Temporary Consolidation of Marine Artifact Based on Polyvinyl Alcohol/Tannic Acid Reversible Hydrogel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
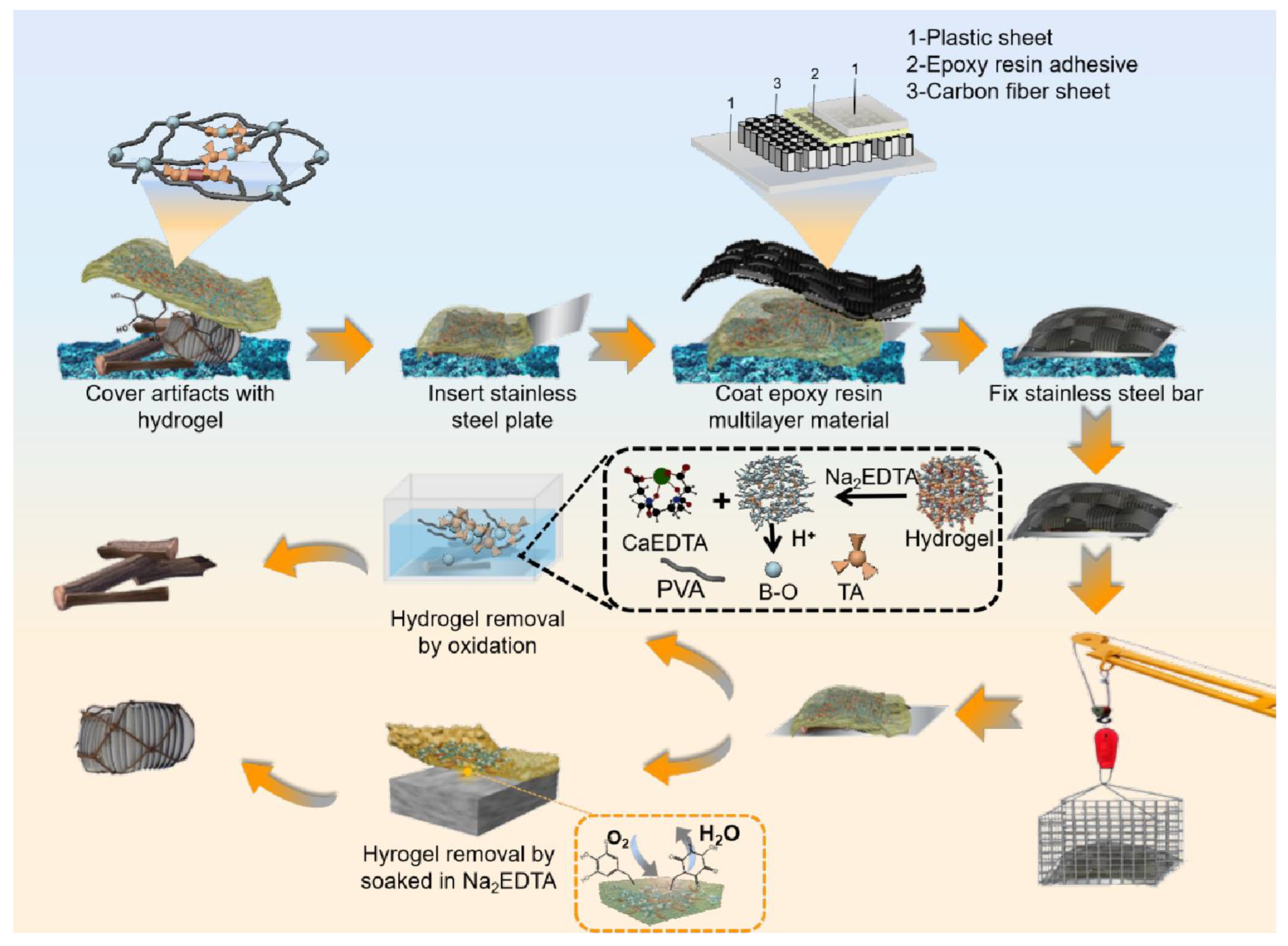
2.2. Methods
2.3. Preparation of Concretion
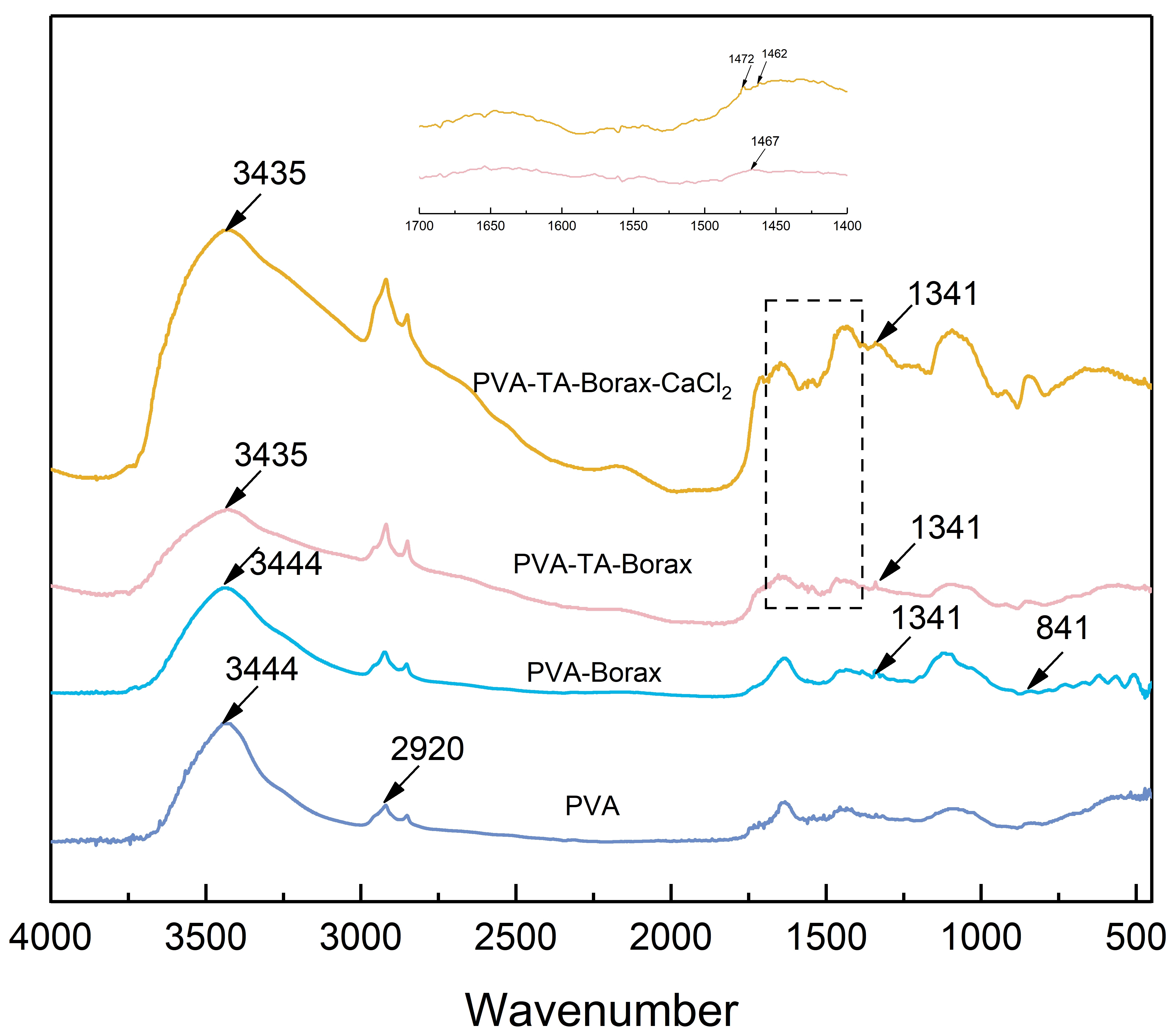
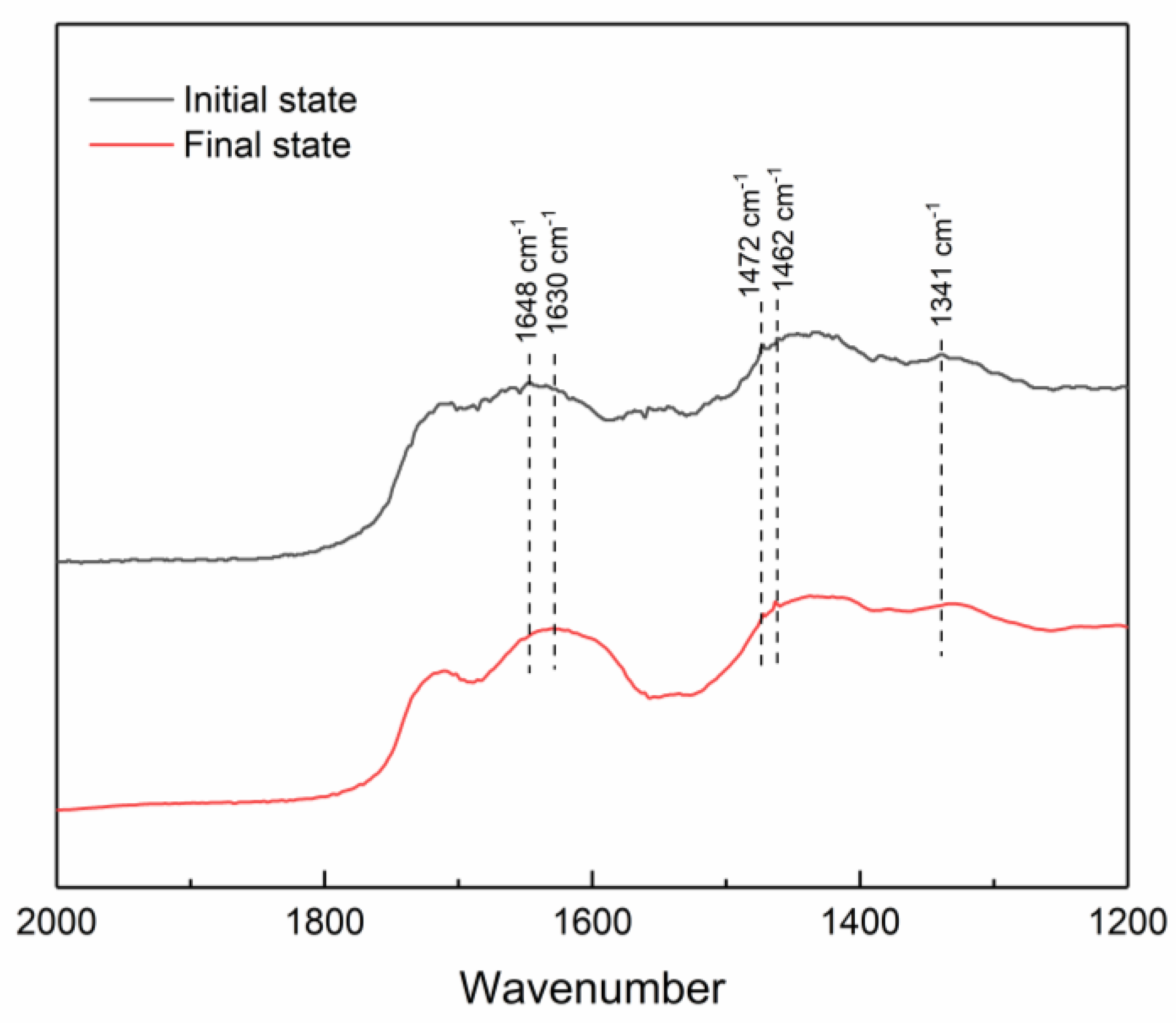
2.4. Fourier Transform Infrared Spectrometer
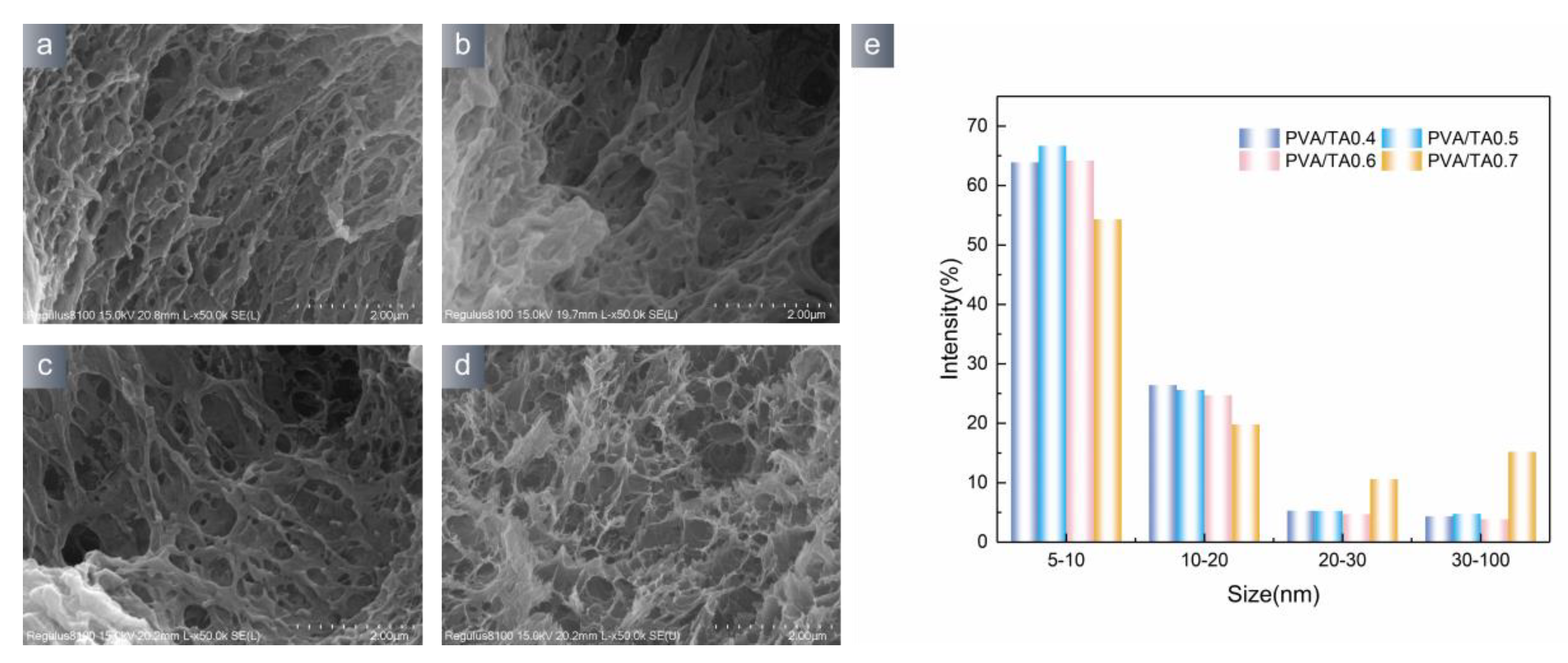
2.5. Scanning Electron Microscope
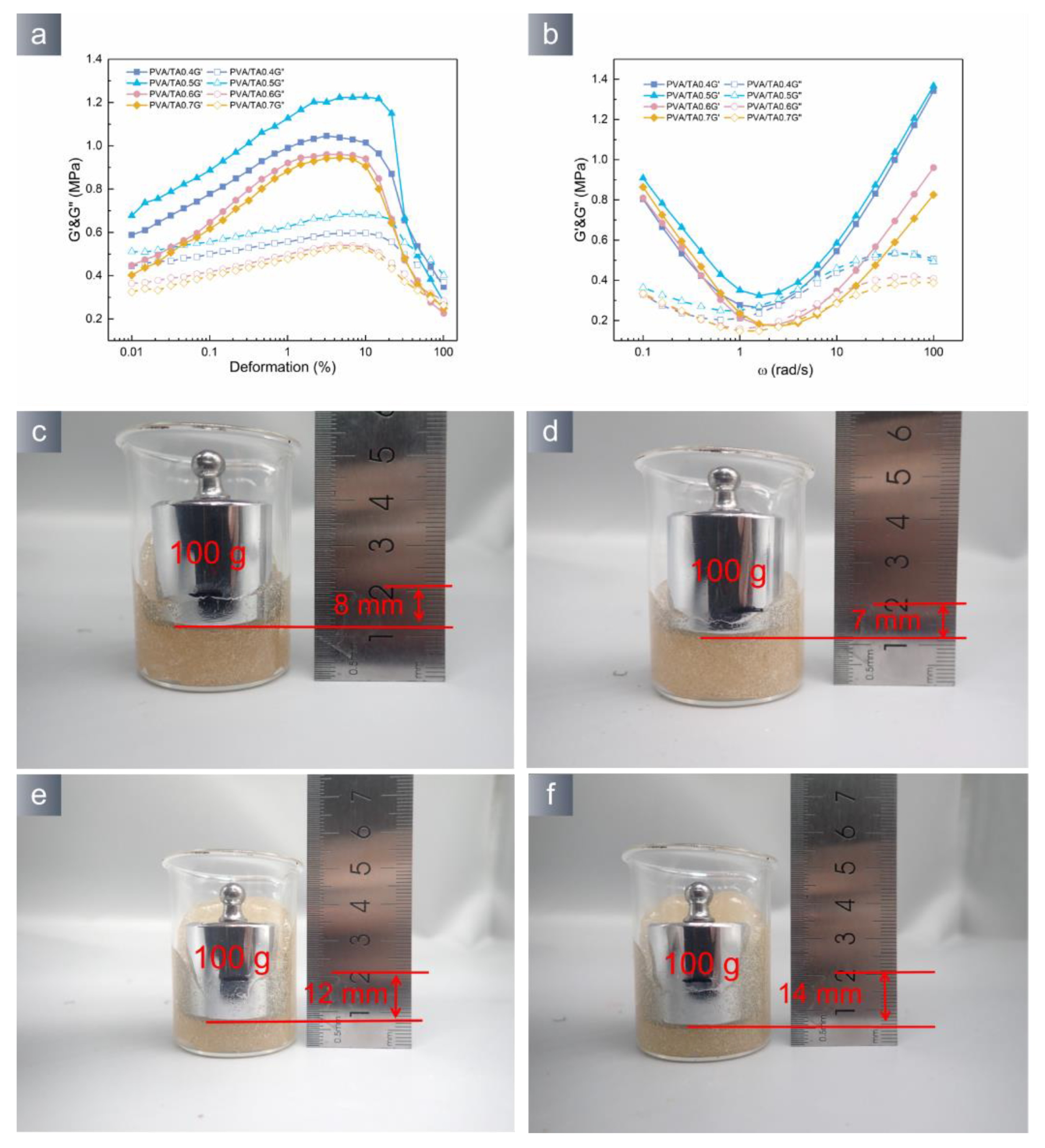
2.6. Adhesion Performance
2.7. Rheological Measurements
2.8. Reversible Performance
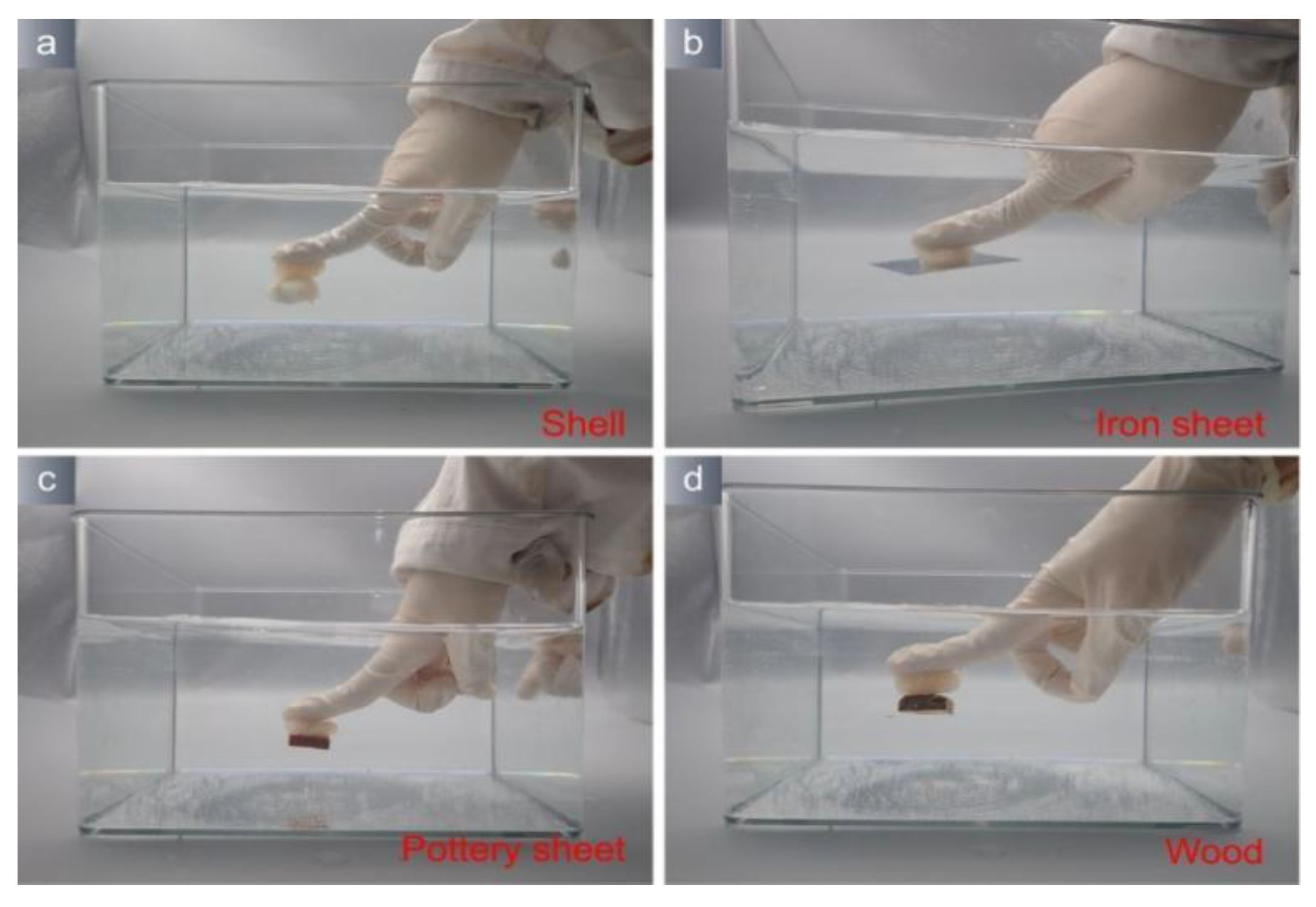
2.9. Simulation Application
3. Results and Discussion
3.1. Hydrogel Characterization
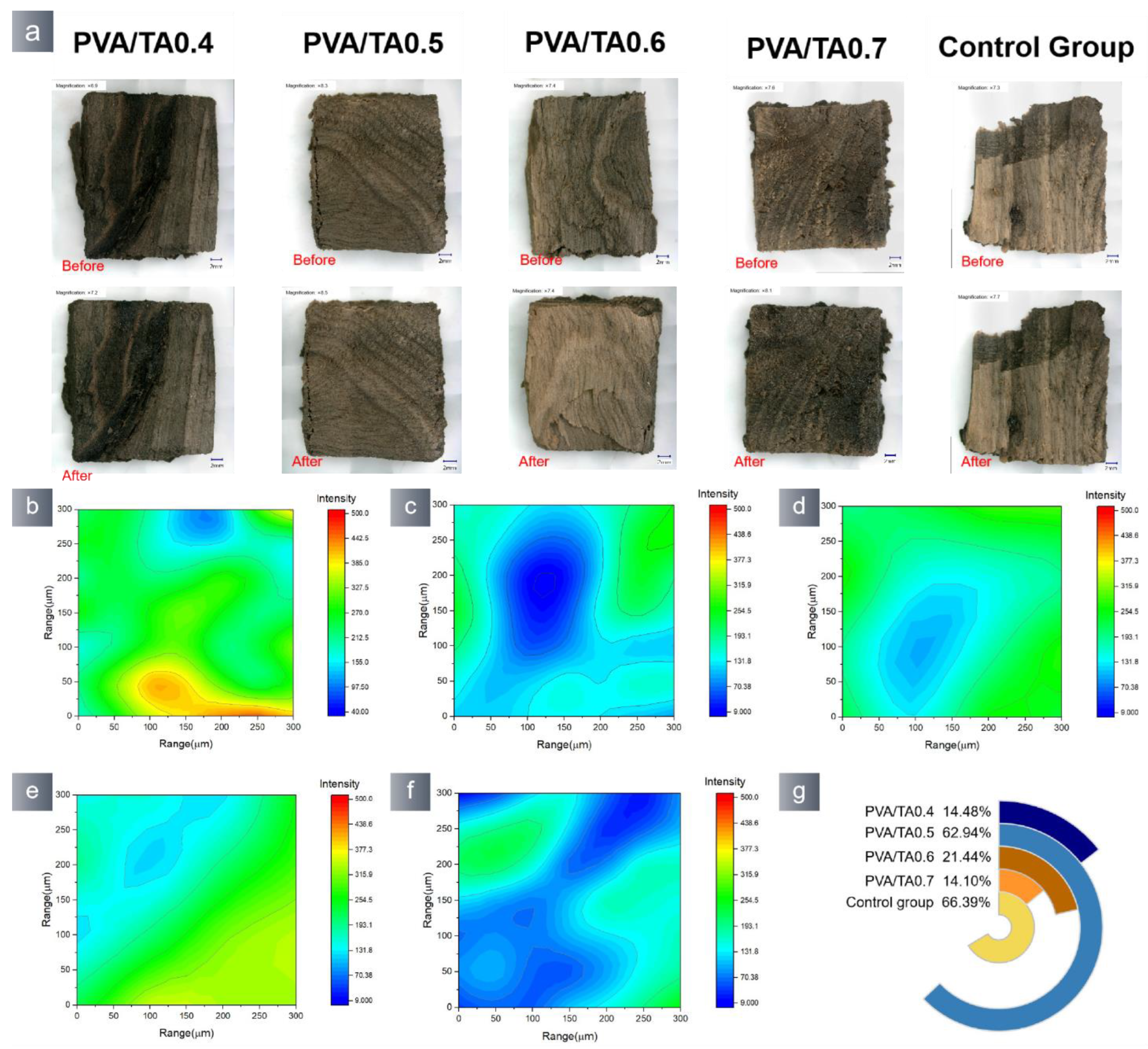
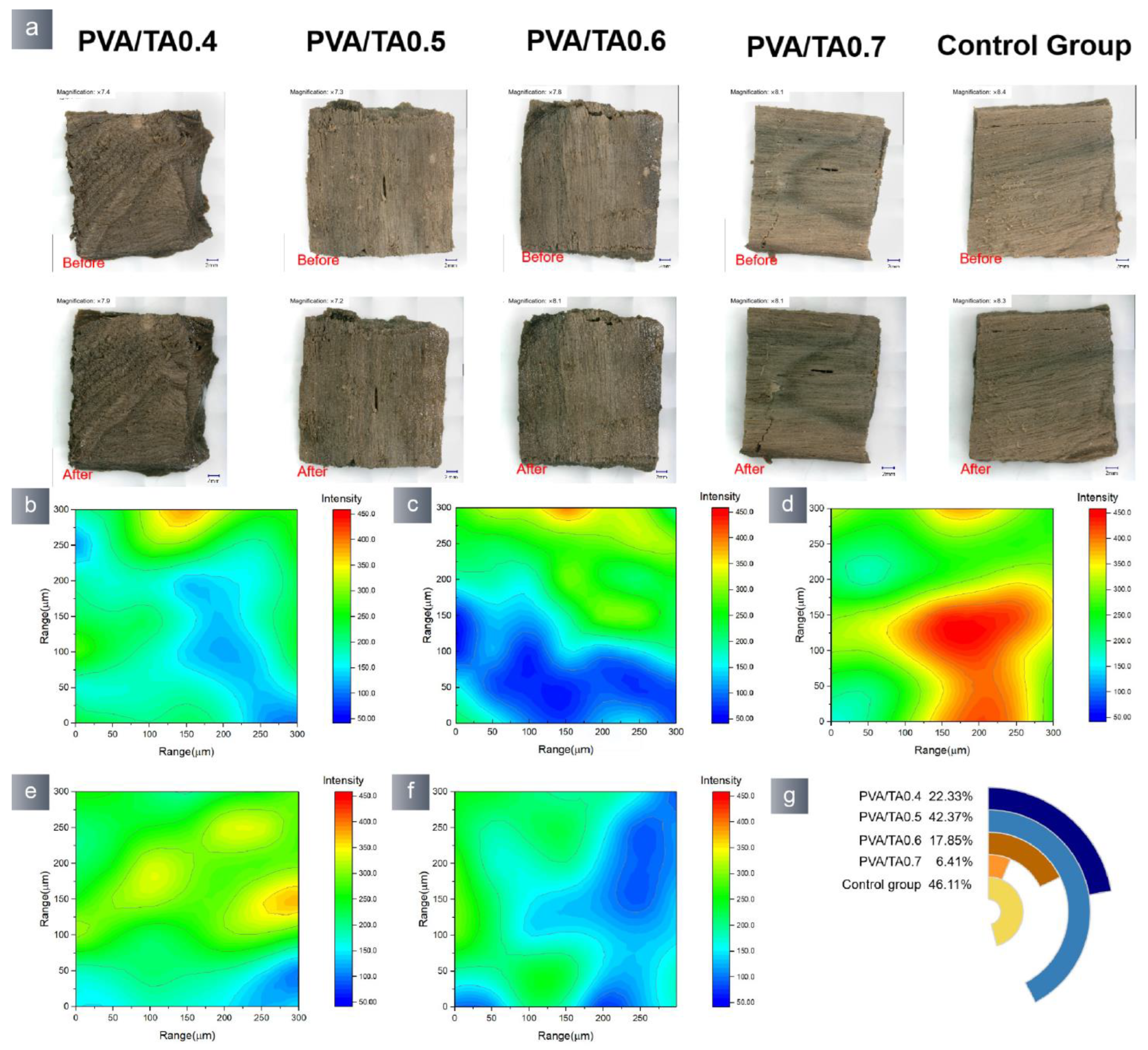
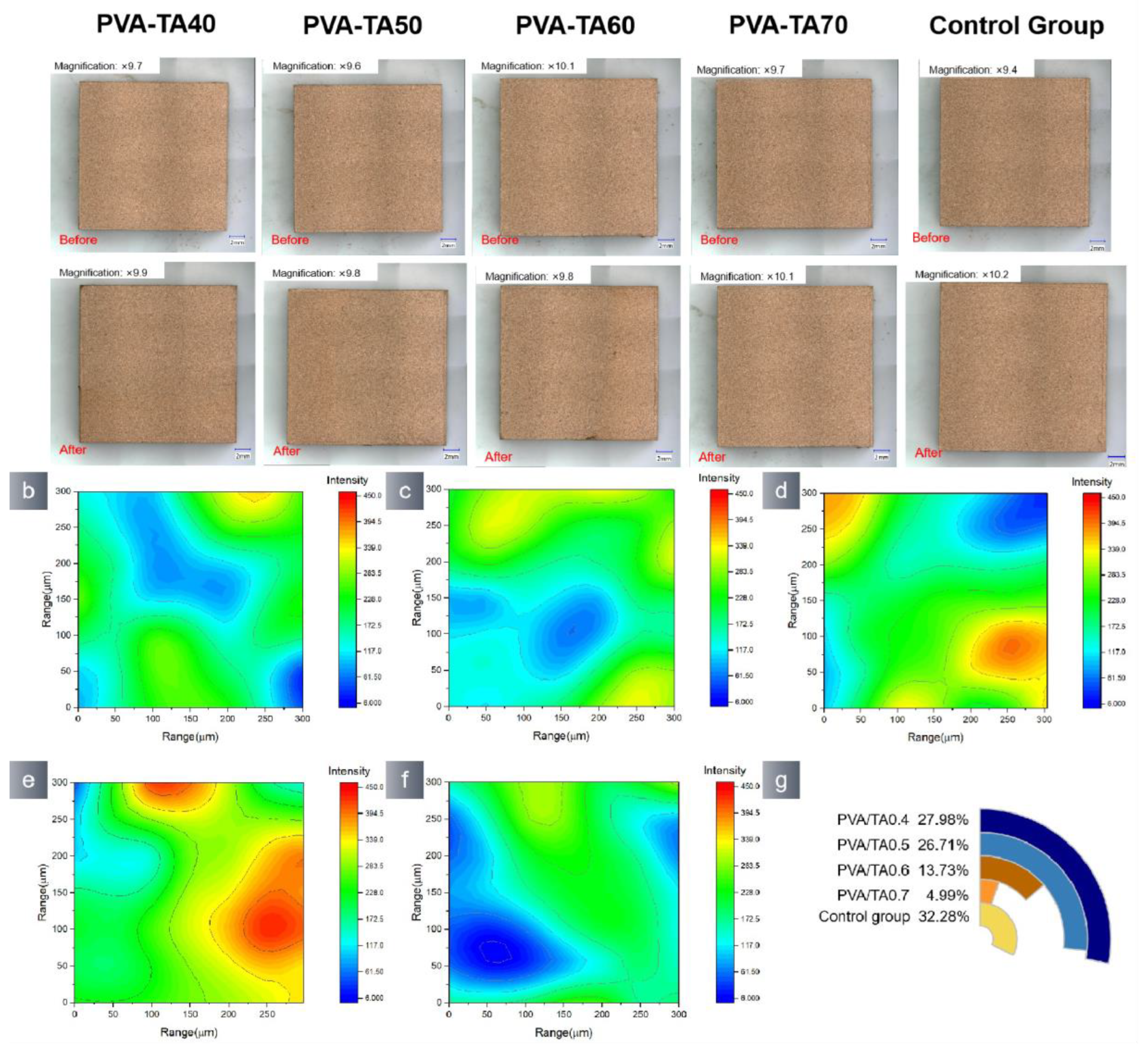
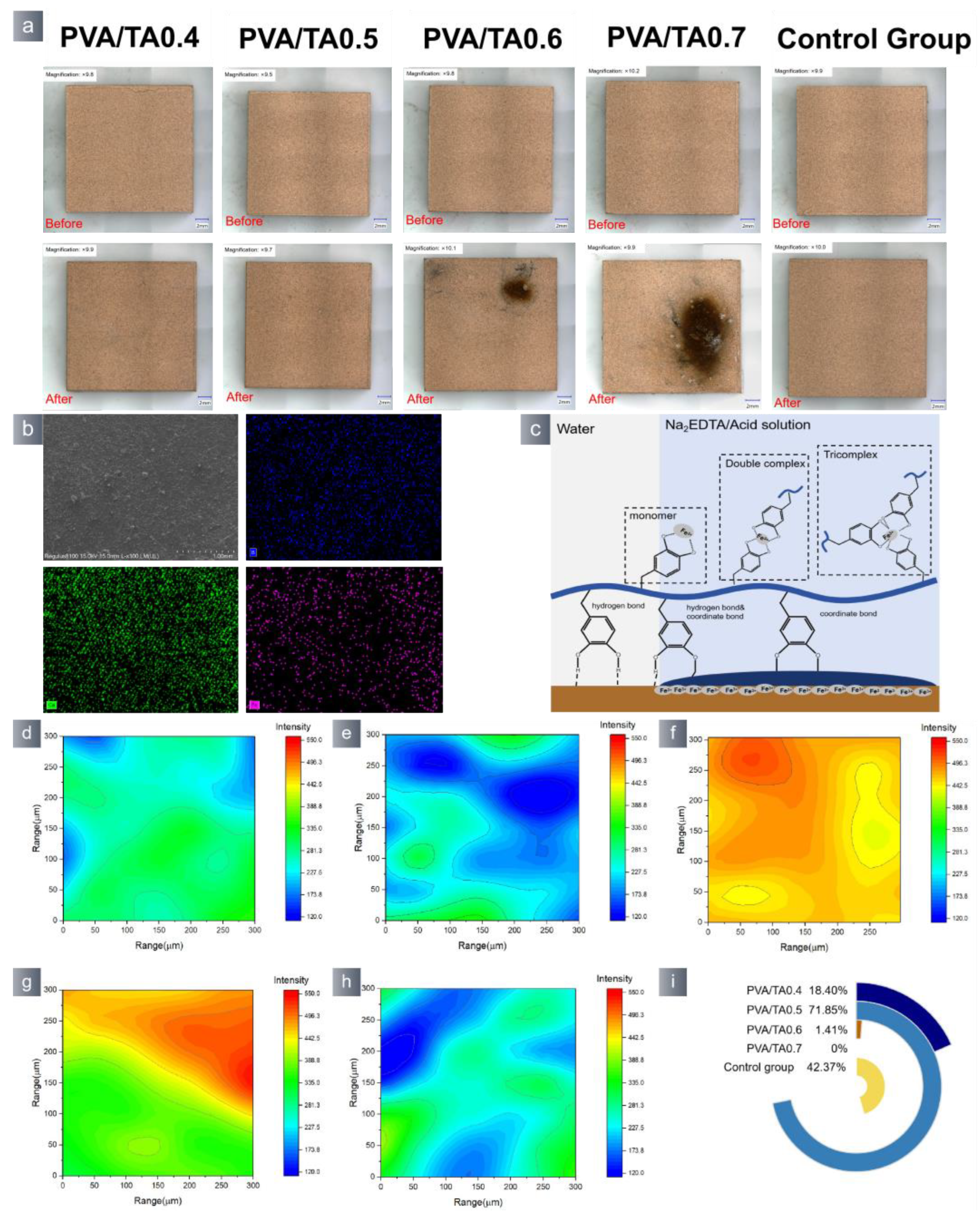
3.2. Application Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prieto, J.; Bruno, F.; Lagudi, A.; Ricca, M.; La Russa, M.F.; Papatheodorou, G.; Mišković, N. Advanced Technologies for Maritime and Underwater Archaeology. J. Mar. Sci. Eng. 2023, 11, 593. [Google Scholar] [CrossRef]
- Kergourlay, F.; Réguer, S.; Neff, D.; Foy, E.; Picca, F.E.; Saheb, M.; Hustache, S.; Mirambet, F.; Dillmann, P. Stabilization treatment of cultural heritage artefacts: In situ monitoring of marine iron objects dechlorinated in alkali solution. Corros. Sci. 2018, 132, 21–34. [Google Scholar] [CrossRef]
