New Poly(lactic acid)–Hydrogel Core–Shell Scaffolds Highly Support MSCs’ Viability, Proliferation and Osteogenic Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
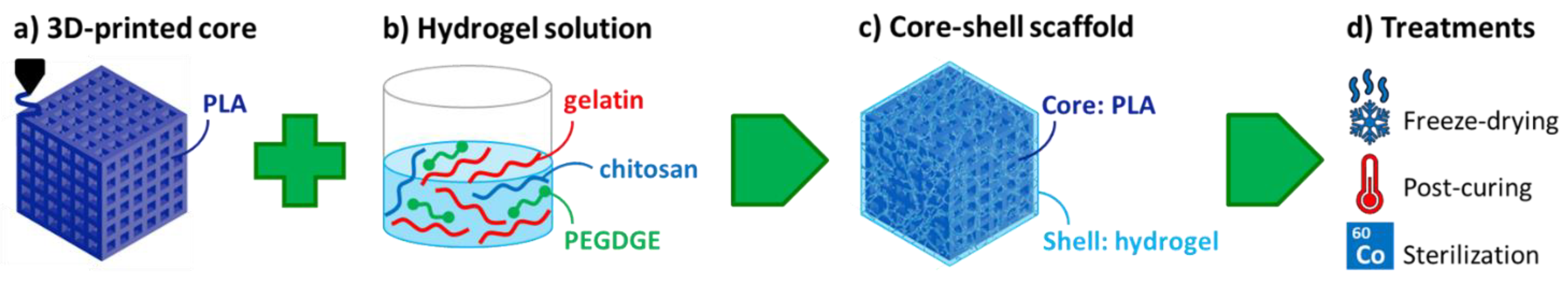
2.2. Preparation of Core–Shell Composite Scaffolds
2.3. Physical and Mechanical Characterization
2.4. Hydrolytic Degradation Experiments
2.5. In Vitro Characterization, Optical and Electron Microscopy
2.5.1. BM-hMSC Culture, Seeding and Osteogenic Differentiation
2.5.2. BM-hMSC Viability and Proliferation Assay
2.5.3. Histomorphological Analysis at Optical Microscope
2.5.4. Scanning Electron Microscopy Observation
2.6. Statistical Analysis
3. Results and Discussion
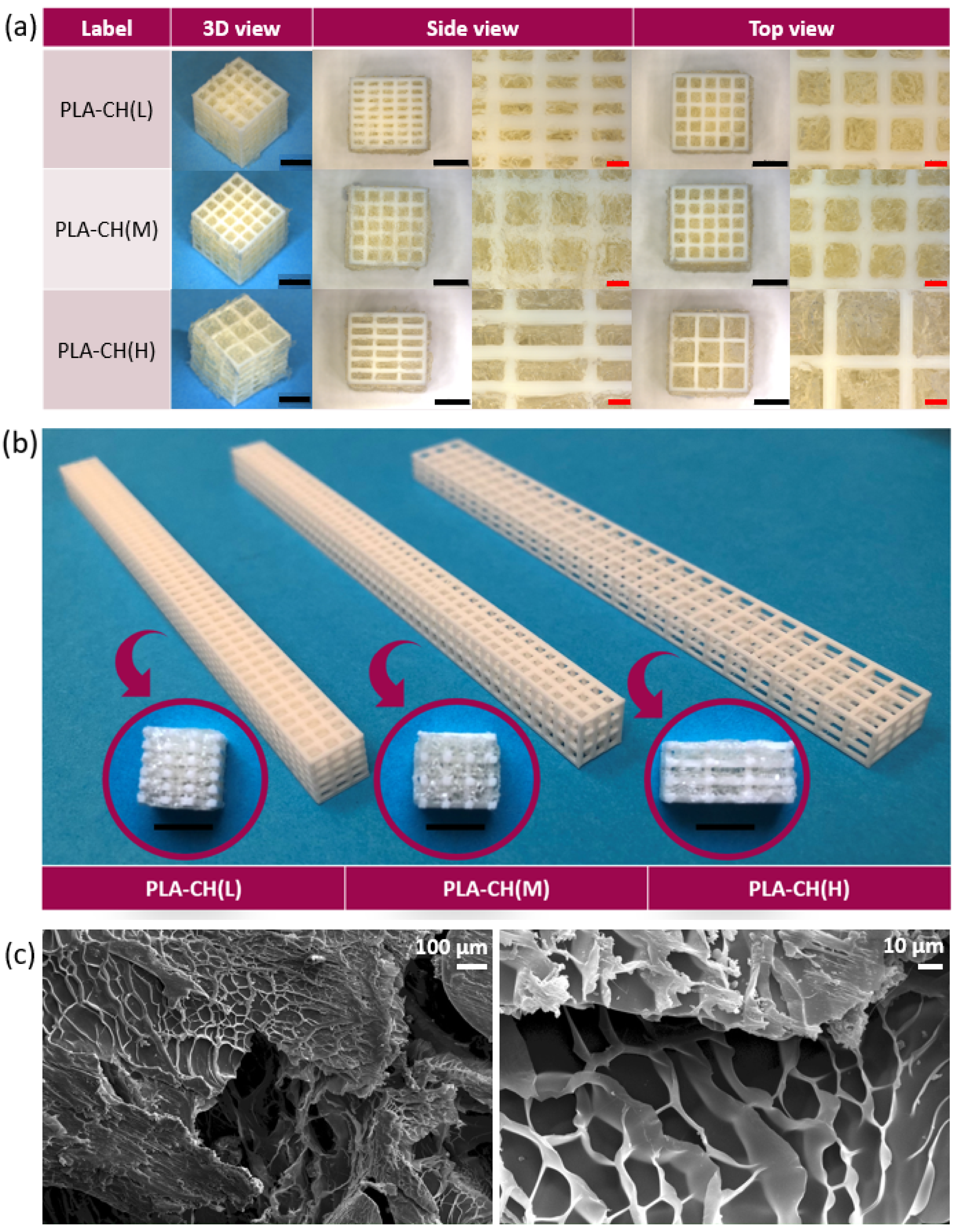
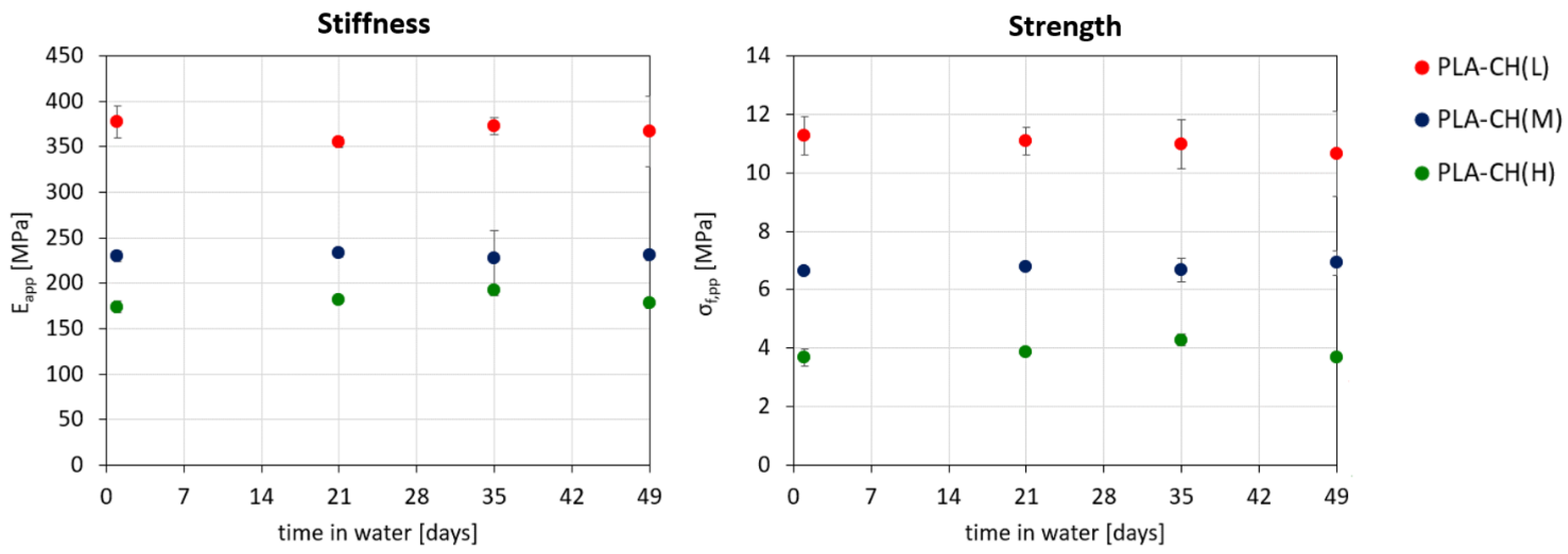
3.1. Physical and Mechanical Characterization
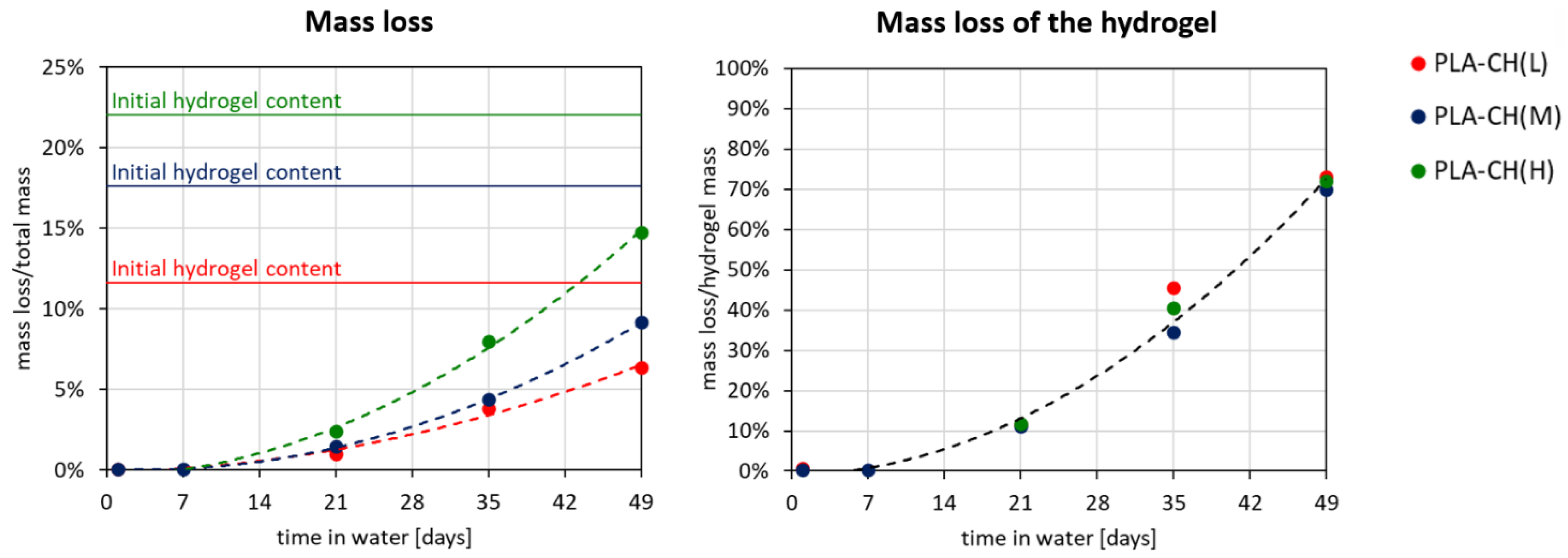
3.2. Hydrolytic Degradation Experiments
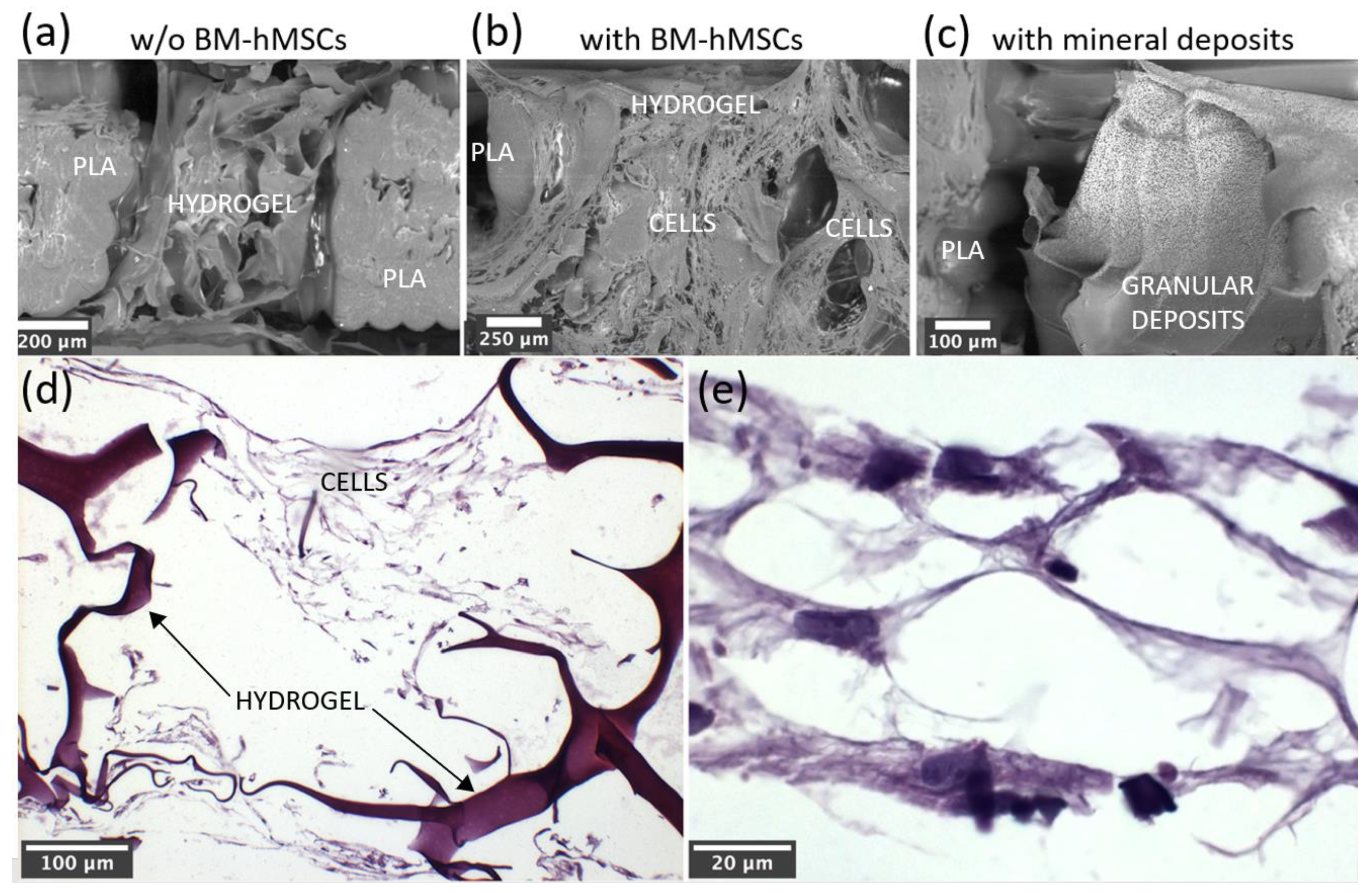
3.3. In vitro Characterization, Optical Microscopy, and SEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and biocompatible polymers for tissue engineering application: A review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef]
- Mabrouk, M.; Beherei, H.H.; Das, D.B. Recent progress in the fabrication techniques of 3D scaffolds for tissue engineering. Mater. Sci. Eng. C 2020, 110, 110716. