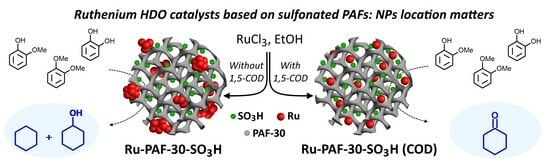
Hydrodeoxygenation of Lignin-Based Compounds over Ruthenium Catalysts Based on Sulfonated Porous Aromatic Frameworks
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Materials
2.2. Synthesis
2.3. Characterization
2.4. Reaction Procedure and Product Analysis
3. Results and Discussion
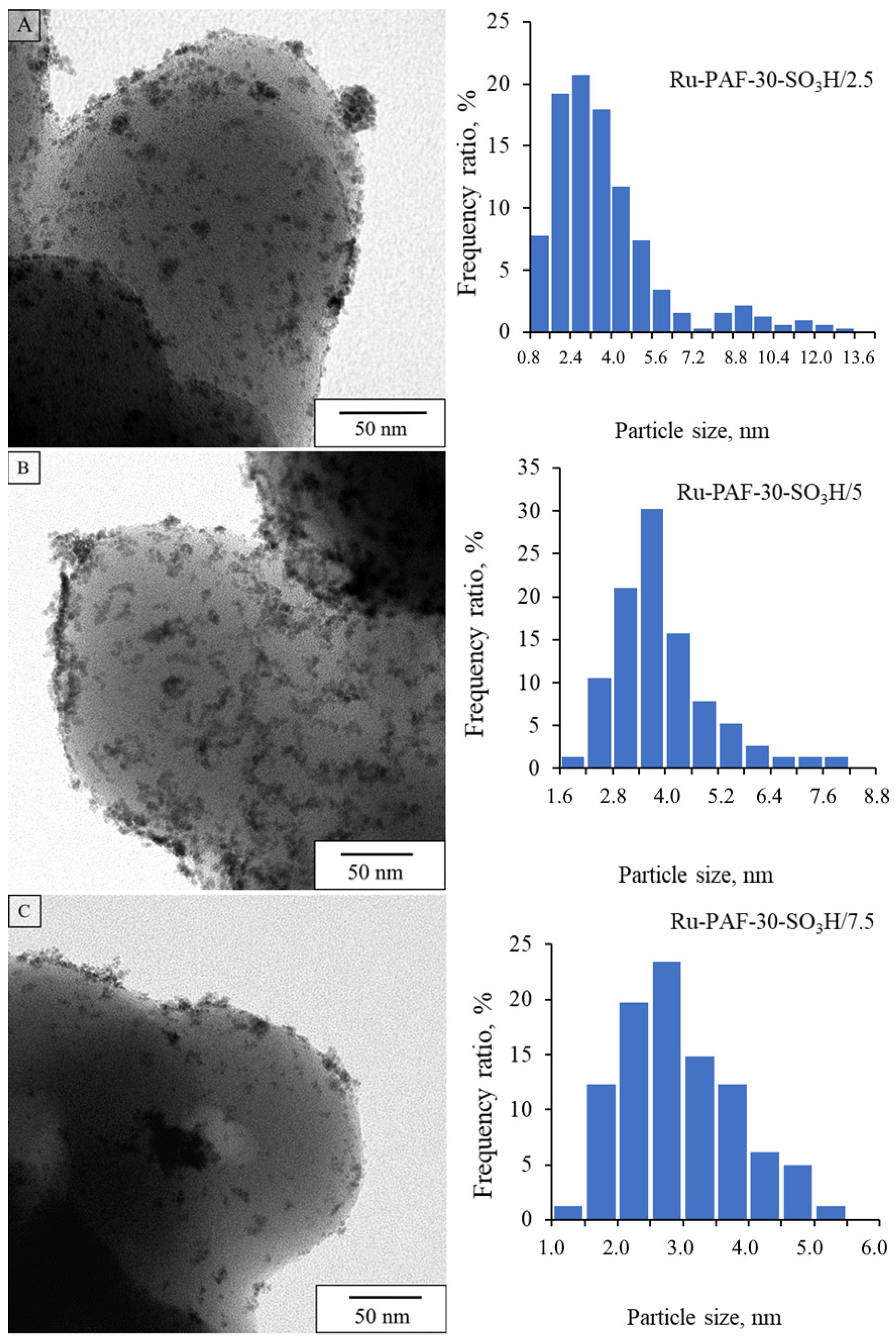
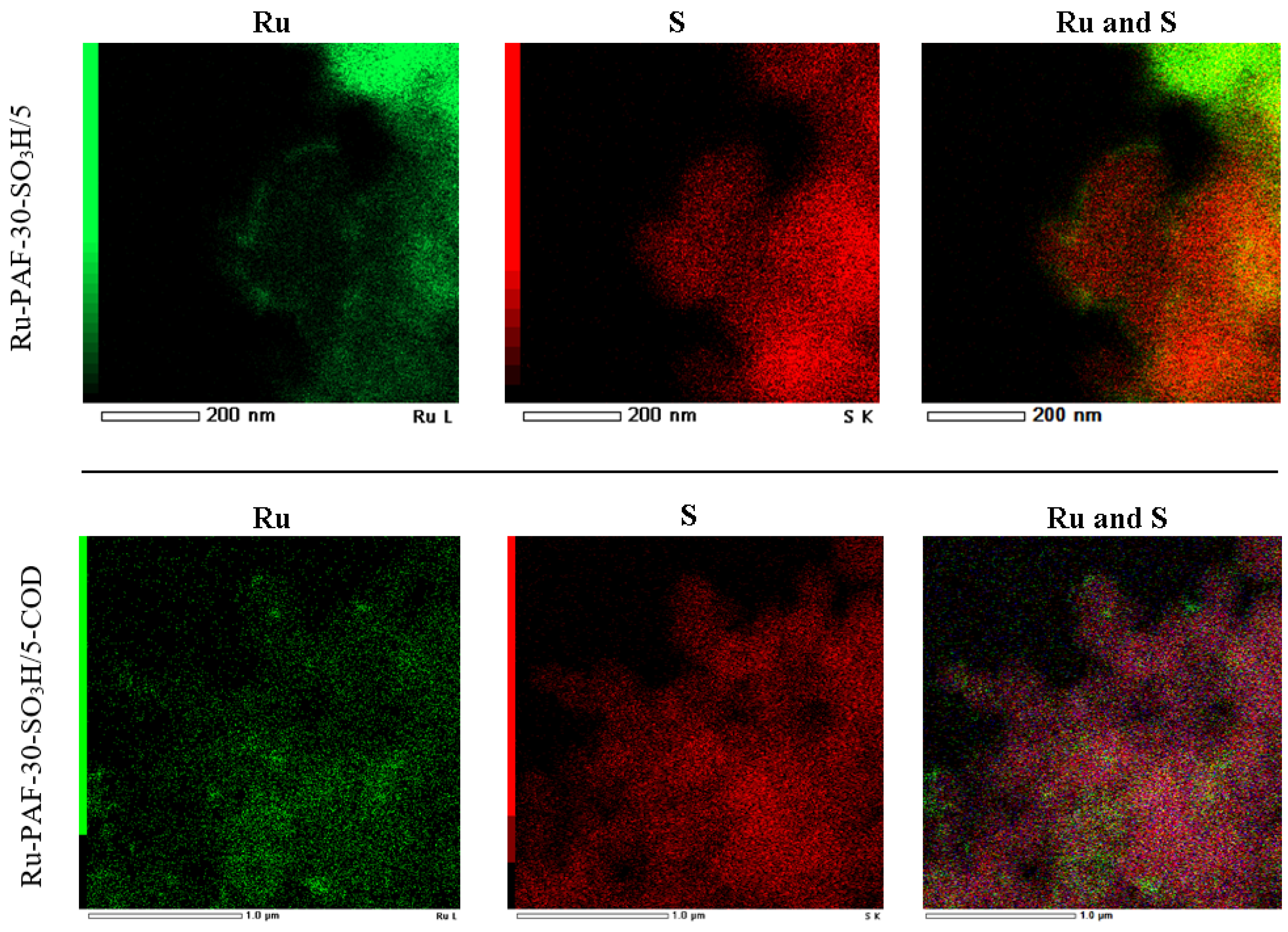
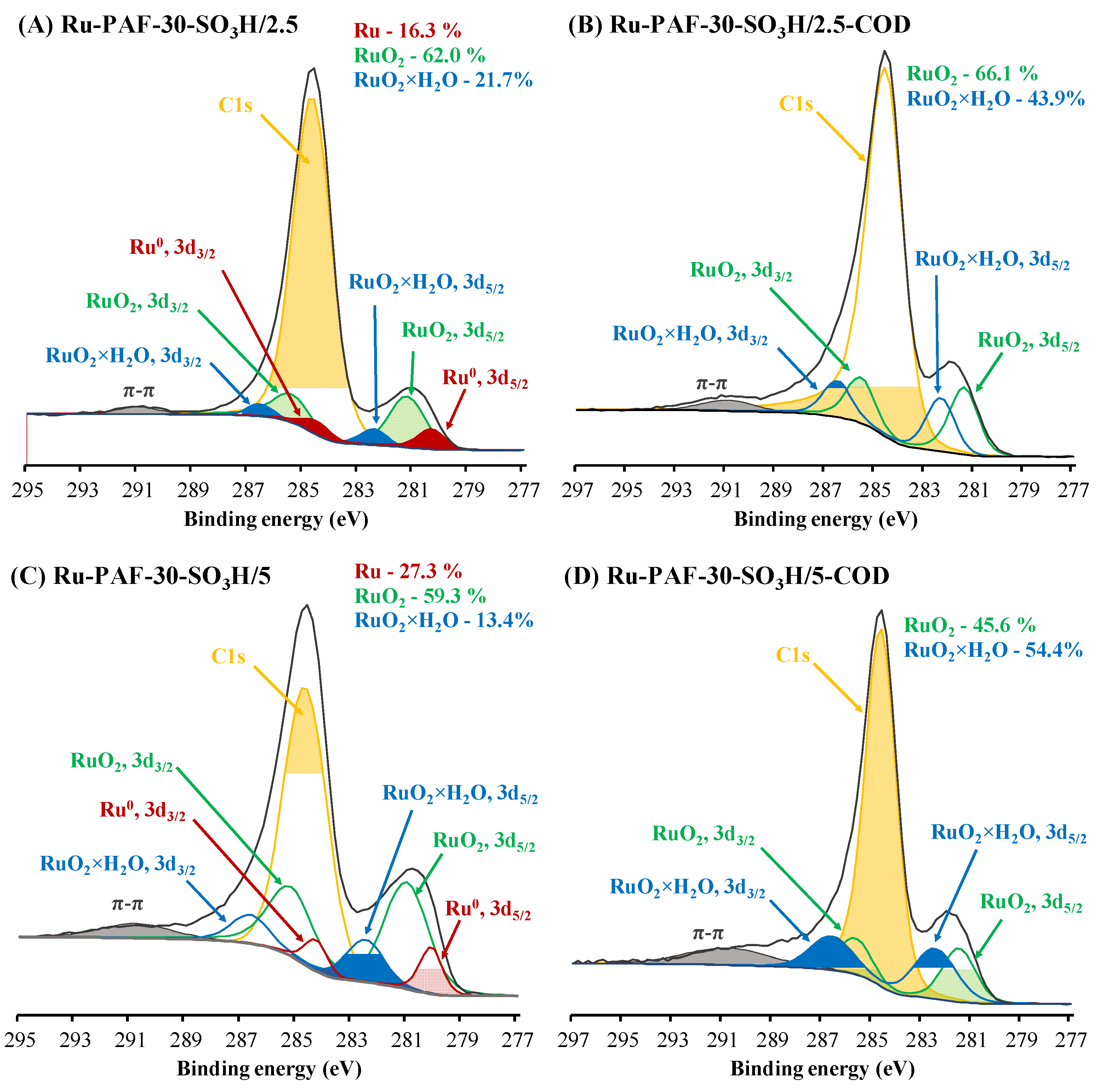
3.1. Characterization of the Materials
3.2. Catalytic Tests
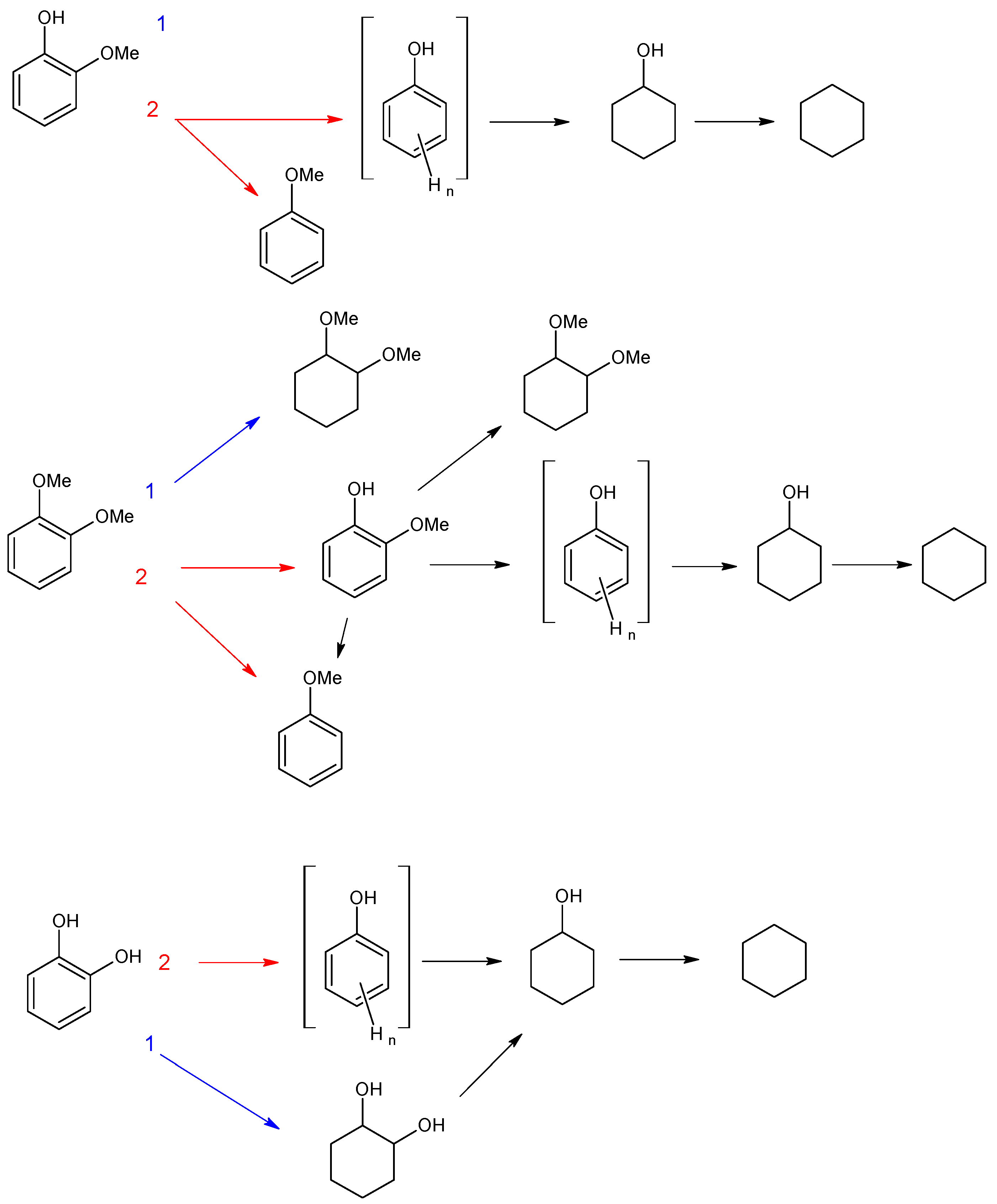
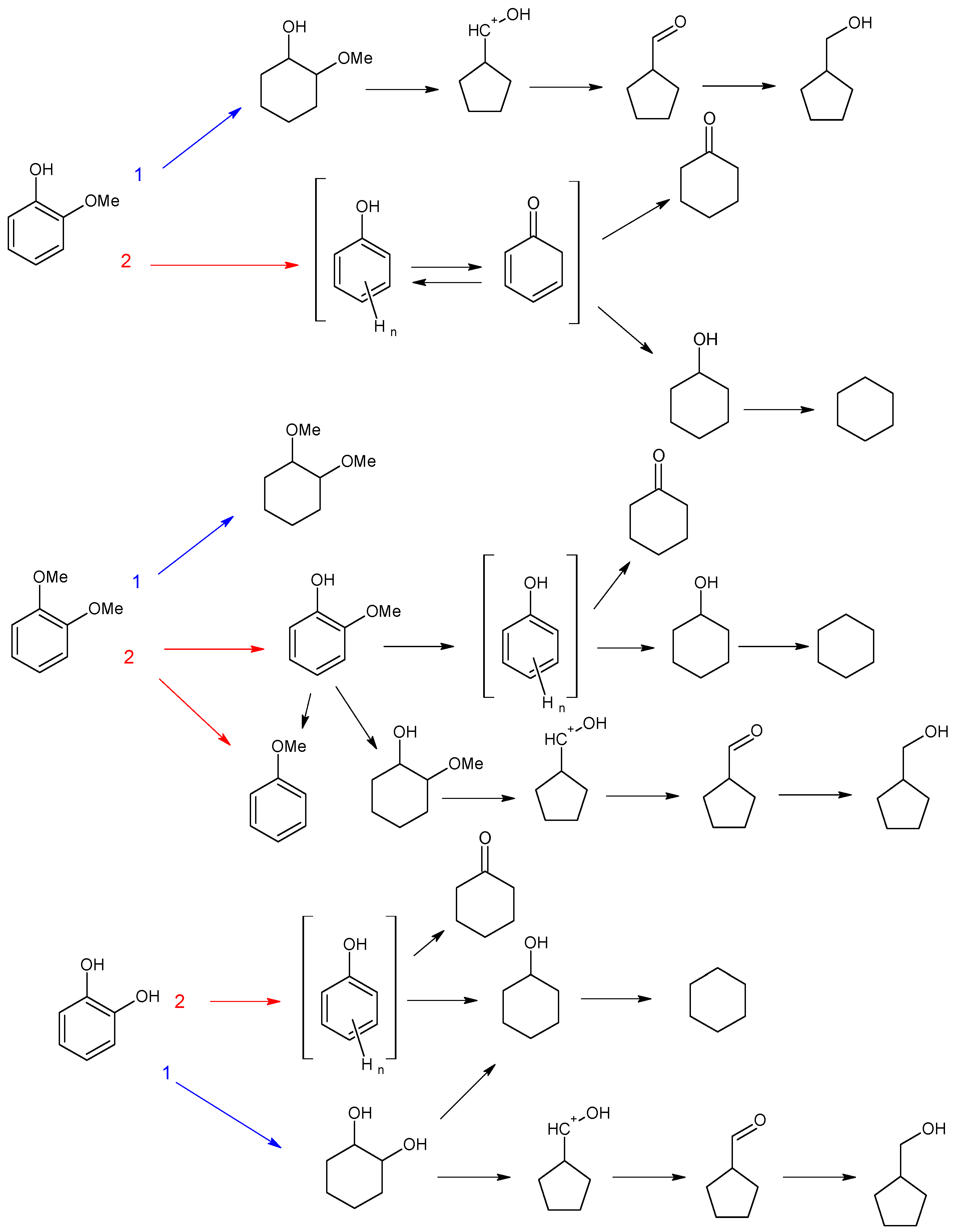
3.2.1. Investigation of the Influence of the Number of Acid Sites
3.2.2. Investigation of the Influence of the Mutual Arrangement of Metal and Acid Centers
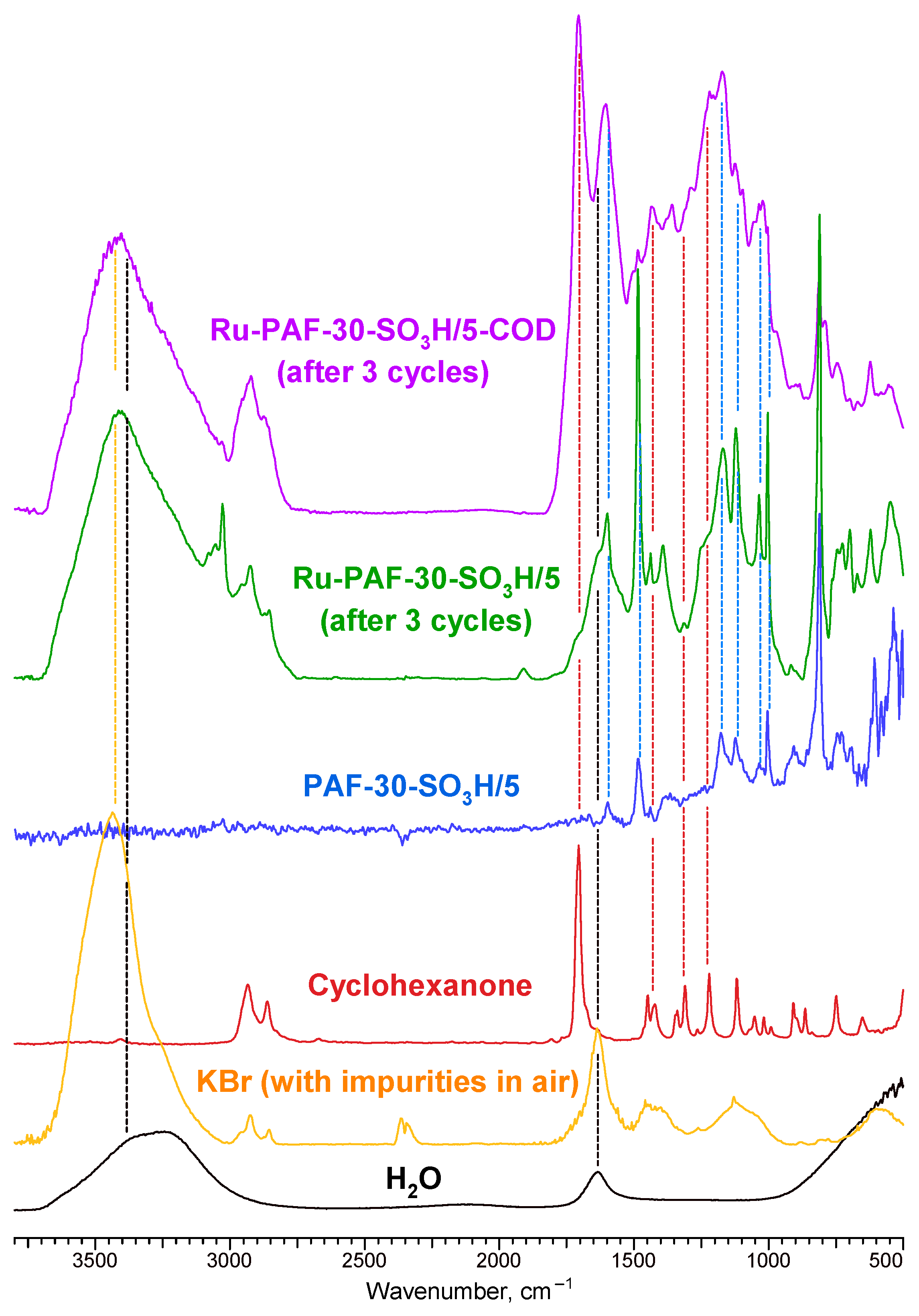
3.2.3. Catalysts Recycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- He, M.; Sun, Y.; Han, B. Green Carbon Science: Efficient Carbon Resource Processing, Utilization, and Recycling towards Carbon Neutrality. Angew. Chem. 2022, 134, e202112835. [Google Scholar] [CrossRef]
- Yang, C.; Chen, H.; Peng, T.; Liang, B.; Zhang, Y.; Zhao, W. Lignin valorization toward value-added chemicals and fuels via electrocatalysis: A perspective. Chin. J. Catal. 2021, 42, 1831–1842. [Google Scholar] [CrossRef]
- Harth, F.M.; Likozar, B.; Grilc, M. Stability of solid rhenium catalysts for liquid-phase biomass valorization–various facets of catalyst deactivation and rhenium leaching. Mater. Today Chem. 2022, 26, 101191. [Google Scholar] [CrossRef]
- Phan, D.P.; Tran, M.H.; Lee, E.Y. Metal-organic framework-based materials as heterogeneous catalysts for biomass upgrading into renewable plastic precursors. Mater. Today Chem. 2023, 33, 101691. [Google Scholar] [CrossRef]
- Hu, Y.; Li, M.; Gao, Z.; Wang, L.; Zhang, J. Leaf-derived sulfonated carbon dots: Efficient and recoverable catalysts to synthesize 5-hydroxymethylfurfural from fructose. Mater. Today Chem. 2021, 20, 100423. [Google Scholar] [CrossRef]
- Pinto, P.C.R.; Da Silva, E.A.B.; Rodrigues, A.E. Insights into Oxidative Conversion of Lignin to High-Added-Value Phenolic Aldehydes. Ind. Eng. Chem. Res. 2010, 50, 741–748. [Google Scholar] [CrossRef]
- Dawange, M.; Galkin, M.V.; Samec, J.S.M. Selective Aerobic Benzylic Alcohol Oxidation of Lignin Model Compounds: Route to Aryl Ketones. ChemCatChem 2015, 7, 401–404. [Google Scholar] [CrossRef]
