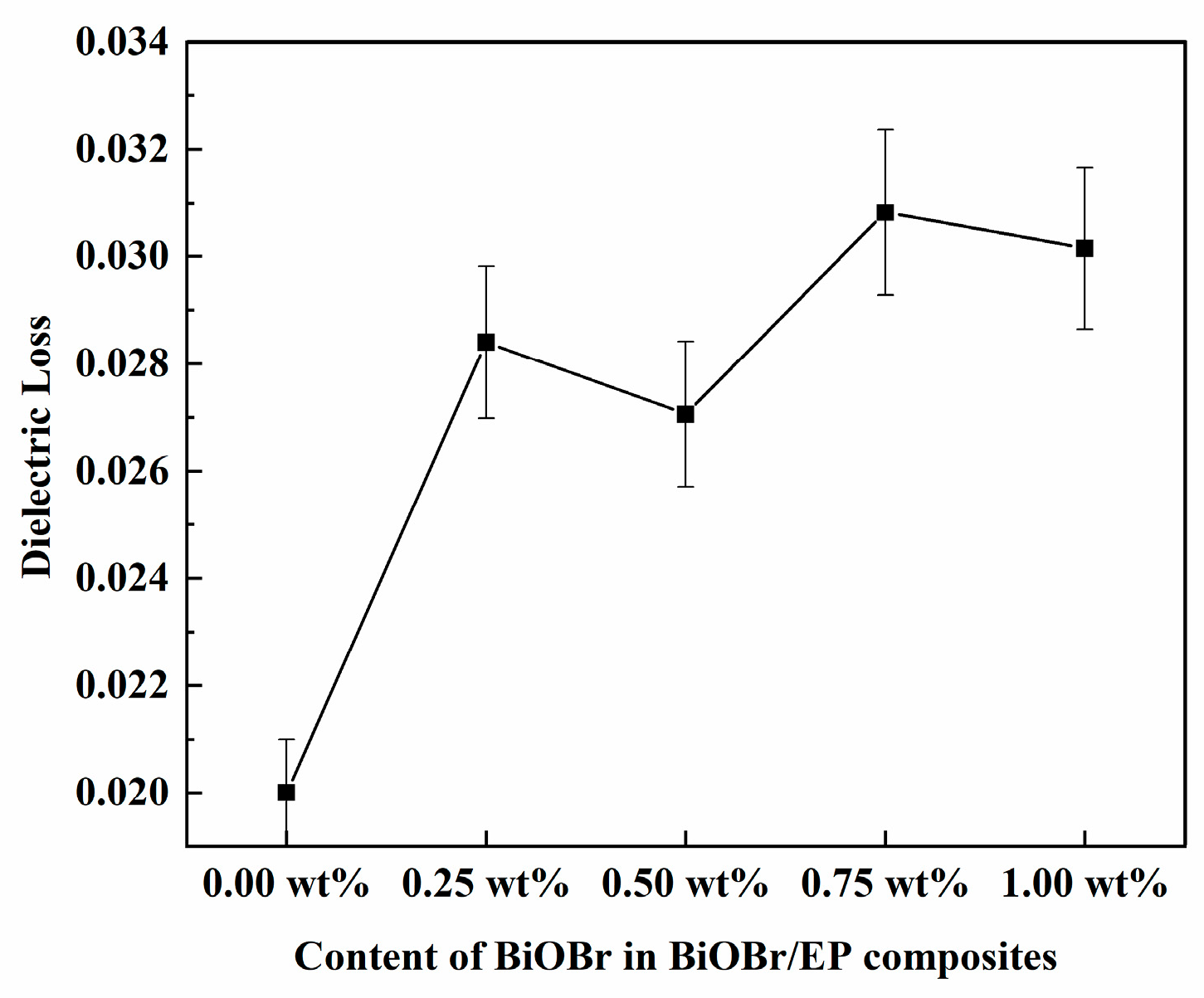
3.2. Dielectric Properties of BiOBr/EP
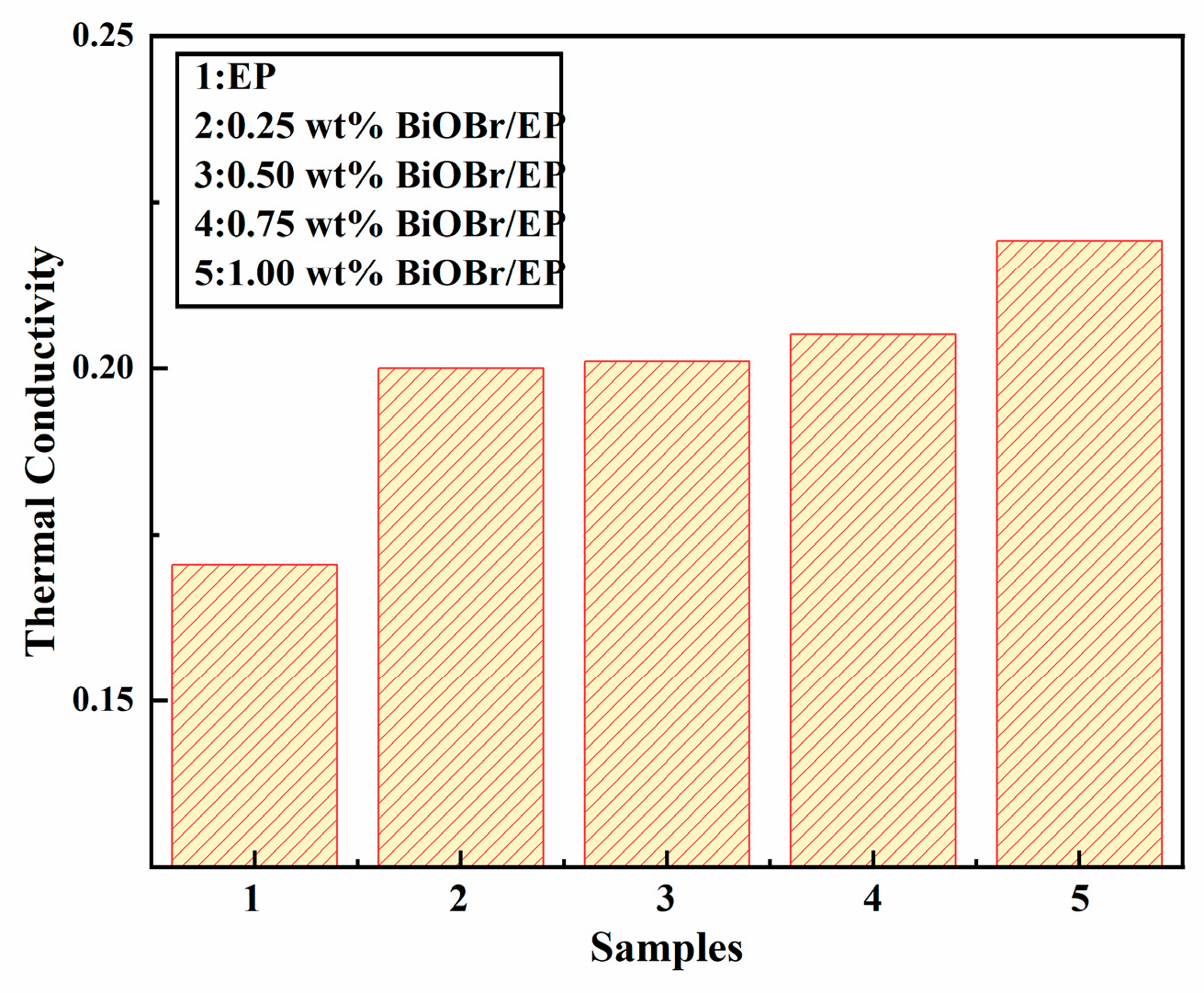
3.3. Thermal Conductivities of the Materials
3.4. Thermal Resistant of the Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, Y.; Ren, Y.; Chen, G.X.; Li, Q. Synthesis of high-k and low dielectric loss polymeric composites from crosslinked divinylbenzene coated carbon nanotubes. Polymer 2016, 100, 179–187. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Negi, A.; He, J.; Hu, G.; Tian, S.; Liu, J. Synergistic Effects of Boron Nitride (BN) Nanosheets and Silver (Ag) Nanoparticles on Thermal Conductivity and Electrical Properties of Epoxy Nanocomposites. Polymers 2020, 12, 426. [Google Scholar] [CrossRef]
- Guo, J.; Saha, P.; Liang, J. Multi-walled carbon nanotubes coated by multi-layer silica for improving thermal conductivity of polymer composites. J. Therm. Anal. Calorim. 2013, 113, 467–474. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Liu, Y.; Teng, C.; Cui, W. Curing mechanism and mechanical properties of Al2O3/cyanate ester-epoxy composites. J. Electron. Mater. 2020, 49, 1473–1481. [Google Scholar] [CrossRef]
- Bai, Y.; Pan, Y.J.; An, N.; Zhang, H.T.; Wang, C.; Tian, W.; Huang, T. Host-guest interactions based supramolecular complexes self-assemblies for amplified chemodynamic therapy with H2O2 elevation and GSH consumption properties. Chin. Chem. Letters. 2023, 34, 107552. [Google Scholar] [CrossRef]
- Fan, B.H.; Zha, J.W.; Wang, D.R.; Zhao, J.; Zhang, Z.F.; Dang, Z.M. Preparation and dielectric behaviors of thermoplastic and thermosetting polymer nanocomposite films containing BaTiO3 nanoparticles with different diameters. Compos. Sci. Technol. 2013, 80, 66–72. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, X.; Yang, G.; Shang, Y.; Su, F.; Hu, Q.; Patil, R.R.; Liu, H.; Liu, C.; Guo, Z. Thermally Conductive Anticorrosive Epoxy Nanocomposites with Tannic Acid-Modified Boron Nitride Nanosheets. Ind. Eng. Chem. Res. 2020, 59, 20371–20381. [Google Scholar] [CrossRef]
