Development and Characterization of Thermoresponsive Smart Self-Adaptive Chitosan-Based Polymer for Wellbore Plugging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation of Chitosan-Based Polymers
- To dissolve the chitosan, we placed 20 g of it into a conical flask. Remembering that chitosan is soluble in acidic solutions, we added 32 g of NaOH as a solvent. This alkaline environment not only ensures sufficient carboxymethylation modification of chitosan but also reduces raw material consumption. After vigorously mixing the contents in an ice-water bath for 30 min, we obtained a well-mixed chitosan suspension.
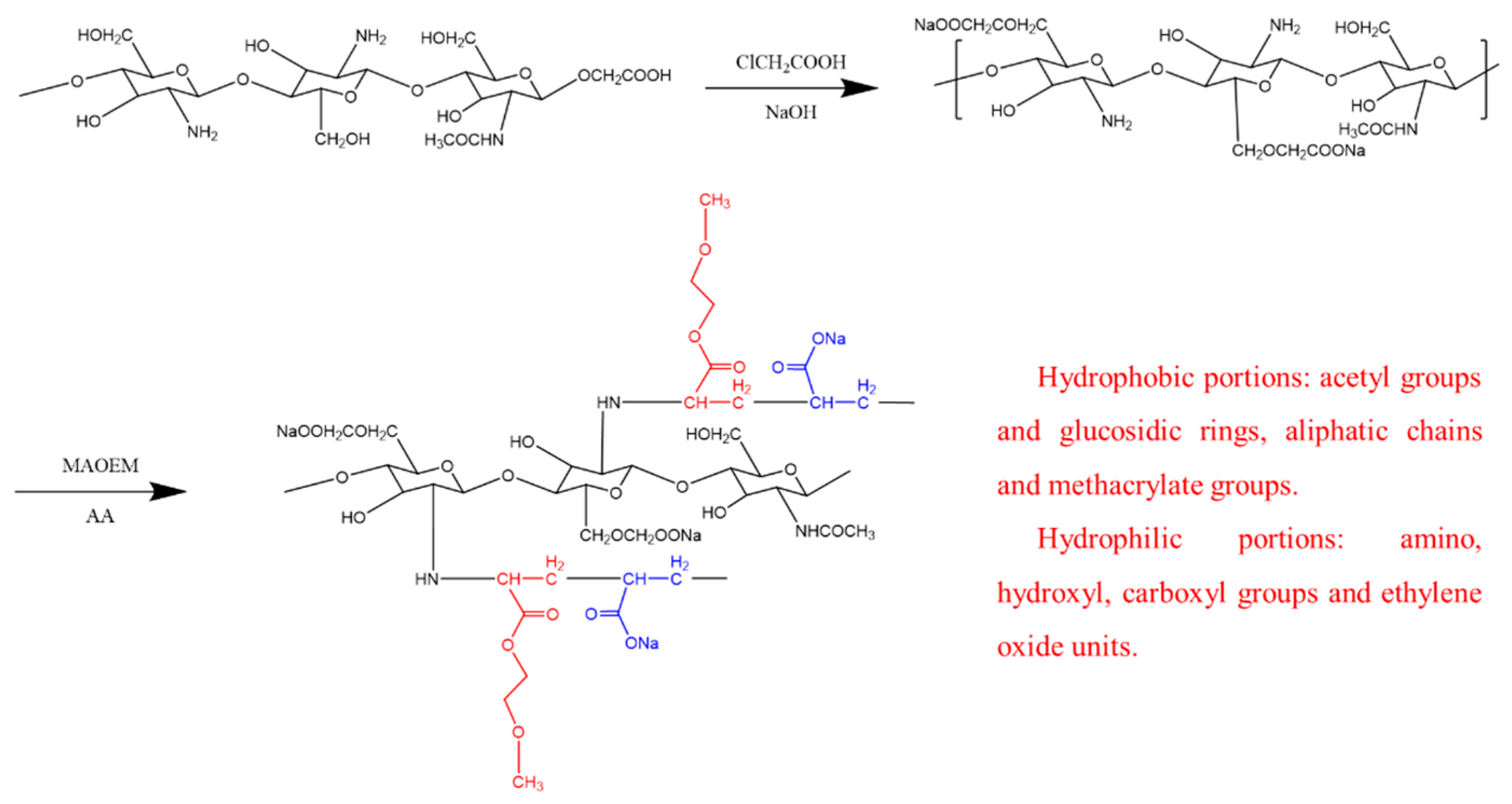
- Chitosan modification: Due to the nucleophilic reaction between the hydroxymethyl groups (-CH2OH) on chitosan and the amide groups (-NH2), chitosan, a biopolymer, possesses multiple hydroxymethyl sites. Gel-like polymer structures can be achieved through crosslinking reactions. To modify the chitosan, 30 g of chloroacetic acid was added as an etherifying agent, followed by 50 milliliters of isopropanol as a solvent to the chitosan suspension. The chitosan suspension was then placed in a 50 °C environment for 10 min. The modification process for chitosan is depicted in Figure 1. Subsequently, 500 milliliters of a 70% ethanol solution was added to the mixture and stirred at room temperature for one hour. The solid material was then filtered out of the suspension.
- Extraction of CMCS: The solid particles were rinsed in a 75% ethanol solution to remove any precipitated sodium salts and any residual water. The product was then thoroughly dried under vacuum to obtain preliminarily purified biomass CMCS. To obtain completely water-soluble CMCS, the water extraction method was employed. For this, the crude salt product was mixed with deionized water in a ratio of 1:30. Then, a 1.3 mol/L HCl neutralization solution was used to remove insoluble impurities (alkali salts) by precipitation. After collecting the solid particles, purified CMCS was obtained through vacuum drying, with a yield of approximately 94%.
- Preparation of CMCS: To prepare CMCS, the material was dissolved in deionized water and nitrogen gas was continuously bubbled through the solution. The solution was then heated to 70 °C for 2 h. After this, 0.2 g of ammonium persulfate (APS) was added as an initiator and the mixture was stirred for 20 min to prevent the aggregation effect of the product.