- Bai, Z.; Phuan, W.C.; Ding, J.; Heng, T.H.; Luo, J.; Zhu, Y. Production of Terephthalic Acid from Lignin-Based Phenolic Acids by a Cascade Fixed-Bed Process. ACS Catal. 2016, 6, 6141–6145. [Google Scholar] [CrossRef]
- Guo, X.; Wang, W.; Wu, K.; Huang, Y.; Shi, Q.; Yang, Y. Preparation of Fe promoted MoS2 catalysts for the hydrodeoxygenation of p-cresol as a model compound of lignin-derived bio-oil. Biomass Bioenergy 2019, 125, 34–40. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Ma, L.; Zhang, Q.; Huang, X.; Yu, Y. Production of cyclohexane from lignin degradation compounds over Ni/ZrO2–SiO2 catalysts. Appl. Energy 2013, 112, 533–538. [Google Scholar] [CrossRef]
- Ma, D.; Lu, S.; Liu, X.; Guo, Y.; Wang, Y. Depolymerization and hydrodeoxygenation of lignin to aromatic hydrocarbons with a Ru catalyst on a variety of Nb-based supports. Chin. J. Catal. 2019, 40, 609–617. [Google Scholar] [CrossRef]
- Yoosuk, B.; Tumnantong, D.; Prasassarakich, P. Amorphous unsupported Ni–Mo sulfide prepared by one step hydrothermal method for phenol hydrodeoxygenation. Fuel 2012, 91, 246–252. [Google Scholar] [CrossRef]
- Wang, D.; Gong, W.; Zhang, J.; Han, M.; Chen, C.; Zhang, Y.; Wang, G.; Zhang, H.; Zhao, H. Encapsulated Ni-Co alloy nanoparticles as efficient catalyst for hydrodeoxygenation of biomass derivatives in water. Chin. J. Catal. 2021, 42, 2027–2037. [Google Scholar] [CrossRef]
- Lee, H.W.; Jun, B.R.; Kim, H.; Kim, D.H.; Jeon, J.K.; Park, S.H.; Ko, C.H.; Kim, T.W.; Park, Y.K. Catalytic hydrodeoxygenation of 2-methoxy phenol and dibenzofuran over Pt/mesoporous zeolites. Energy 2015, 81, 33–40. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Yohe, S.L.; Choudhari, H.J.; Mehta, D.D.; Dietrich, P.J.; Detwiler, M.D.; Akatay, C.M.; Stach, E.A.; Miller, J.T.; Delgass, W.N.; Agrawal, R.; et al. High-pressure vapor-phase hydrodeoxygenation of lignin-derived oxygenates to hydrocarbons by a PtMo bimetallic catalyst: Product selectivity, reaction pathway, and structural characterization. J. Catal. 2016, 344, 535–552. [Google Scholar] [CrossRef]
- Dong, P.; Lu, G.P.; Cai, C. Effective hydrodeoxygenation of dibenzofuran by a bimetallic catalyst in water. New J. Chem. 2016, 40, 1605–1609. [Google Scholar] [CrossRef]
- He, Z.; Wang, X. Hydrodeoxygenation of model compounds and catalytic systems for pyrolysis bio-oils upgrading. Catal. Sustain. Energy 2012, 1, 28–52. [Google Scholar] [CrossRef]