- Li, X.Y.; Zha, J.W.; Wang, S.J.; Zhong, S.L.; Zhang, C.; Dang, Z.M. Effect of high-thermal conductivity epoxy resin on heat dissipation performance of saturated reactor. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 3898–3905. [Google Scholar] [CrossRef]
- Qiang, D.; Wang, Y.; Chen, G.; Andritsch, T. Dielectric properties of epoxy silica and boron nitride nanocomposites and moisture/temperature influence. IET Nanodielectrics 2018, 1, 48–59. [Google Scholar] [CrossRef]
- Yu, B.; Shi, Y.; Yuan, B.; Qiu, S.; Xing, W.; Hu, W.; Song, L.; Lo, S.; Hu, Y. Enhanced Thermal and Flame Retardant Properties of Flame-retardant-wrapped Graphene/Epoxy Resin Nanocomposites. J. Mater. Chem. A 2015, 3, 8034–8044. [Google Scholar] [CrossRef]
- Awale, A.G.; Gholse, S.B.; Utale, P.S. Synthesis, spectral properties and applications of some mordant and disperse mono azo dyes derived from 2-amino-1,3-benzo-thiazole. Res. J. Chem. Sci. 2013, 3, 81–87. [Google Scholar]
- Gao, N.; Liu, W.; Yan, Z.; Wang, Z. Synthesis and properties of transparent cycloaliphatic epoxy-silicone resins for opto-electronic devices packaging. Opt. Mater. 2013, 35, 567–575. [Google Scholar] [CrossRef]
- Deng, J.; Liu, X.; Li, C.; Jiang, Y.; Zhu, J. Synthesis and properties of a bio-based epoxy resin from 2,5-furandicarboxylic acid (FDCA). RSC Adv. 2015, 5, 15930–15939. [Google Scholar] [CrossRef]
- Weng, L.; Zhang, Y.; Zhang, X.; Liu, L.; Zhang, H. Synthesis and properties of cured epoxy mixed resin systems modified by poly-phenylene oxide for production of high-frequency copper clad laminates. Polym. Compos. 2018, 39, 2831–2840. [Google Scholar] [CrossRef]
- Chon, J.; Ye, S.; Cha, K.J.; Lee, S.C.; Koo, Y.S.; Jung, J.H.; Kwon, Y.K. High-K dielectric sol-gel hybrid materials containing barium titanate nanoparticles. Chem. Mater. 2010, 22, 5445–5452. [Google Scholar] [CrossRef]
- Qiao, Y.; Islam, M.S.; Han, K.; Leonhardt, E.; Zhang, J.; Wang, Q.; Ploehn, H.J.; Tang, C. Polymers containing highly polarizable conjugated side chains as high-performance all-organic nanodielectric materials. Adv. Funct. Mater. 2013, 23, 5638–5646. [Google Scholar] [CrossRef]