- Jia, Y.; Yin, L.; Zhang, F.; Wang, M.; Sun, M.; Hu, C.; Liu, Z.; Chen, Y.; Liu, J.; Pan, J. Fungal Community Analysis and Biodeterioration of Waterlogged Wooden Lacquerware from the Nanhai No. 1 Shipwreck. Appl. Sci. 2020, 10, 3797. [Google Scholar] [CrossRef]
- López-Arce, P.; Zornoza-Indart, A.; Gomez-Villalba, L.; Pérez-Monserrat, E.M.; Alvarez de Buergo, M.; Vivar, G.; Fort, R. Archaeological ceramic amphorae from underwater marine environments: Influence of firing temperature on salt crystallization decay. J. Eur. Ceram. Soc. 2013, 33, 2031–2042. [Google Scholar] [CrossRef]
- Junwen, S.; Maolin, Z.; Qijiang, L.; Feng, Y. Research Progress on Surface Sediment Diseases of Ceramic Relics. J. Ceram. 2021, 42, 256–262. [Google Scholar] [CrossRef]
- Geoffrey, Q.C.B. Salvage and the underwater cultural heritage. Mar. Policy 1996, 20, 337–342. [Google Scholar] [CrossRef]
- Rémazeilles, C.; Neff, D.; Kergourlay, F.; Foy, E.; Conforto, E.; Guilminot, E.; Reguer, S.; Refait, P.; Dillmann, P. Mechanisms of long-term anaerobic corrosion of iron archaeological artefacts in seawater. Corros. Sci. 2009, 51, 2932–2941. [Google Scholar] [CrossRef]