- Free radical grafting of CMCS: Different ratios of solutions of MAOEM and AA were mixed and added to the CMCS solution. To enhance the grafting reaction, nitrogen gas was bubbled through the mixture while maintaining it at 70 °C. To account for the varying proportions of MAOEM in the solvent system, experimental groups were designated as CCMMA-A to CCMMA-F, as shown in Table 1. The grafting process is schematically represented in Figure 1.
- Purification of CCMMA: The grafted CCMMA polymer was completely immersed in deionized water. The deionized water was replaced every 5 h to remove unreacted CMCS and chitosan monomers. After purification, the product was dried in a vacuum oven at 60 °C for 3 h to obtain purified CCMMA [24].
2.3. Characterization of Chitosan-Based Polymers
2.3.1. Microstructure Characterization
2.3.2. Self-Assembly Behavior Testing
2.3.3. Turbidity Testing
2.3.4. Drilling Fluid Performance Testing
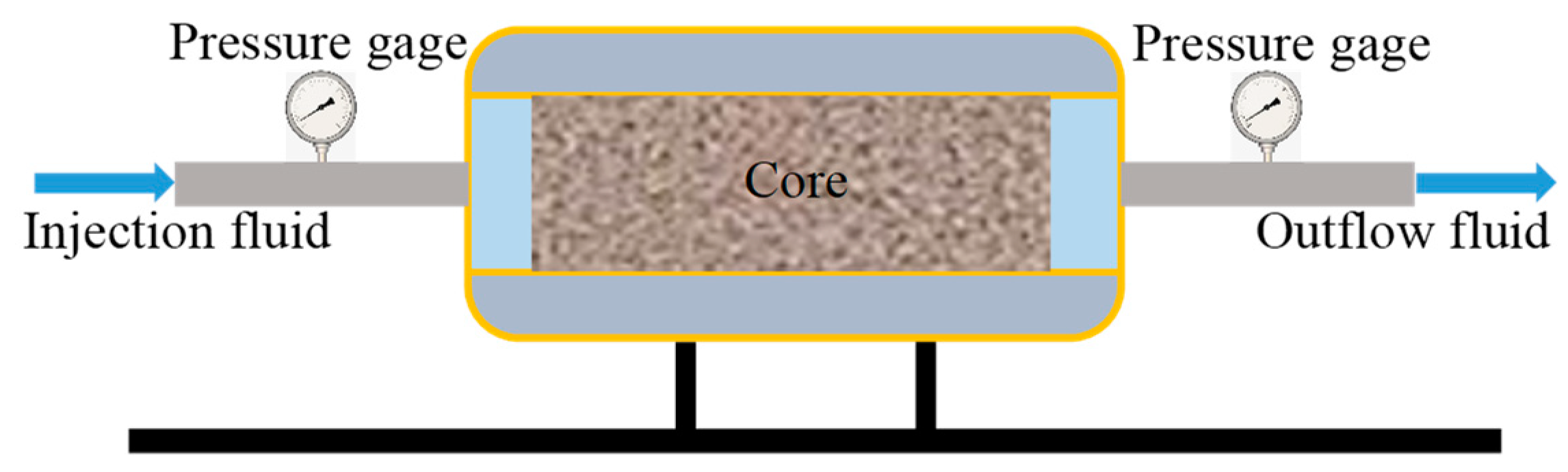
2.3.5. Formation Plugging Performance Testing
3. Results and Discussion
3.1. Microstructure Characterization
3.1.1. FT-IR Spectrum Analysis
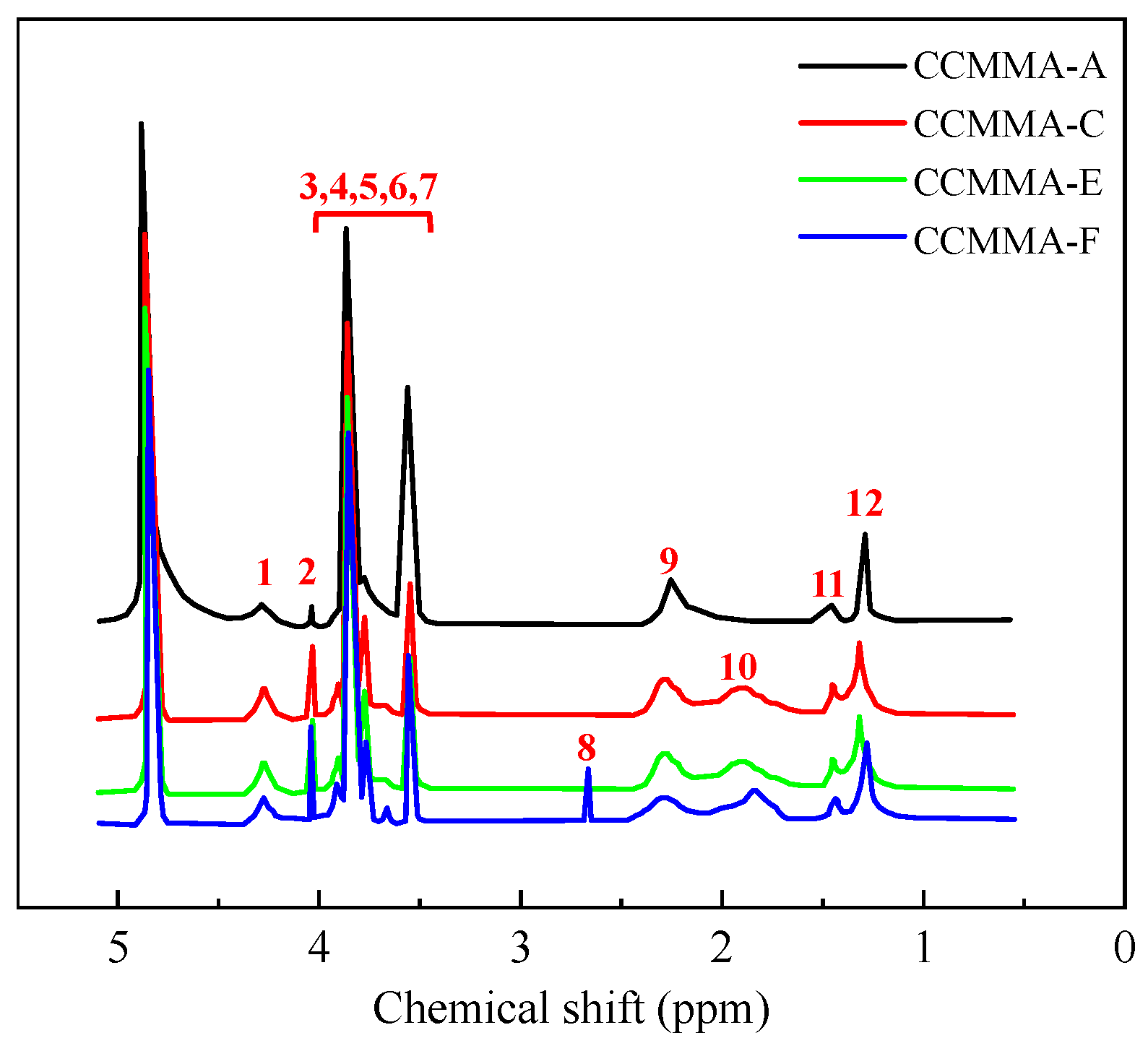
3.1.2. 1H NMR Analysis
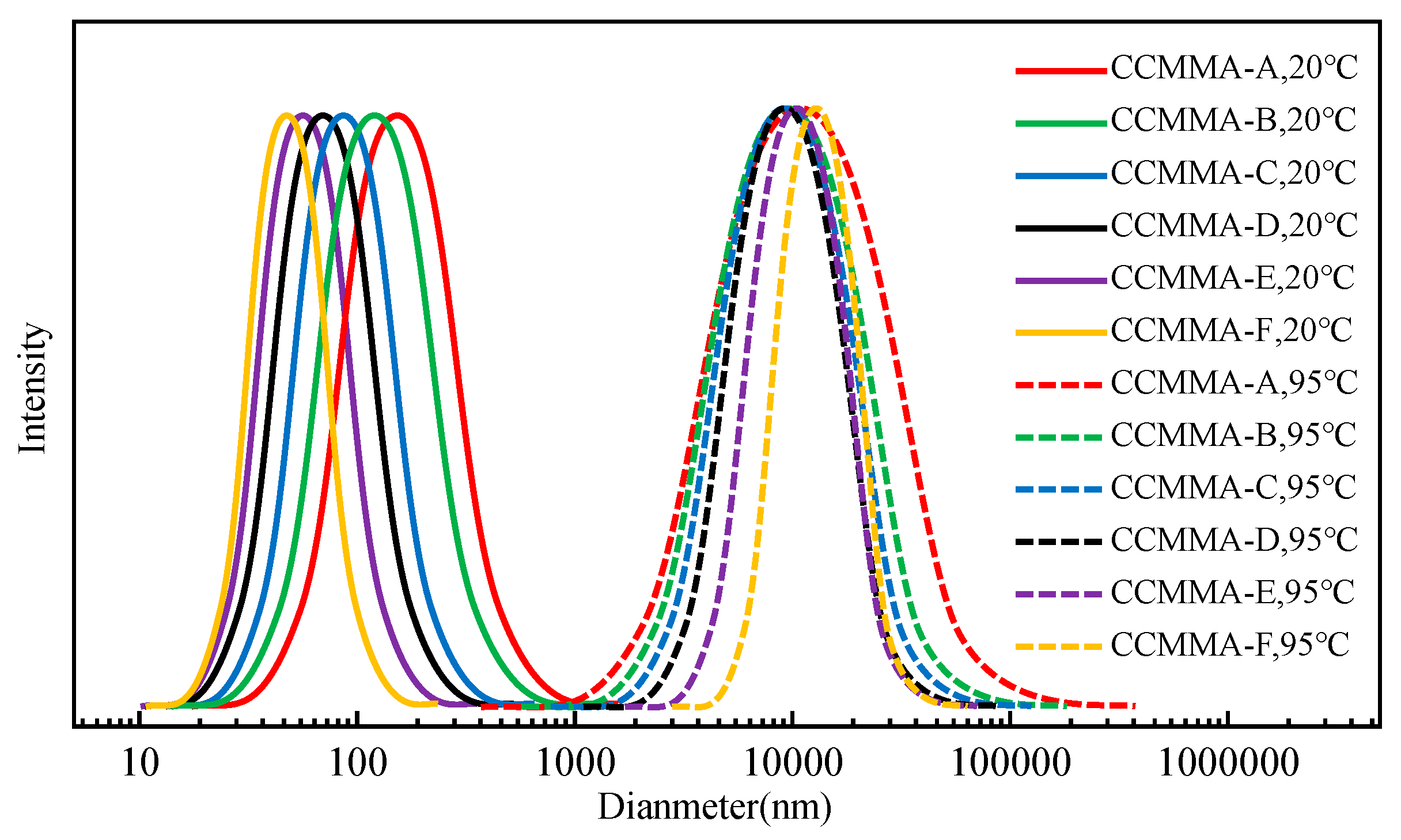
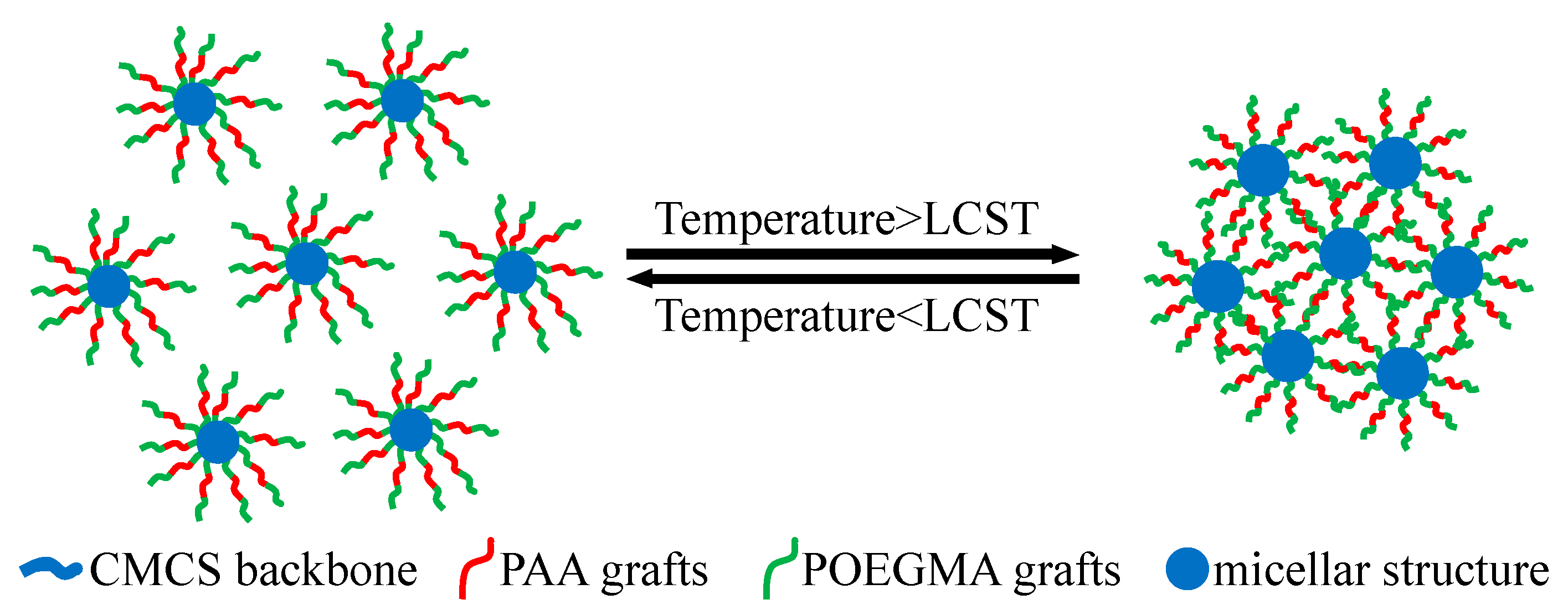
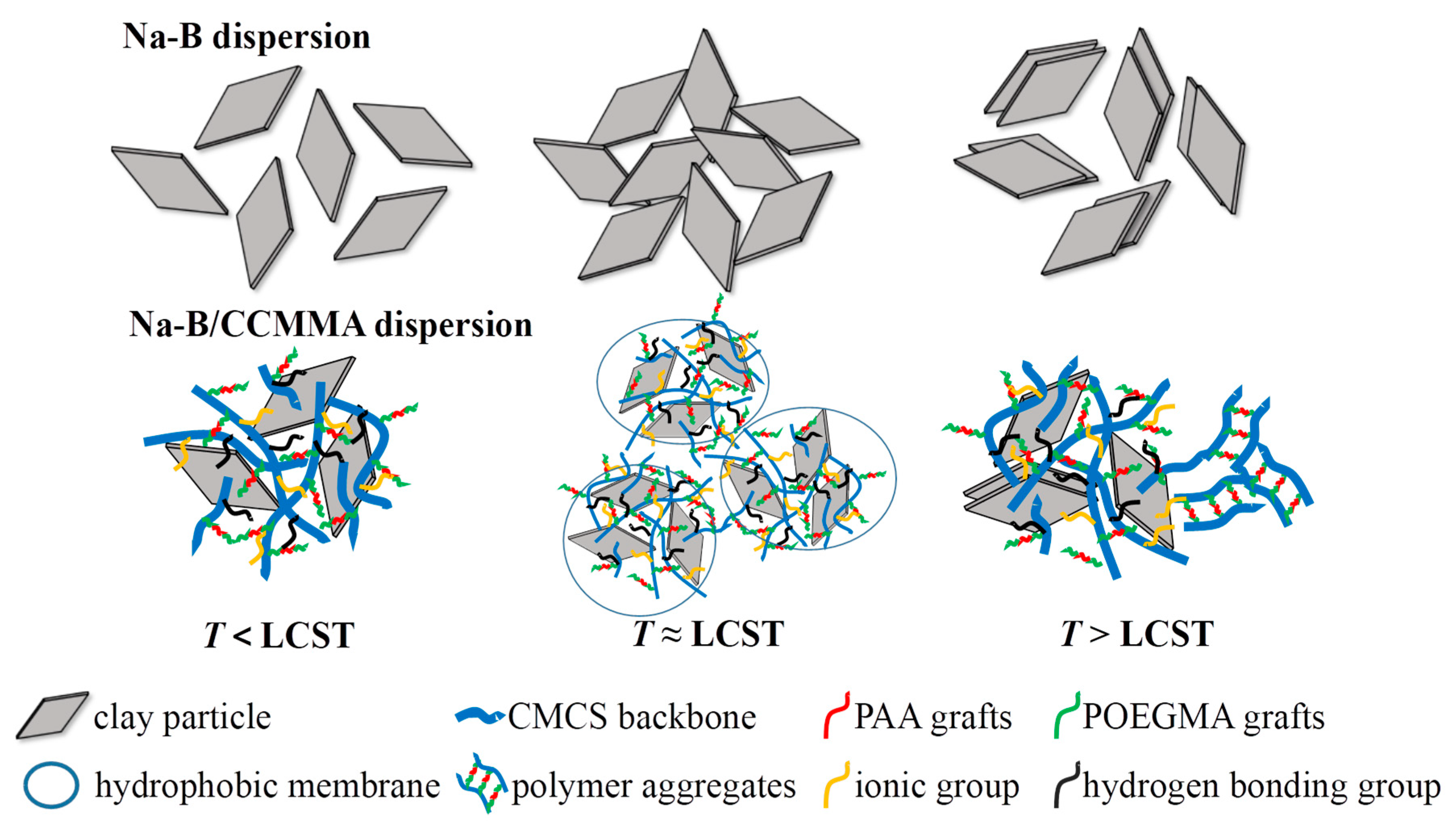