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Abalymov, A.; Parakhonskiy, B.; Skirtach, A.G. Polymer- and Hybrid-Based Biomaterials for Interstitial, Connective, Vascular, Nerve, Visceral and Musculoskeletal Tissue Engineering. Polymers 2020, 12, 620. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Biol. 2021, 13, 100186. [Google Scholar] [CrossRef]
- Pereira, H.F.; Cengiz, I.F.; Silva, F.S.; Reis, R.L.; Oliveira, J.M. Scaffolds and coatings for bone regeneration. J. Mater. Sci. Mater. Med. 2020, 31, 27. [Google Scholar] [CrossRef]
- Vining, K.H.; Mooney, D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef]
- Darie-Niță, R.N.; Râpă, M.; Frąckowiak, S. Special Features of Polyester-Based Materials for Medical Applications. Polymers 2022, 14, 951. [Google Scholar] [CrossRef] [PubMed]
- Richbourg, N.R.; Peppas, N.A.; Sikavitsas, V.I. Tuning the biomimetic behavior of scaffolds for regenerative medicine through surface modifications. J. Tissue Eng. Regen. Med. 2019, 13, 1275–1293. [Google Scholar] [CrossRef]
- Priya, M.V.; Sivshanmugam, A.; Boccaccini, A.R.; Goudouri, O.M.; Sun, W.; Hwang, N.; Deepthi, S.; Nair, S.V.; Jayakumar, R. Injectable osteogenic and angiogenic nanocomposite hydrogels for irregular bone defects. Biomed. Mater. 2016, 11, 035017. [Google Scholar] [CrossRef]
- Li, T.T.; Zhang, Y.; Ren, H.T.; Peng, H.K.; Lou, C.W.; Lin, J.H. Two-step strategy for constructing hierarchical pore structured chitosan–hydroxyapatite composite scaffolds for bone tissue engineering. Carbohydr. Polym. 2021, 260, 117765. [Google Scholar] [CrossRef]