- Izquierdo, A.; Fernández-Montblanc, T.; Bethencourt, M.; Mañanes, R. ARQUEOMONITOR: Study of the influence of physical, chemical and biological conditions in the damage and protection of underwater historical heritage. Constructing the bases for in situ protection. In Proceedings of the 5th International Congress on Underwater Archaeology: A Heritage for Mankind, Cartagena, Colombia, 15–18 October 2014. [Google Scholar]
- Petriaggi, B.D.; Gregory, D.J.; Dencker, J. Recovery of Fragile Objects from Underwater Archaeological Excavations: New Materials and Techniques by SASMAP Project. In Proceedings of the Digital Heritage. Progress in Cultural Heritage: Documentation, Preservation, and Protection, Limassol, Cyprus, 3–8 November 2014; pp. 625–634. [Google Scholar]
- EU Sasmap Project Website. Available online: http://sasmap.eu/ (accessed on 6 June 2012).
- Chen, X.; Zhang, B.; Zhang, Z. Application of veratraldehyde as a temporary consolidant for relics at underwater cultural heritage sites. Archaeometry 2019, 61, 1417–1429. [Google Scholar] [CrossRef]
- Yao, L.; Zhu, L.; Zhang, B. Application of 4-dihydrochromone as a temporary consolidant in underwater archeology. J. Cult. Herit. 2022, 57, 235–242. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, B.; Xie, L.; Wang, F. MWCNTs polyurethane sponges with enhanced super-hydrophobicity for selective oil–water separation. Surf. Eng. 2020, 36, 651–659. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, B.; Zhang, Z. A novel method of temporary solidification and extraction of underwater fragile relics in their original state. Int. J. Adhes. 2021, 104, 102724. [Google Scholar] [CrossRef]
- Chen, X.; Xie, L.; Wang, F.; Wu, Y.; Zhang, B.; Zhu, L. Temporary consolidation and packaging of fragile cultural relics at underwater archaeological sites to maintain their original state during extraction. Archaeometry 2020, 62, 1067–1077. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, B.; Hu, Y. Research on extraction of fragile bamboo slips by underwater temporary solidification in original state. J. Cult. Herit. 2021, 51, 174–181. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Zhang, B.; Hu, Y.; Zhu, L. Temporary solidification and extraction of marine archaeological wood underwater at a depth of seven meters: A further contribution. J. Cult. Herit. 2023, 62, 13–20. [Google Scholar] [CrossRef]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hsu, S.-H. Synthesis and Biomedical Applications of Self-healing Hydrogels. Front. Chem. 2018, 6, 449. [Google Scholar] [CrossRef] [PubMed]
- Nuthanakanti, A.; Srivatsan, S.G. Multi-stimuli responsive heterotypic hydrogels based on nucleolipids show selective dye adsorption. Nanoscale Adv. 2020, 2, 4161–4171. [Google Scholar] [CrossRef]