3.2. Analysis of Self-Assembly Behavior
3.3. Analysis of Temperature-Responsive Behavior
3.4. Evaluation of Drilling Fluid Performance
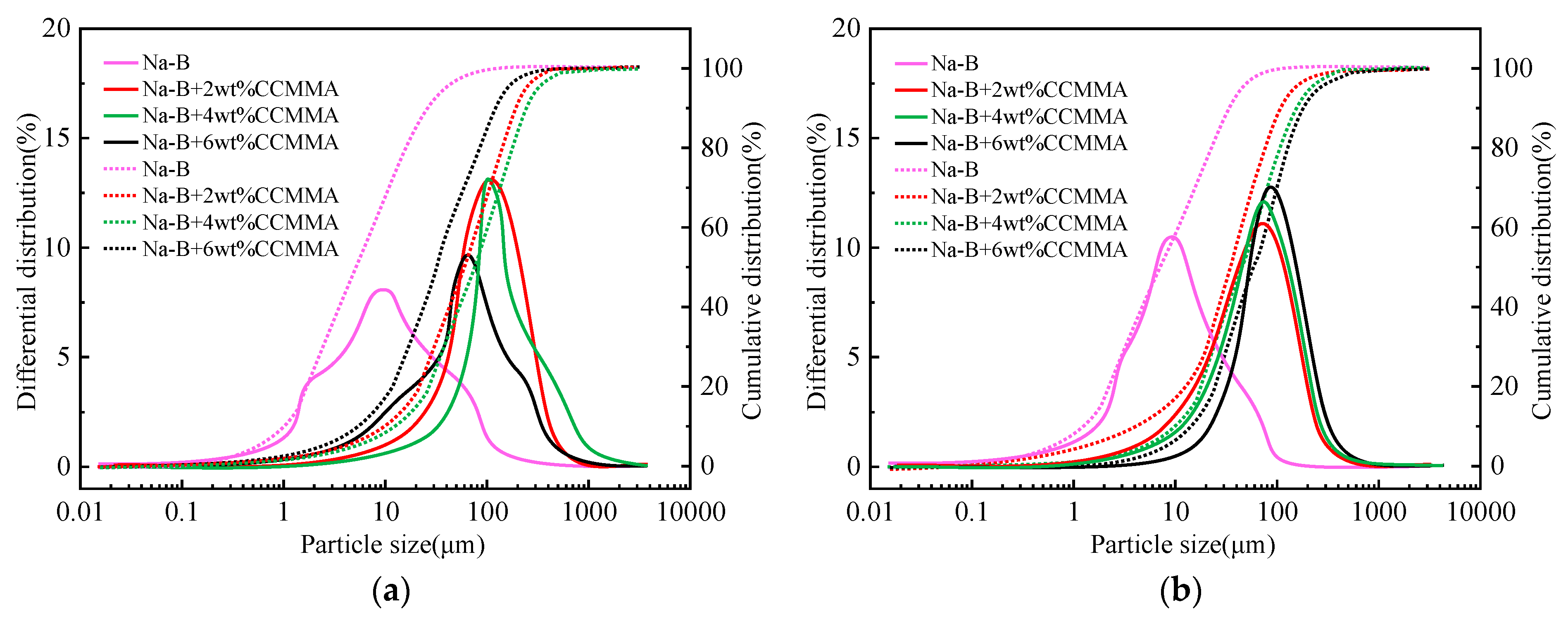
3.4.1. Rheological and Filtration Evaluation
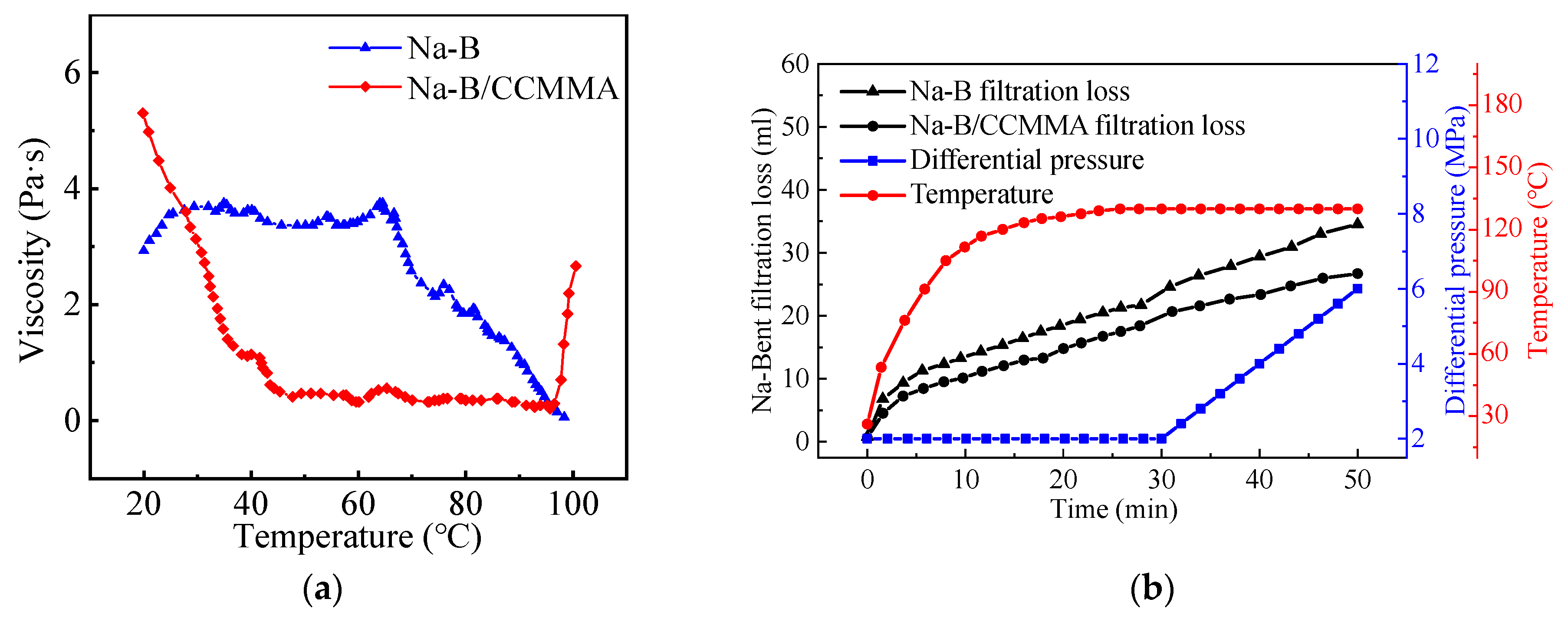
3.4.2. High-Temperature Rheological and Filtration Evaluation
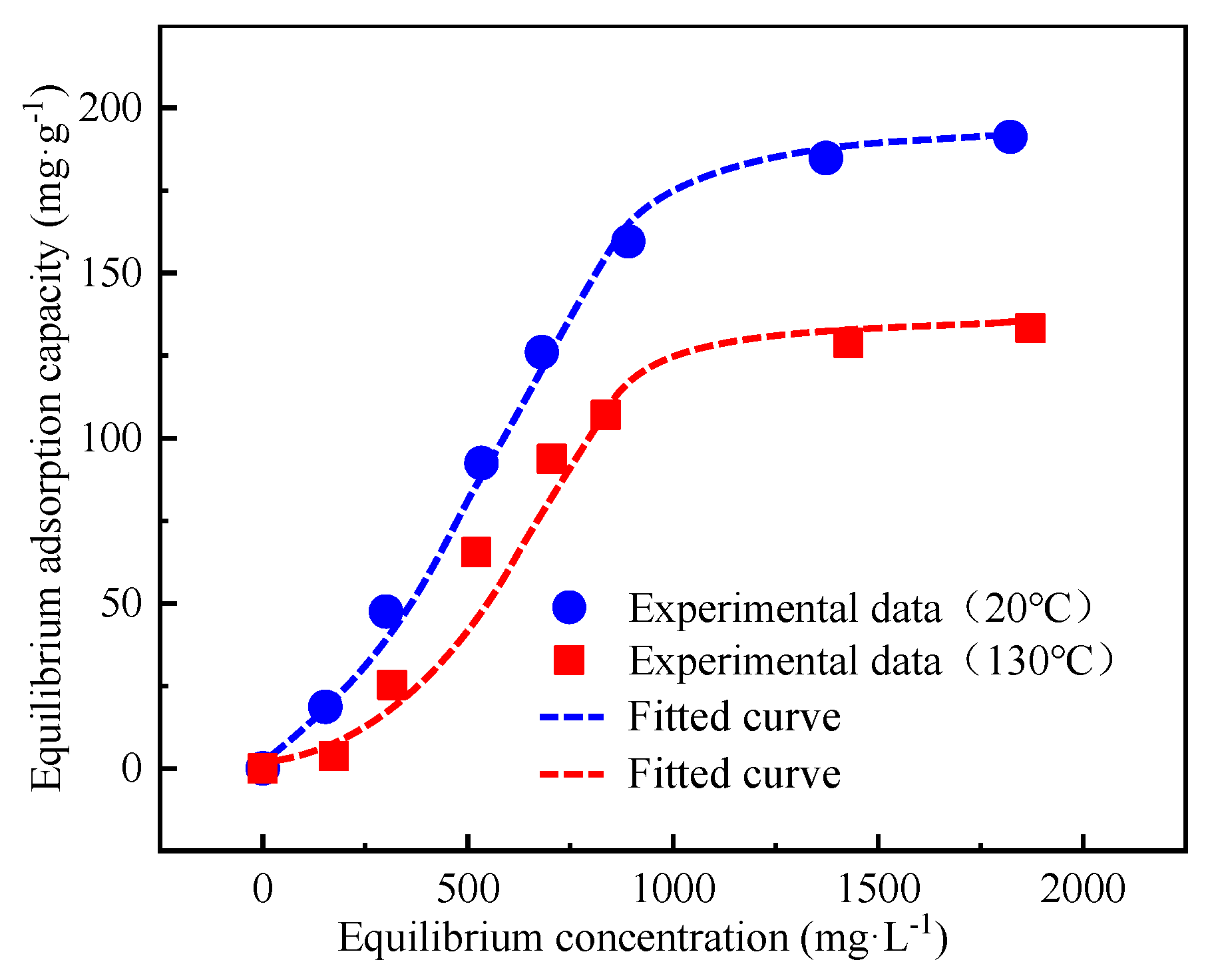
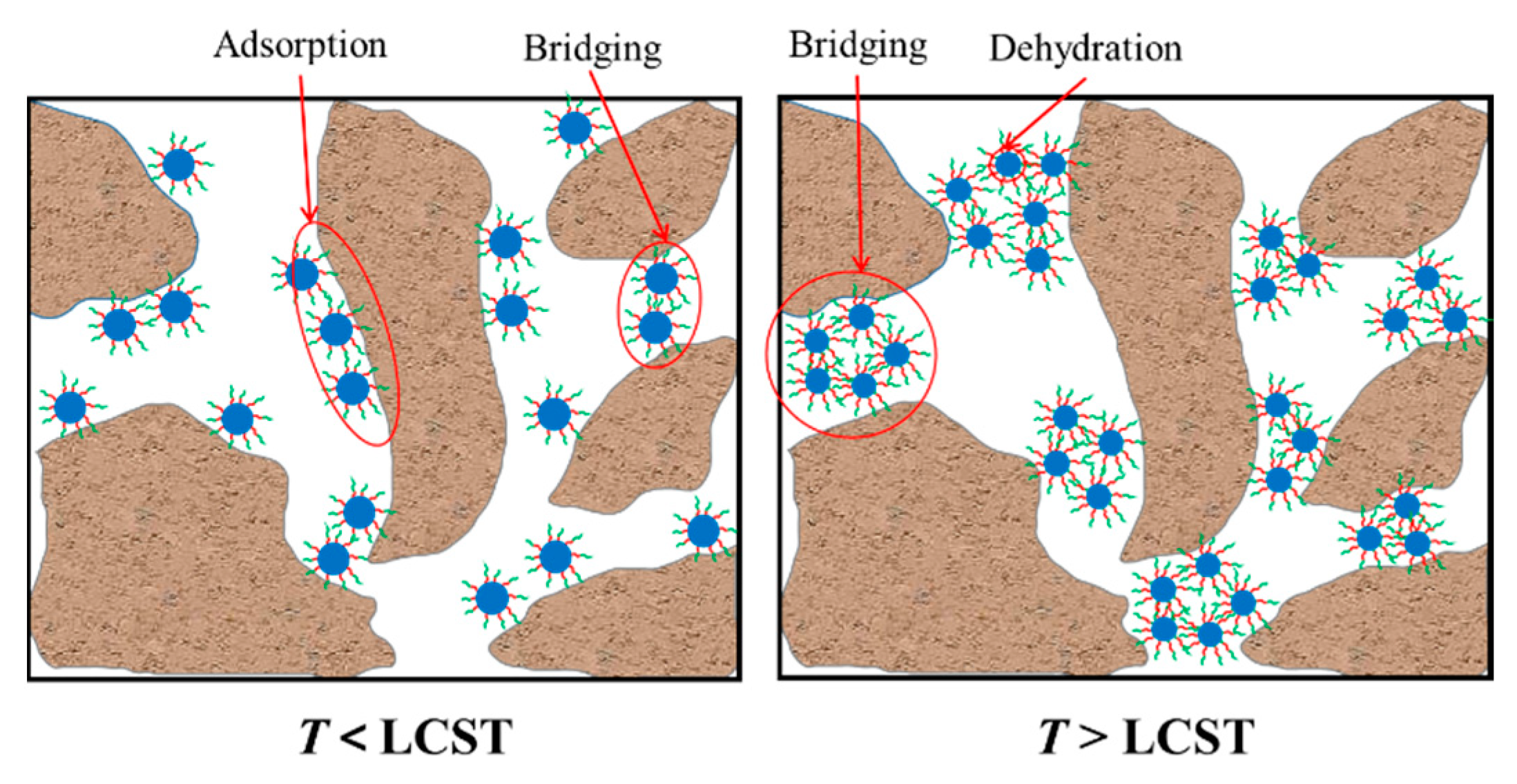
3.5. Intelligent Plugging Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, L.; Luo, P.; He, Y.; Zhang, L.; Fan, Y.; Jiang, Z. Improving the stability of multiwalled carbon nano-tubes in extremely environments: Applications as nano plugging additives in drilling fluids. J. Nat. Gas Sci. Eng. 2019, 74, 103082. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Jaafar, M.Z.; Yusof, M.A.M.; Idris, A.K. A review on the effect of nanoparticle in drilling fluid on filtration and formation damage. J. Pet. Sci. Eng. 2022, 217, 110922. [Google Scholar] [CrossRef]
- Saleh, T.A.; Ibrahim, M.A. Advances in functionalized Nanoparticles based drilling inhibitors for oil production. Energy Rep. 2019, 5, 1293–1304. [Google Scholar] [CrossRef]
- Adil, A.; Baig, T.; Jamil, F.; Farhan, M.; Shehryar, M.; Ali, H.M.; Khushnood, S. Nanoparticle-based cutting fluids in drilling: A recent review. Int. J. Adv. Manuf. Technol. 2023, 1–18. [Google Scholar] [CrossRef]