- Hennemann, K.K.; Lenz, D.M. Structural methacrylate/epoxy based adhesives for aluminIum joints. Int. J. Adhes. Adhes. 2019, 89, 11–18. [Google Scholar] [CrossRef]
- Xue, G.; Zhang, B.; Sun, M.; Zhang, X.; Li, J.; Wang, L.; Song, C. Morphology, thermal and mechanical properties of epoxy adhesives containing well-dispersed graphene oxide. Int. J. Adhes. Adhes. 2019, 88, 11–18. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Pei, Q.X.; Zhang, Y. Some aspects on thermal transport across the interface between graphene and epoxy in nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 8272–8279. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Z.; Zhi, C.; Zhang, W. High thermal conductivity and temperature probing of copper nanowire/upconversion nanoparticles/epoxy composite. Compos. Sci. Technol. 2016, 130, 63–69. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Z.; Kang, R.; Chen, Y.; Hou, X.; Wu, Y.; Wang, M.; Wang, B.; Cui, J.; Jiang, N.; et al. Enhanced thermal conductivity of epoxy composites filled with tetrapod-shaped ZnO. RSC Adv. 2018, 8, 12337–12343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, L.; Wu, H.; Guo, S. Enhanced thermally conductivity and mechanical properties of polyethylene (PE)/boron nitride (BN) composites through multistage stretching extrusion. Compos. Sci. Technol. 2013, 89, 24–28. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Lin, J.; Cui, P.; Li, M.; Du, X. Thermal conductivity of epoxy composites filled by thermally reduced graphite oxide with different reduction degree. J. Compos. Mater. 2017, 51, 1743–1752. [Google Scholar] [CrossRef]
- Ye, L.; Liu, J.; Jiang, Z.; Peng, T.; Zan, L. Facets coupling of BiOBr-g-C3N4 composite photocatalyst for enhanced visible-light-driven photocatalytic activity. Appl. Catal. B Environ. 2013, 142, 1–7. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ji, M.; Wang, B.; Yin, S.; Zhang, Q.; Chen, Z.; Li, H. Advanced photocatalytic performance of graphene-like BN modified BiOBr flower-like materials for the removal of pollutants and mechanism insight. Appl. Catal. B Environ. 2016, 183, 254–262. [Google Scholar] [CrossRef]