- Fan, X.; Liu, H.; Wang, J.; Tang, K. Investigation of double network hydrogel with controllable swelling behavior by response surface methodology. J. Appl. Polym. Sci. 2019, 137, 48805. [Google Scholar] [CrossRef]
- Zhou, C.; Dai, S.; Zhou, X.; Zhu, H.; Cheng, G.; Ding, J. Ethanol stimuli-responsive toughening PNIPAM/PVA self-healing hydrogel thermal actuator. New J. Chem. 2023, 47, 5825–5831. [Google Scholar] [CrossRef]
- Wolbers, R.C. Recent developments in the use of gel formulations for the cleaning of paintings. In Proceedings of the In Restoration ’92: Conservation, Training, Materials and Techniques: Latest Developments, Amsterdam, The Netherlands, 20–22 October 1992; pp. 74–75. [Google Scholar]
- Yang, Y.; Lian, X.; Yang, Z.; Zhou, Y.; Zhang, X.; Wang, Y. Self-Shaping Microemulsion Gels for Cultural Relic Cleaning. Langmuir 2021, 37, 11474–11483. [Google Scholar] [CrossRef]
- Olvera-Sosa, M.; Guerra-Contreras, A.; Gómez-Durán, C.F.A.; González-García, R.; Palestino, G. Tuning the pH-responsiveness capability of poly(acrylic acid-co-itaconic acid)/NaOH hydrogel: Design, swelling, and rust removal evaluation. J. Appl. Polym. Sci. 2019, 137, 48403. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.; Wang, J.; Ding, J.; Zhang, P.; Dong, W.; Zhao, X.; Lu, Z.; Li, X. Effectively removing animal glue coated on the surface of ancient mural via dissolution of PVA hydrogel induced by thermal treatment. J. Cult. Herit. 2022, 55, 179–184. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, F.; Jiang, X.; Zhang, W. Thermoreversible Tissue Adhesion with Hydrogel Through a Topological Entanglement Approach. Macromol. Rapid Commun. 2023, 44, 2300144. [Google Scholar] [CrossRef]
- Guo, M.; Li, G.; Cai, M.; Hou, X.; Huang, K.; Tang, J.; Guo, C.F. A Tough Hydrogel Adhesive for the Repair of Archeological Pottery. Nano Lett. 2023, 23, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Gong, J.P. Bioinspired Underwater Adhesives. Adv. Mater. 2021, 33, 2102983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, B.; Zhou, Y.; Zhou, F.; Liu, W.; Wang, Z. Mussel-inspired hydrogels: From design principles to promising applications. Chem. Soc. Rev. 2020, 49, 3605–3637. [Google Scholar] [CrossRef] [PubMed]
- Statz, A.; Finlay, J.; Dalsin, J.; Callow, M.; Callow, J.A.; Messersmith, P.B. Algal antifouling and fouling-release properties of metal surfaces coated with a polymer inspired by marine mussels. Biofouling 2006, 22, 391–399. [Google Scholar] [CrossRef]
- Zha, J.; Huang, Q.; Liu, X.; Han, X.; Guo, H. Removal of Calcareous Concretions from Marine Archaeological Ceramics by Means of a Stimuli-Responsive Hydrogel. Polymers 2023, 15, 2929. [Google Scholar] [CrossRef]