- Kareem, M.M.; Hodgkinson, T.; Sanchez, M.S.; Dalby, M.J.; Tanner, K.E. Hybrid core–shell scaffolds for bone tissue engineering. Biomed. Mater. 2019, 14, 025008. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Liu, L.; Pan, J.; Mei, J.; Li, C.; Zheng, Y. Biomimetic composite scaffold of hydroxyapatite/gelatin-chitosan core-shell nanofibers for bone tissue engineering. Mater. Sci. Eng. C 2018, 97, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Zhang, Y.; Zheng, L.; Zhang, H.; Shi, H.H.; Zhang, X.C.; Liu, B. Mineralized self-assembled silk fibroin/cellulose interpenetrating network aerogel for bone tissue engineering. Mater. Sci. Eng. C 2021, 134, 112549. [Google Scholar] [CrossRef] [PubMed]
- El-Habashy, S.E.; El-Kamel, A.H.; Essawy, M.M.; Abdelfattah, E.-Z.A.; Eltaher, H.M. Engineering 3D-printed core–shell hydrogel scaffolds reinforced with hybrid hydroxyapatite/polycaprolactone nanoparticles for in vivo bone regeneration. Biomater. Sci. 2021, 9, 4019–4039. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, E.; Huang, Y.; Li, X. Hybrid hydrogel based on stereocomplex PDLA/PLLA and gelatin for bone regeneration. J. Appl. Polym. Sci. 2020, 137, 49571. [Google Scholar] [CrossRef]
- Dong, L.; Wang, S.J.; Zhao, X.R.; Zhu, Y.F.; Yu, J.K. 3D-Printed Poly(ε-caprolactone) Scaffold Integrated with Cell-laden Chitosan Hydrogels for Bone Tissue Engineering. Sci. Rep. 2017, 7, 4–12. [Google Scholar] [CrossRef]