- Pu, L.; Xu, P.; Xu, M.; Song, J.; He, M. Lost circulation materials for deep and ultra-deep wells: A review. J. Pet. Sci. Eng. 2022, 214, 110404. [Google Scholar] [CrossRef]
- Li, H.; Lv, K.; Huang, X.; Lu, Z.; Dong, X. The synthesis of polymeric nanospheres and the application as high-temperature Nano-Plugging Agent in Water Based Drilling Fluid. Front. Chem. 2020, 8, 247. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, J.; Liu, J.; Lv, K.; Zhang, T.; Sun, Y.; Xiu, Z.; Huang, N. Preparation and Evaluation of Nanopolymer Microsphere Plugging Agents for Ultrahigh-Temperature Water-Based Drilling Fluids. Energy Fuels 2023, 37, 13093–13103. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Liu, Z.Y.; Zhang, J.; Chen, C.; Ma, M. Investigation on matching relationship and plugging mechanism of self-adaptive micro-gel (SMG) as a profile control and oil displacement agent. Powder Technol. 2020, 364, 774–784. [Google Scholar] [CrossRef]
- Wang, Z.; Bai, B.; Sun, X.; Wang, J. Effect of multiple factors on preformed particle gel placement, dehydration, and plugging performance in partially open fractures. Fuel 2019, 251, 73–81. [Google Scholar] [CrossRef]