- Luo, B.; Li, R.; Shu, R.; Wang, C.; Zhang, J.; Chen, Y. Boric acid as a novel homogeneous catalyst coupled with Ru/C for hydrodeoxygenation of phenolic compounds and raw lignin oil. Ind. Eng. Chem. Res. 2020, 59, 17192–17199. [Google Scholar] [CrossRef]
- Yang, Z.; Luo, B.; Shu, R.; Zhong, Z.; Tian, Z.; Wang, C.; Chen, Y. Synergistic effect of active metal–acid sites on hydrodeoxygenation of lignin-derived phenolic compounds under mild conditions using Ru/C-HPW catalyst. Fuel 2022, 319, 123617. [Google Scholar] [CrossRef]
- Bazhenova, M.A.; Kulikov, L.A.; Bolnykh, Y.S.; Maksimov, A.L.; Karakhanov, E.A. Palladium catalysts based on porous aromatic frameworks for vanillin hydrogenation: Tuning the activity and selectivity by introducing functional groups. Catal. Commun. 2022, 170, 106486. [Google Scholar] [CrossRef]
- Yan, P.; Mensah, J.; Drewery, M.; Kennedy, E.; Maschmeyer, T.; Stockenhuber, M. Role of metal support during ru-catalysed hydrodeoxygenation of biocrude oil. Appl. Catal. B. 2021, 281, 119470. [Google Scholar] [CrossRef]
- Yati, I.; Ridwan, M.; Jeong, G.E.; Lee, Y.; Choi, J.W.; Yoon, C.W.; Suh, D.J.; Ha, J.M. Effects of sintering-resistance and large metal-support interface of alumina nanorod-stabilized Pt nanoparticle catalysts on the improved high temperature water gas shift reaction activity. Catal. Commun. 2014, 56, 11–16. [Google Scholar] [CrossRef]
- Dwiatmoko, A.A.; Kim, I.; Zhou, L.; Choi, J.W.; Suh, D.J.; Jae, J.; Ha, J.M. Hydrodeoxygenation of guaiacol on tungstated zirconia supported Ru catalysts. Appl. Catal. A Gen. 2017, 543, 10–16. [Google Scholar] [CrossRef]
- Song, W.; Liu, Y.; Baráth, E.; Zhao, C.; Lercher, J.A. Synergistic effects of Ni and acid sites for hydrogenation and C–O bond cleavage of substituted phenols. Green. Chem. 2015, 17, 1204. [Google Scholar] [CrossRef]
- Wang, X.; Lv, Y.; Zhu, S.; Wang, X.; Deng, C. Phosphoric acid modification of hβ zeolite for guaiacol hydrodeoxygenation. Catalysts 2021, 11, 962. [Google Scholar] [CrossRef]
- Duong, N.; Tan, Q.; Resasco, D.E. Controlling phenolic hydrodeoxygenation by tailoring metal–O bond strength via specific catalyst metal type and particle size selection. Comptes Rendus Chim. 2018, 21, 155–163. [Google Scholar] [CrossRef]
- Rochmadi, S.; Mulyono, P.; Aziz, M.; Budiman, A. Kinetic study of catalytic cracking of bio-oil over silica-alumina catalyst. Bioresources 2018, 13, 1917–1929. [Google Scholar] [CrossRef]
- Nie, L.; Resasco, D.E. Kinetics and mechanism of m-cresol hydrodeoxygenation on a Pt/SiO2 catalyst. J. Catal. 2014, 317, 22–29. [Google Scholar] [CrossRef]