- Lei, B.; Guo, B.; Rambhia, K.J.; Ma, P.X. Hybrid polymer biomaterials for bone tissue regeneration. Front. Med. 2018, 13, 189–201. [Google Scholar] [CrossRef]
- Re, F.; Borsani, E.; Rezzani, R.; Sartore, L.; Russo, D. Bone Regeneration Using Mesenchymal Stromal Cells and Biocompatible Scaffolds: A Concise Review of the Current Clinical Trials. Gels 2023, 9, 389. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Sipp, D.; Robey, P.G.; Turner, L. Clear up this stem-cell mess. Nature 2018, 561, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Sartore, L.; Pasini, C.; Pandini, S.; Dey, K.; Ferrari, M.; Taboni, S.; Chan, H.H.L.; Townson, J.; Viswanathan, S.; Mathews, S.; et al. Hybrid Core-Shell Polymer Scaffold for Bone Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 4533. [Google Scholar] [CrossRef]
- Sartore, L.; Pandini, S.; Dey, K.; Bignotti, F.; Chiellini, F. A versatile cell-friendly approach to produce PLA-based 3D micro-macro-porous blends for tissue engineering scaffolds. Materialia 2020, 9, 100615. [Google Scholar] [CrossRef]
- Dey, K.; Agnelli, S.; Re, F.; Russo, D.; Lisignoli, G.; Manferdini, C.; Bernardi, S.; Gabusi, E.; Sartore, L. Rational Design and Development of Anisotropic and Mechanically Strong Gelatin-Based Stress Relaxing Hydrogels for Osteogenic/Chondrogenic Differentiation. Macromol. Biosci. 