- Saleh, T.A.; Rana, A.; Arfaj, M.K.; Ibrahim, M.A. Hydrophobic polymer-modified nanosilica as effective shale inhibitor for water-based drilling mud. J. Pet. Sci. Eng. 2022, 209, 109868. [Google Scholar] [CrossRef]
- Liu, F.; Zheng, Z.; Wang, X.; Li, X.; Zhang, Z.; Wang, X.; Dai, X.; Xin, Y.; Liu, Q.; Yao, H.; et al. Novel modified nanosilica/polymer composite in water-based drilling fluids to plug shale pores. Energy Sources. Part A Recover. Util. Env. Eff. 2021, 44, 8662–8678. [Google Scholar] [CrossRef]
- Martin, C.; Babaie, M.; Nourian, A.; Nasr, G.G. Rheological Properties of the Water-Based Muds Composed of Silica Nanoparticle Under High Pressure and High Temperature. SPE J. 2022, 27, 2563–2576. [Google Scholar] [CrossRef]
- Lei, M.; Huang, W.; Sun, J.; Shao, Z.; Chen, Z.; Chen, W. Synthesis and characterization of high-temperature self-crosslinking polymer latexes and their application in water-based drilling fluid. Powder Technol. 2021, 389, 392–405. [Google Scholar] [CrossRef]
- Lei, M.; Huang, W.; Sun, J.; Jin, Z.; Huang, X. The Utilization of Self-crosslinkable nanoparticles as high-temperature plugging agent in water-based drilling fluid. SPE J. 2022, 27, 2628–2641. [Google Scholar] [CrossRef]