- Tran, Q.K.; Han, S.; Ly, H.V.; Kim, S.S.; Kim, J. Hydrodeoxygenation of a bio-oil model compound derived from woody biomass using spray-pyrolysis-derived spherical γ-Al2O3-SiO2 catalysts. J. Ind. Eng. Chem. 2020, 92, 243–251. [Google Scholar] [CrossRef]
- Kulikov, L.; Kalinina, M.; Makeeva, D.; Maximov, A.; Kardasheva, Y.; Terenina, M.; Karakhanov, E. Palladium Catalysts Based on Porous Aromatic Frameworks, Modified with Ethanolamino-Groups, for Hydrogenation of Alkynes, Alkenes and Dienes. Catalysts 2020, 10, 1106. [Google Scholar] [CrossRef]
- Kulikov, L.A.; Bazhenova, M.A.; Bolnykh, Y.S.; Makeeva, D.A.; Terenina, M.V.; Kardasheva, Y.S.; Maximov, A.L.; Karakhanov, E.A. Alkylation of Guaiacol with Alcohols on Porous Aromatic Frameworks Modified with Sulfo Groups. Pet. Chem. 2022, 62, 1195–1203. [Google Scholar] [CrossRef]
- Ben, T.; Qiu, S. Porous aromatic frameworks: Synthesis, structure and functions. CrystEngComm 2012, 15, 17–26. [Google Scholar] [CrossRef]
- Maximov, A.; Zolotukhina, A.; Kulikov, L.; Kardasheva, Y.; Karakhanov, E. Ruthenium catalysts based on mesoporous aromatic frameworks for the hydrogenation of arenes, Reaction Kinetics. Mech. Catal. 2016, 117, 729–743. [Google Scholar] [CrossRef]
- Wang, F.; Mielby, J.; Richter, F.H.; Wang, G.; Prieto, G.; Kasama, T.; Weidenthaler, C.; Bongard, H.J.; Kegnæs, S.; Fürstner, A.; et al. A polyphenylene support for pd catalysts with exceptional catalytic activity. Angew. Chem. Int. Ed. 2014, 53, 8645–8648. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Sun, F.; Ren, H.; Jing, X.; Wang, W.; Ma, H.; Zhao, H.; Zhu, G. Targeted synthesis of a porous aromatic framework with a high adsorption capacity for organic molecules. J. Mater. Chem. 2011, 21, 13498–13502. [Google Scholar] [CrossRef]
- Yang, J.; Huang, W.; Liu, Y.; Zhou, T. Enhancing the conversion of ethyl levulinate to γ-valerolactone over Ru/UiO-66 by introducing sulfonic groups into the framework. RSC Adv. 2018, 8, 16611–16618. [Google Scholar] [CrossRef]
- Pan, H.; Shen, S.; Li, T.; Wen, X.; Ma, X.; Zhou, Z.; Li, J.; Wang, C.; Jing, S. A simple strategy for the preparation of chlorine functionalized coal-based solid acid with rich carboxyl to improve the activity for hydrolysis of cellulose. Mol. Catal. 2020, 492, 111015. [Google Scholar] [CrossRef]