2019, 19, 1900099. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Domingos, M.A.N.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- Mirkhalaf, M.; Men, Y.; Wang, R.; No, Y.; Zreiqat, H. Personalized 3D printed bone scaffolds: A review. Acta Biomater. 2023, 156, 110–124. [Google Scholar] [CrossRef]
- Moroni, L.; de Wijn, J.; van Blitterswijk, C. 3D fiber-deposited scaffolds for tissue engineering: Influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials 2006, 27, 974–985. [Google Scholar] [CrossRef]
- Bittner, S.M.; Smith, B.T.; Diaz-Gomez, L.; Hudgins, C.D.; Melchiorri, A.J.; Scott, D.W.; Fisher, J.P.; Mikos, A.G. Fabrication and mechanical characterization of 3D printed vertical uniform and gradient scaffolds for bone and osteochondral tissue engineering. Acta Biomater. 2019, 90, 37–48. [Google Scholar] [CrossRef]
- Velioglu, Z.B.; Pulat, D.; Demirbakan, B.; Ozcan, B.; Bayrak, E.; Erisken, C. 3D-printed poly(lactic acid) scaffolds for trabecular bone repair and regeneration: Scaffold and native bone characterization. Connect. Tissue Res. 2019, 60, 274–282. [Google Scholar] [CrossRef]
- Nyberg, E.; O’sullivan, A.; Grayson, W. scafSLICR: A MATLAB-based slicing algorithm to enable 3D-printing of tissue engineering scaffolds with heterogeneous porous microarchitecture. PLoS ONE 2019, 14, e0225007. [Google Scholar] [CrossRef] [PubMed]