- Jiang, G.; Dong, T.; Cui, K.; He, Y.; Quan, X.; Yang, L.; Fu, Y. Research status and development directions of intelligent drilling fluid technologies. Pet. Explor. Dev. 2022, 49, 660–670. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Olayiwola, T.; Elkatatny, S.; Haq, B.; Patil, S. Insights into the application of surfactants and nanomaterials as shale inhibitors for water-based drilling fluid: A review. J. Nat. Gas. Sci. Eng. 2021, 92, 103987. [Google Scholar] [CrossRef]
- Li, M.-C.; Wu, Q.; Lei, T.; Mei, C.; Xu, X.; Lee, S.-Y.; Gwon, J. Thermothickening Drilling Fluids Containing Bentonite and Dual-Functionalized Cellulose Nanocrystals. Energy Fuels 2020, 34, 8206–8215. [Google Scholar] [CrossRef]
- Xie, B.; Liu, X. Thermo-thickening behavior of LCST-based copolymer viscosifier for water-based drilling fluids. J. Pet. Sci. Eng. 2017, 154, 244–251. [Google Scholar] [CrossRef]
- Du, G.; Peng, Y.; Pei, Y.; Zhao, L.; Wen, Z.; Hu, Z. Thermo-responsive temporary plugging agent based on multiple phase transition supramolecular gel. Energy Fuels 2017, 31, 9283–9289. [Google Scholar] [CrossRef]
- Dong, W.; Pu, X.; Ren, Y.; Zhai, Y.; Gao, F.; Xie, W. Thermoresponsive bentonite for water-based drilling fluids. Materials 2019, 12, 2115. [Google Scholar] [CrossRef]