- Duncan, T.T.; Chan, E.P.; Beers, K.L. Quantifying the ‘press and peel’ removal of particulates using elastomers and gels. J. Cult. Herit. 2021, 48, 236–243. [Google Scholar] [CrossRef]
- Brewer, S.H.; Allen, A.M.; Lappi, S.E.; Chasse, T.L.; Briggman, K.A.; Gorman, C.B.; Franzen, S. Infrared Detection of a Phenylboronic Acid Terminated Alkane Thiol Monolayer on Gold Surfaces. Langmuir 2004, 20, 5512–5520. [Google Scholar] [CrossRef]
- Sever, M.J.; Weisser, J.T.; Monahan, J.; Srinivasan, S.; Wilker, J.J. Metal-Mediated Cross-Linking in the Generation of a Marine-Mussel Adhesive. Angew. Chem. Int. Ed. 2004, 43, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Meng, L.; Wang, M.; Cui, C.; Wang, B.; Han, C.-R.; Xu, F.; Yang, J. Mimicking Dynamic Adhesiveness and Strain-Stiffening Behavior of Biological Tissues in Tough and Self-Healable Cellulose Nanocomposite Hydrogels. ACS Appl. Mater. Interfaces 2019, 11, 5885–5895. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-P.; Hao, D.-Z.; Hao, W.-J.; Guo, X.-L.; Jiang, L. Hydrogel with Ultrafast Self-Healing Property Both in Air and Underwater. ACS Appl. Mater. Interfaces 2017, 10, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Kubinyi, M.; Varsányi, G. Infrared and Far Infrared Spectra of Crystalline Phenol-p-benzoquinone Complexes. Spectrosc. Lett. 2006, 9, 689–696. [Google Scholar] [CrossRef]