- Lin, X.; Cheng, S.; Wu, F.; Li, Y.; Zhuang, Q.; Dong, W.; Xie, A. Connecting of conjugate microporous polymer nanoparticles by polypyrrole via sulfonic acid doping to form conductive nanocomposites for excellent microwaves absorption. Compos. Sci. Technol. 2022, 221, 109350. [Google Scholar] [CrossRef]
- Lebedeva, A.; Albuquerque, B.L.; Domingos, J.B.; Lamonier, J.F.; Giraudon, J.M.; Lecante, P.; Denicourt-Nowicki, A.A. Roucoux, Ruthenium Trichloride Catalyst in Water: Ru Colloids versus Ru Dimer Characterization Investigations. Inorg. Chem. 2019, 58, 4141–4151. [Google Scholar] [CrossRef]
- Malenov, D.P.; Zarić, S.D. Stacking interactions of aromatic ligands in transition metal complexes. Coord. Chem. Rev. 2020, 419, 213338. [Google Scholar] [CrossRef]
- Pan, C.; Pelzer, K.; Philippot, K.; Chaudret, B.; Dassenoy, F.; Lecante, P.; Casanove, M.J. Ligand-stabilized ruthenium nanoparticles: Synthesis, organization, and dynamics. J. Am. Chem. Soc. 2001, 123, 7584–7593. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.J. Resolving ruthenium: XPS studies of common ruthenium materials. Surf. Interface Anal. 2015, 47, 1072–1079. [Google Scholar] [CrossRef]
- Shangguan, J.; Pfriem, N.; Chin, Y.H.C. Mechanistic details of CO bond activation in and H-addition to guaiacol at water-Ru cluster interfaces. J. Catal. 2019, 370, 186–199. [Google Scholar] [CrossRef]
- Luo, Z.; Zheng, Z.; Wang, Y.; Sun, G.; Jiang, H.; Zhao, C. Hydrothermally stable Ru/HZSM-5-catalyzed selective hydrogenolysis of lignin-derived substituted phenols to bio-arenes in water. Green. Chem. 2016, 18, 5845–5858. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Ishikawa, M.; Tamura, M.; Tomishige, K. Selective production of cyclohexanol and methanol from guaiacol over Ru catalyst combined with MgO. Green. Chem. 2014, 16, 2197–2203. [Google Scholar] [CrossRef]
- Kulikov, L.A.; Makeeva, D.A.; Kalinina, M.A.; Cherednichenko, K.A.; Maximov, A.L.; Karakhanov, E.A. Pt and Ru catalysts based on porous aromatic frameworks for hydrogenation of lignin biofuel components. Pet. Chem. 2021, 61, 711–720. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, A.; Yang, L.; Fan, G.; Li, F. Supported Ru nanocatalyst over phosphotungstate intercalated Zn-Al layered double hydroxide derived mixed metal oxides for efficient hydrodeoxygenation of guaiacol. Mol. Catal. 2022, 528, 112503. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Li, X.; Yan, P.; Han, W.; Sheng, T.; Deng, T.; Zhu, W.; Wang, H. Regulating the nanoscale intimacy of metal and acidic sites in Ru/γ-Al2O3 for the selective conversions of lignin-derived phenols to jet fuels. J. Energy Chem. 2022, 66, 576–586. [Google Scholar] [CrossRef]