- Bai, X.; Yong, X.; Luo, Y.; Deng, L.; Li, K.; Zhou, Y. Synthesis and application of temperature-sensitive polymer as a novel plugging agent for water-based drilling fluids. J. Appl. Polym. Sci. 2022, 139, e52524. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Zhang, K.; Lv, K.; Huang, X.; Wang, J.; Wang, R.; Meng, X. A temperature-sensitive polymeric rheology modifier used in water-based drilling fluid for deepwater drilling. Gels 2022, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Huang, W.; Sun, J.; Shao, Z.; Zhao, L.; Zheng, K.; Fang, Y. Synthesis and characterization of thermo-responsive polymer based on carboxymethyl chitosan and its potential application in water-based drilling fluid. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127478. [Google Scholar] [CrossRef]
- Arslan, H.; Aytaç, U.S.; Bilir, T.; Şen, Ş. The synthesis of a new chitosan based superplasticizer and investigation of its effects on concrete properties. Constr. Build. Mater. 2019, 204, 541–549. [Google Scholar] [CrossRef]
- Singh, V.; Tripathi, D.N.; Tiwari, A.; Sanghi, R. Microwave synthesized chitosangraft-poly(methylmethacrylate): An efficient Zn2+ ion binder. Carbohydr. Polym. 2006, 65, 35–41. [Google Scholar] [CrossRef]
- Han, S.K.; Jhun, B.H. Effect of additives on the cloud point of polyethylene glycols. Arch. Pharm. Res. 1984, 7, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Qiu, Z.S.; Zhong, H.Y.; Zhao, X.; Liu, Z.K.; Huang, W.A. Effects of water-based drilling fluid on properties of mud cake and wellbore stability. J. Pet. Sci. Eng. 2022, 208, 109704–109724. [Google Scholar] [CrossRef]