- Foreman, M.M.; Terry, L.M.; Weber, J.M. Binding Pocket Response of EDTA Complexes with Alkaline Earth Dications to Stepwise Hydration─Structural Insight from Infrared Spectra. J. Phys. Chem. A 2023, 127, 5374–5381. [Google Scholar] [CrossRef]





| Number | PVA (g) | TA (g) | H2O (mL) | Borax (mL) | CaCl2 (mL) |
|---|---|---|---|---|---|
| PVA/TA0.4 | 12 | 0.4 | 73 | 10 | 5 |
| PVA/TA0.5 | 12 | 0.5 | 73 | 10 | 5 |
| PVA/TA0.6 | 12 | 0.6 | 73 | 10 | 5 |
| PVA/TA0.7 | 12 | 0.7 | 73 | 10 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Zha, J.; Han, X.; Wang, H. Temporary Consolidation of Marine Artifact Based on Polyvinyl Alcohol/Tannic Acid Reversible Hydrogel. Polymers 2023, 15, 4621. https://doi.org/10.3390/polym15244621
Huang Q, Zha J, Han X, Wang H. Temporary Consolidation of Marine Artifact Based on Polyvinyl Alcohol/Tannic Acid Reversible Hydrogel. Polymers. 2023; 15(24):4621. https://doi.org/10.3390/polym15244621
Chicago/Turabian StyleHuang, Qijun, Jianrui Zha, Xiangna Han, and Hao Wang. 2023. "Temporary Consolidation of Marine Artifact Based on Polyvinyl Alcohol/Tannic Acid Reversible Hydrogel" Polymers 15, no. 24: 4621. https://doi.org/10.3390/polym15244621