- Shu, R.; Xu, Y.; Ma, L.; Zhang, Q.; Chen, P.; Wang, T. Synergistic effects of highly active Ni and acid site on the hydrodeoxygenation of syringol. Catal. Commun. 2017, 91, 1–5. [Google Scholar] [CrossRef]
- Dong, L.; Yin, L.L.; Xia, Q.; Liu, X.; Gong, X.Q.; Wang, Y. Size-dependent catalytic performance of ruthenium nanoparticles in the hydrogenolysis of a β-O-4 lignin model compound. Catal. Sci. Technol. 2018, 8, 735–745. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, B.; Zhou, R.; Zhong, Z.; Chen, P.; Hou, Z. Selective hydrogenation of phenol to cyclohexanone over small amount of Ru-promoted Pd/ZrHP catalysts. Appl. Catal. A Gen. 2023, 657, 119144. [Google Scholar] [CrossRef]
- Kamal, S. Catalyst deactivation of cation-exchange resin in cross-aldol condensation of acetaldehyde to methyl pentenone. Flavour. Fragr. J. 2023, 38, 312–325. [Google Scholar] [CrossRef]
- Luska, K.L.; Migowski, P.; El Sayed, S.; Leitner, W. Synergistic Interaction within Bifunctional Ruthenium Nanoparticle/SILP Catalysts for the Selective Hydrodeoxygenation of Phenols. Angew. Chem. Int. Ed. 2015, 54, 15750–15755. [Google Scholar] [CrossRef] [PubMed]
- Dwiatmoko, A.A.; Zhou, L.; Kim, I.; Choi, J.W.; Suh, D.J.; Ha, J.M. Hydrodeoxygenation of Lignin-Derived Monomers and Lignocellulose Pyrolysis Oil on the Carbon-Supported Ru Catalysts. Catal Today. 2016, 265, 192–198. [Google Scholar] [CrossRef]
- Wang, H.; Ruan, H.; Feng, M.; Qin, Y.; Job, H.; Luo, L.; Wang, C.; Engelhard, M.H.; Kuhn, E.; Chen, X.; et al. One-Pot Process for Hydrodeoxygenation of Lignin to Alkanes Using Ru-Based Bimetallic and Bifunctional Catalysts Supported on Zeolite Y. ChemSusChem. 2017, 10, 1846–1856. [Google Scholar] [CrossRef] [PubMed]
- Boronoev, M.P.; Shakirov, I.I.; Ignat’eva, V.I.; Maximov, A.L.; Karakhanov, E.A. A Nanospherical Mesoporous Ruthenium-Containing Polymer as a Guaiacol Hydrogenation Catalyst. Pet. Chem. 2019, 59, 1300–1306. [Google Scholar] [CrossRef]
- Long, W.; Liu, P.; Xiong, W.; Hao, F.; Luo, H. Conversion of Guaiacol as Lignin Model Component Using Acid-Treated, Multi-Walled Carbon Nanotubes Supported Ru–MnO Bimetallic Catalysts. Can J. Chem. 2020, 98, 57–65. [Google Scholar] [CrossRef]