- Kolan, K.C.R.; Huang, Y.-W.; Semon, J.A.; Leu, M.C. 3D-printed Biomimetic Bioactive Glass Scaffolds for Bone Regeneration in Rat Calvarial Defects. Int. J. Bioprinting 2020, 6, 274. [Google Scholar] [CrossRef] [PubMed]
- Egan, P.; Wang, X.; Greutert, H.; Shea, K.; Wuertz-Kozak, K.; Ferguson, S. Mechanical and Biological Characterization of 3D Printed Lattices. 3D Print. Addit. Manuf. 2019, 6, 73–81. [Google Scholar] [CrossRef]
- Heo, D.N.; Castro, N.J.; Lee, S.-J.; Noh, H.; Zhu, W.; Zhang, L.G. Enhanced bone tissue regeneration using a 3D printed microstructure incorporated with a hybrid nano hydrogel. Nanoscale 2017, 9, 5055–5062. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.L.; Jacobsen, T.D.; Emsbo, E.; Murali, A.; Anton, K.; Liu, J.Z.; Lu, H.H.; Chahine, N.O. Three-Dimensional-Printed Flexible Scaffolds Have Tunable Biomimetic Mechanical Properties for Intervertebral Disc Tissue Engineering. ACS Biomater. Sci. Eng. 2021, 7, 5836–5849. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Mandal, N.; Mondal, S.; Mukherjee, K.; Mukhopadhyay, S.; Dey, A. Optimisation of process parameters for fabrication of nanocrystalline TiO2–hydoxyapatite based scaffold using response surface methodology. Adv. Appl. Ceram. 2014, 113, 129–138. [Google Scholar] [CrossRef]
- Pasini, C.; Sartore, L.; Pandini, S.; Ramorino, G. Hybrid scaffolds with a 3D-printed polymer lattice core and a bioactive hydrogel shell for bone regeneration. Mater. Today Proc. 2022, 70, 230–236. [Google Scholar] [CrossRef]