- Xu, J.; Li, L.; Guo, C.; Zhang, Y.; Wang, S. Removal of benzotriazole from solution by Bi OBr photocatalysis under simulated solar irradiation. Chem. Eng. J. 2013, 221, 230–237. [Google Scholar] [CrossRef]
- Shahil, K.M.F.; Balandin, A.A. Graphene–multilayer graphene nanocomposites as highly efficient thermal interface materials. Nano Lett. 2012, 12, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, B.; Zhao, Y.; Niu, J.; Zhu, Y. Solvothermal synthesis and photocatalytic activity of Al-doped BiOBr microspheres. Ceram. Int. 2014, 40, 5597–5603. [Google Scholar] [CrossRef]
- Cao, G.; Liu, Z.S. Floatable graphitic carbon nitride foam-supported Bi OBr composites with high photocatalytic activity. Mater. Lett. 2017, 202, 32–35. [Google Scholar] [CrossRef]
- Guo, W.; Qin, Q.; Geng, L.; Wang, D.; Guo, Y.; Yang, Y. Morphology-controlled preparation and plasmon-enhanced photocatalytic activity of Pt-Bi OBr heterostructures. J. Hazard. Mater. 2016, 308, 374–385. [Google Scholar] [CrossRef]
- Jia, Y.; Ma, H.; Yang, J.; Liu, Z. Preparation of molybdenum disulfde microspheres and their efect on the thermal conductivity of epoxy resin. Polym. Bull. 2023. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Li, B.; Ma, H.; Yang, J.; Liu, Z. The Enhancement of the Thermal Conductivity of Epoxy Resin Reinforced by Bromo-Oxybismuth. Polymers 2023, 15, 4616. https://doi.org/10.3390/polym15234616
Jia Y, Li B, Ma H, Yang J, Liu Z. The Enhancement of the Thermal Conductivity of Epoxy Resin Reinforced by Bromo-Oxybismuth. Polymers. 2023; 15(23):4616. https://doi.org/10.3390/polym15234616
Chicago/Turabian StyleJia, Yuan, Beibei Li, Huan Ma, Juxiang Yang, and Zhen Liu. 2023. "The Enhancement of the Thermal Conductivity of Epoxy Resin Reinforced by Bromo-Oxybismuth" Polymers 15, no. 23: 4616. https://doi.org/10.3390/polym15234616
APA StyleJia, Y., Li, B., Ma, H., Yang, J., & Liu, Z. (2023). The Enhancement of the Thermal Conductivity of Epoxy Resin Reinforced by Bromo-Oxybismuth. Polymers, 15(23), 4616. https://doi.org/10.3390/polym15234616