- Zasypalov, G.; Vutolkina, A.; Klimovsky, V.; Abramov, E.; Vinokurov, V.; Glotov, A. Hydrodeoxygenation of Guaiacol over Halloysite Nanotubes Decorated with Ru Nanoparticles: Effect of Alumina Acid Etching on Catalytic Behavior and Reaction Pathways. Appl. Catal. B 2024, 342, 123425. [Google Scholar] [CrossRef]
- Lin, B.; Li, R.; Shu, R.; Wang, C.; Yuan, Z.; Liu, Y.; Chen, Y. Synergistic Effect of Highly Dispersed Ru and Moderate Acid Site on the Hydrodeoxygenation of Phenolic Compounds and Raw Bio-Oil. J. Energy Inst. 2020, 93, 847–856. [Google Scholar] [CrossRef]
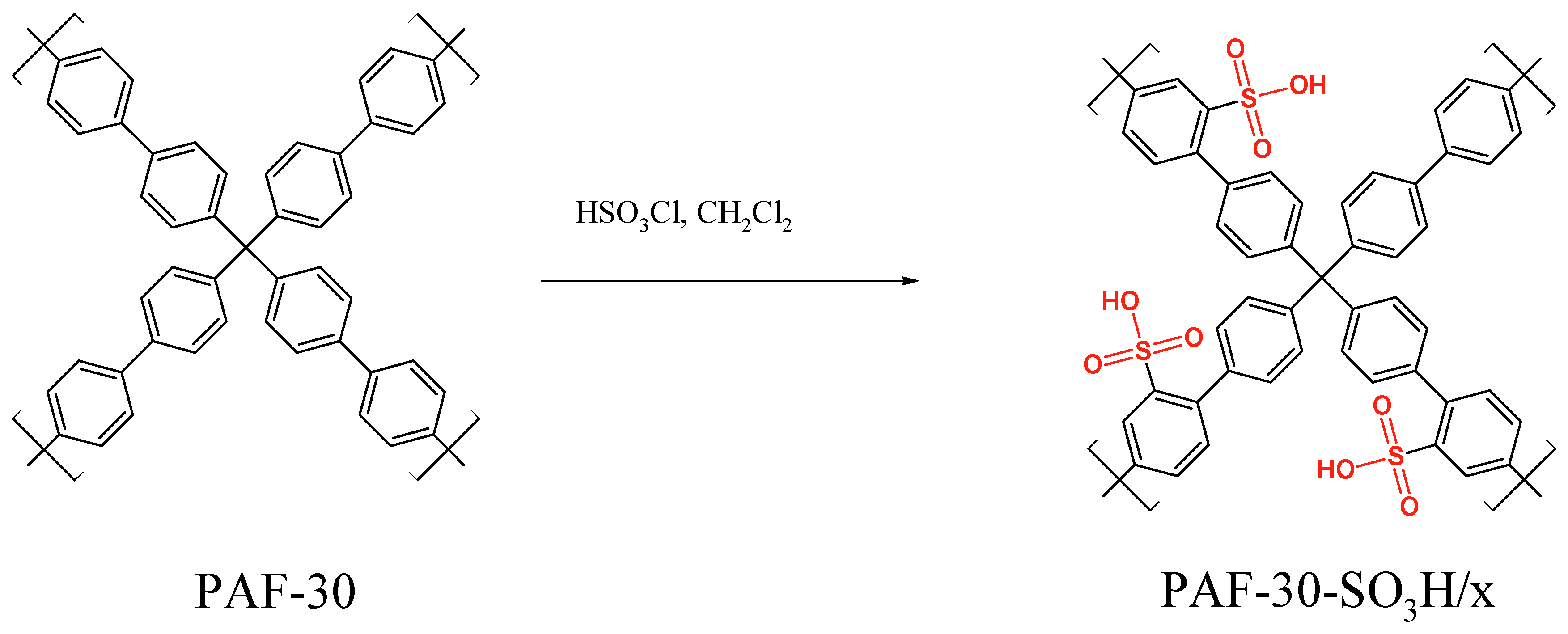

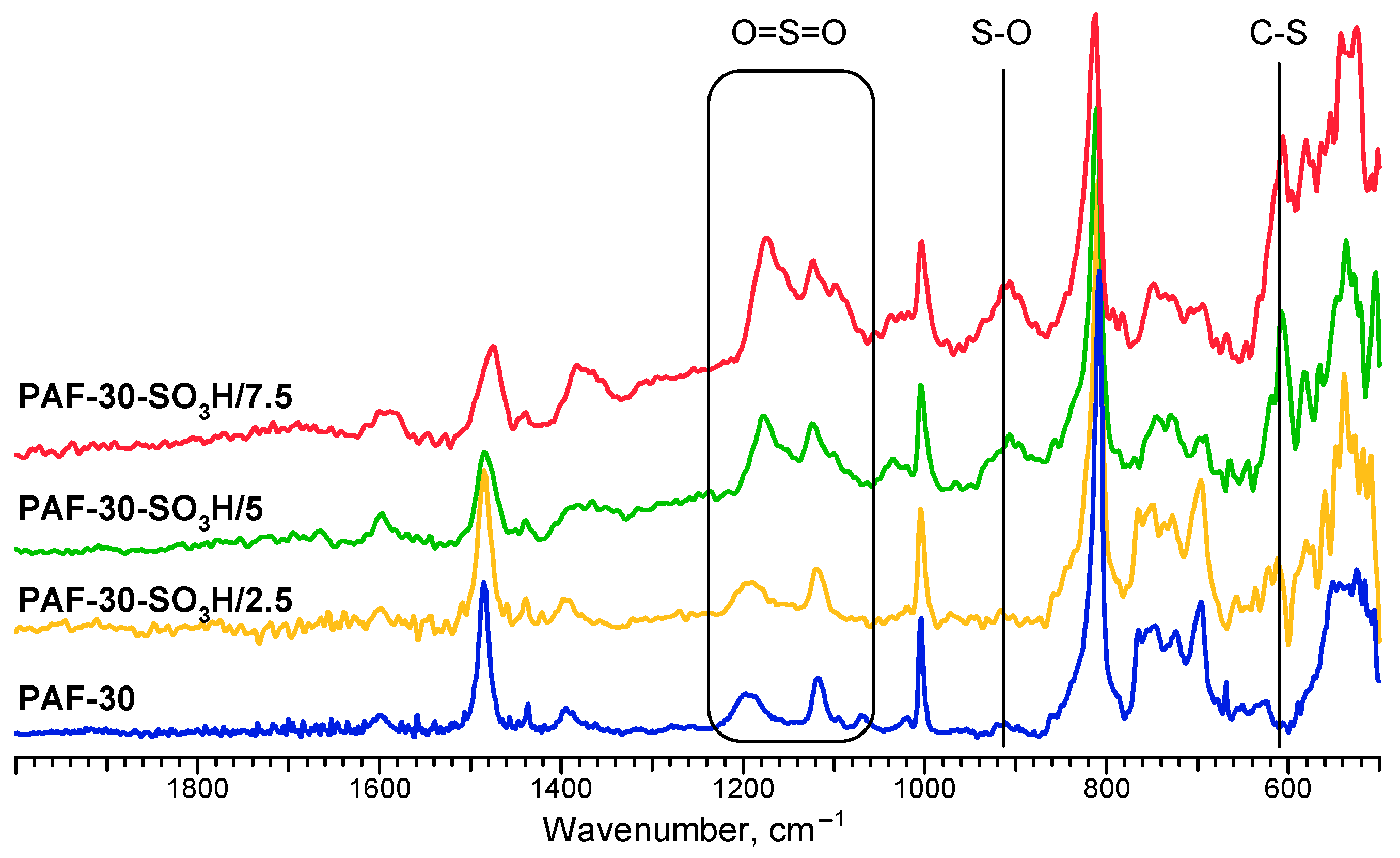




| Material | SBET, m2/g | Total Pore Volume, cm3/g | Sulfur Content, wt. % | Acidity, mmol/g |
|---|---|---|---|---|
| PAF-30 | 484 | 0.28 | - | - |
| PAF-30-SO3H/2.5 | 427 | 0.19 | 2.5 | 0.82 |
| PAF-30-SO3H/5 | 369 | 0.09 | 5 | 1.64 |
| PAF-30-SO3H/7.5 | 197 | 0.08 | 7.5 | 2.34 |
| Catalyst | dav, nm | Ru Content, wt.% | S/Ru, mol:mol | SBET, m2/g |
|---|---|---|---|---|
| Ru-PAF-30-SO3H/2.5 | 2.8 | 1.47 | 5.36 | 410 |
| Ru-PAF-30-SO3H/5 | 3.8 | 4.67 | 3.47 | 359 |
| Ru-PAF-30-SO3H/7.5 | 2.7 | 0.50 | 47.28 | 153 |
| Ru-PAF-30-SO3H/2.5-COD | 2.2 | 0.79 | 9.98 | 388 |
| Ru-PAF-30-SO3H/5-COD | 2.5 | 0.76 | 20.73 | 354 |
| Ru-PAF-30-SO3H/7.5-COD | 2.8 | 0.47 | 50.29 | 94 |
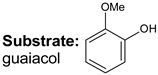 |  | 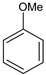 | 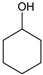 | 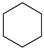 | Conversion, % | ||||
| * Ru-PAF-30 | 63 | — | 34 | — | 97 | ||||
| Ru-PAF-30-SO3H/2.5 | 56 | — | 26 | 2 | 84 | ||||
| Ru-PAF-30-SO3H/5 | 35 | 2 | 8 | 55 | 100 | ||||
| Ru-PAF-30-SO3H/7.5 | — | 4 | 7 | 19 | 30 | ||||
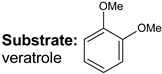 | 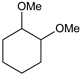 |  |  | 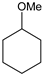 |  | 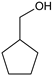 |  | Conversion, % | |
| * Ru-PAF-30 | 95 | 1 | — | — | 3 | — | — | 99 | |
| Ru-PAF-30-SO3H/2.5 | 66 | 22 | — | 7 | 2 | — | 3 | 100 | |
| Ru-PAF-30-SO3H/5 | 46 | 7 | — | 6 | 18 | 2 | 18 | 97 | |
| Ru-PAF-30-SO3H/7.5 | 2 | 2 | 2 | — | — | 11 | 57 | 78 | |
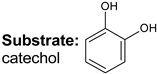 | 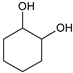 |  | 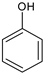 |  | 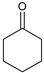 |  |  | Conversion, % | |
| * Ru-PAF-30 | 58 | — | 1 | 41 | — | — | 0.2 | 100 | |
| Ru-PAF-30-SO3H/2.5 | 47 | 13 | 4 | 24 | 1 | 8 | 2 | 99 | |
| Ru-PAF-30-SO3H/5 | 13 | — | — | 73 | 1 | 12 | — | 99 | |