- UNI EN ISO 11137-1:2020; Sterilization of Health Care Products—Radiation—Part 1: Requirements for Development, Validation and Routine Control of a Sterilization Process for Medical Devices. Ente Nazionale Italiano di Unificazione (UNI): Milan, Italy, 2020.
- Re, F.; Sartore, L.; Moulisova, V.; Cantini, M.; Almici, C.; Bianchetti, A.; Chinello, C.; Dey, K.; Agnelli, S.; Manferdini, C.; et al. 3D gelatin-chitosan hybrid hydrogels combined with human platelet lysate highly support human mesenchymal stem cell proliferation and osteogenic differentiation. J. Tissue Eng. 2019, 10. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure & Properties, 2nd ed.; Cambridge University Press: Oxford, UK, 1988. [Google Scholar] [CrossRef]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Re, F.; Sartore, L.; Borsani, E.; Ferroni, M.; Baratto, C.; Mahajneh, A.; Smith, A.; Dey, K.; Almici, C.; Guizzi, P.; et al. Mineralization of 3D Osteogenic Model Based on Gelatin-Dextran Hybrid Hydrogel Scaffold Bioengineered with Mesenchymal Stromal Cells: A Multiparametric Evaluation. Materials 2021, 14, 3852. [Google Scholar] [CrossRef]
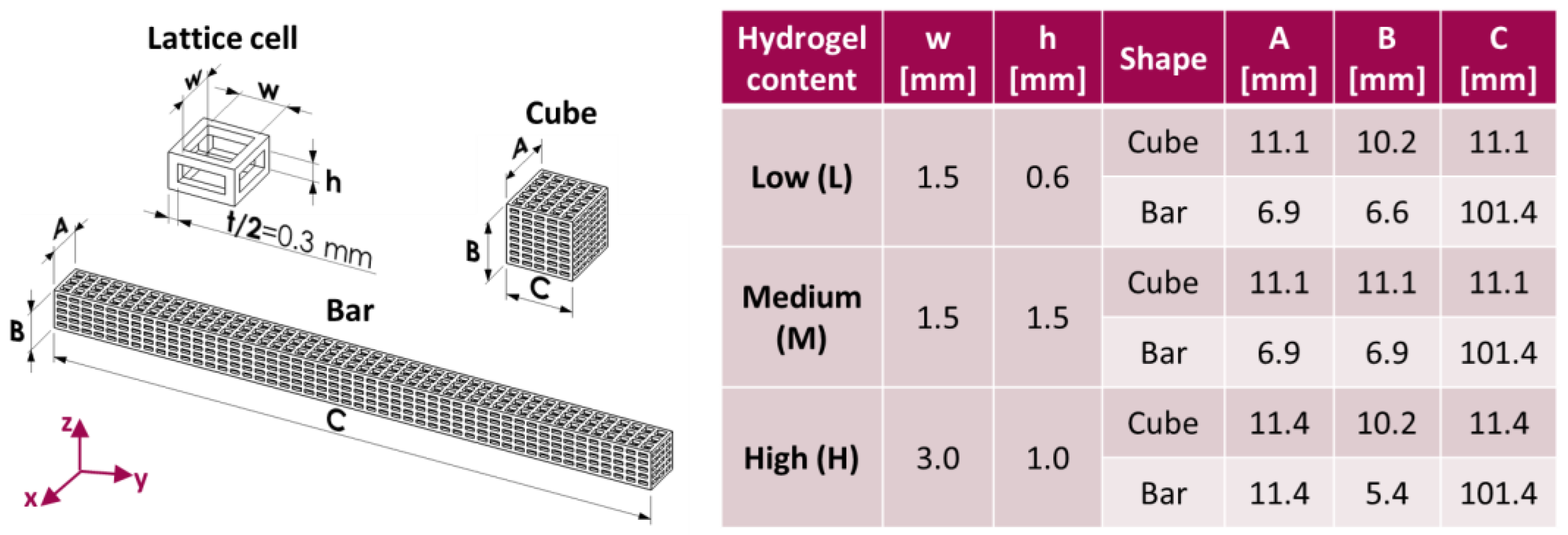





| Label | w × h [mm] | Core Void Volume Fraction, th [%] | Core Void Volume Fraction, exp [%] | Hydrogel Content, exp [%] | Water Uptake (24 h) [%] |
|---|---|---|---|---|---|