| Ru-PAF-30-SO3H/7.5 | — | — | — | — | 5 | — | — | 5 | |
 |  |  |  | 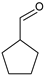 |  |  | Alkylation products | Conversion, % | ||
| Ru-PAF-30-SO3H/2.5-COD | 33 | 40 | — | — | — | 16 | — | 89 | ||
| Ru-PAF-30-SO3H/5-COD | — | — | 44 | 8 | 11 | 1 | 19 | 83 | ||
| Ru-PAF-30-SO3H/7.5-COD | — | — | — | — | — | — | — | 0 | ||
 |  |  |  |  |  |  |  | 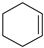 |  | Conversion, % |
| Ru-PAF-30-SO3H/2.5-COD | 26 | 10 | 7 | 6 | — | — | — | — | 13 | 62 |
| Ru-PAF-30-SO3H/5 -COD | 3 | — | — | — | 18 | 2 | 7 | 11 | 38 | 79 |
| Ru-PAF-30-SO3H/7.5-COD | — | — | — | — | — | — | — | — | — | 0 |
 |  |  |  |  |  |  | Conversion, % | |||
| Ru-PAF-30-SO3H/2.5-COD | 7 | 20 | — | — | 1 | 1 | 29 | |||
| Ru-PAF-30-SO3H/5 -COD | 10 | — | 70 | 15 | 5 | — | 100 | |||
| Ru-PAF-30-SO3H/7.5-COD | — | — | — | — | — | — | 0 | |||
 |  |  |  |  | Alkylation products | Conversion, % | |||
| Ru-PAF-30-SO3H/5 | Run 1 | 35 | 2 | 8 | 55 | — | 100 | ||
| Run 2 | 39 | — | 12 | 35 | — | 86 | |||
| Run 3 | 27 | — | 14 | 11 | 6 | 58 | |||
 |  |  |  |  |  |  | Alkylation products | Conversion, % | |
| Ru-PAF-30-SO3H/5 -COD | Run 1 | — | — | 44 | 8 | 11 | 1 | 19 | 83 |
| Run 2 | 4 | 2 | 31 | 2 | — | 1 | 11 | 52 | |
| Run 3 | 2 | 2 | 12 | — | — | 2 | 8 | 28 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazhenova, M.A.; Kulikov, L.A.; Makeeva, D.A.; Maximov, A.L.; Karakhanov, E.A. Hydrodeoxygenation of Lignin-Based Compounds over Ruthenium Catalysts Based on Sulfonated Porous Aromatic Frameworks. Polymers 2023, 15, 4618. https://doi.org/10.3390/polym15234618
Bazhenova MA, Kulikov LA, Makeeva DA, Maximov AL, Karakhanov EA. Hydrodeoxygenation of Lignin-Based Compounds over Ruthenium Catalysts Based on Sulfonated Porous Aromatic Frameworks. Polymers. 2023; 15(23):4618. https://doi.org/10.3390/polym15234618
Chicago/Turabian StyleBazhenova, Maria A., Leonid A. Kulikov, Daria A. Makeeva, Anton L. Maximov, and Eduard A. Karakhanov. 2023. "Hydrodeoxygenation of Lignin-Based Compounds over Ruthenium Catalysts Based on Sulfonated Porous Aromatic Frameworks" Polymers 15, no. 23: 4618. https://doi.org/10.3390/polym15234618
APA StyleBazhenova, M. A., Kulikov, L. A., Makeeva, D. A., Maximov, A. L., & Karakhanov, E. A. (2023). Hydrodeoxygenation of Lignin-Based Compounds over Ruthenium Catalysts Based on Sulfonated Porous Aromatic Frameworks. Polymers, 15(23), 4618. https://doi.org/10.3390/polym15234618