| PLA-CH(L) | 1.5 × 0.6 | 66.3 | 68.8 ± 0.3 | 11.6 ± 0.4 | 102.5 ± 7.4 |
| PLA-CH(M) | 1.5 × 1.5 | 75.3 | 77.0 ± 0.3 | 17.6 ± 0.4 | 137.2 ± 4.3 |
| PLA-CH(H) | 3.0 × 1.0 | 81.9 | 83.4 ± 0.2 | 22.0 ± 0.9 | 201.4 ± 8.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasini, C.; Pandini, S.; Re, F.; Ferroni, M.; Borsani, E.; Russo, D.; Sartore, L. New Poly(lactic acid)–Hydrogel Core–Shell Scaffolds Highly Support MSCs’ Viability, Proliferation and Osteogenic Differentiation. Polymers 2023, 15, 4631. https://doi.org/10.3390/polym15244631
Pasini C, Pandini S, Re F, Ferroni M, Borsani E, Russo D, Sartore L. New Poly(lactic acid)–Hydrogel Core–Shell Scaffolds Highly Support MSCs’ Viability, Proliferation and Osteogenic Differentiation. Polymers. 2023; 15(24):4631. https://doi.org/10.3390/polym15244631
Chicago/Turabian StylePasini, Chiara, Stefano Pandini, Federica Re, Matteo Ferroni, Elisa Borsani, Domenico Russo, and Luciana Sartore. 2023. "New Poly(lactic acid)–Hydrogel Core–Shell Scaffolds Highly Support MSCs’ Viability, Proliferation and Osteogenic Differentiation" Polymers 15, no. 24: 4631. https://doi.org/10.3390/polym15244631
APA StylePasini, C., Pandini, S., Re, F., Ferroni, M., Borsani, E., Russo, D., & Sartore, L. (2023). New Poly(lactic acid)–Hydrogel Core–Shell Scaffolds Highly Support MSCs’ Viability, Proliferation and Osteogenic Differentiation. Polymers, 15(24), 4631. https://doi.org/10.3390/polym15244631