- Luo, X.W.; Jiang, G.C.; Wang, G.S.; Yang, L.L.; He, Y.B.; Cui, K.X.; Yang, J. Novel approach to improve shale stability usingsuper-amphiphobic nanoscale materials in water-based drilling fluids and its field application. Rev. Adv. Mater. Sci. 2022, 61, 41–54. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N.; Tiwari, A. Carboxymethyl chitosan and its applications. Adv. Mater. Lett. 2010, 1, 11–33. [Google Scholar] [CrossRef]
- de Abreu, F.R.; Campana-Filho, S.P. Characteristics and properties of carboxymethylchitosan. Carbohydr. Polym. 2009, 75, 214–221. [Google Scholar] [CrossRef]
- Izyurov, V.; Kharitonov, A.; Semenikhin, I.; Korsunov, E.; Gassan, A.; Tikhonov, E.; Jadan, G.; Stashko, V.; Blagonadeshniy, I.; Manikhin, A.; et al. Selecting bridging agents’ particle size distribution for optimum plugging while drilling in permeable zones. In Proceedings of the Society of Petroleum Engineers—SPE Russian Petroleum Technology Conference, Moscow, Russia, 22–24 October 2019. [Google Scholar] [CrossRef]
- Zhu, A.; Chan-Park, M.B.; Dai, S.; Li, L. The aggregation behavior of 0-carboxymethylchitosan in dilute aqueous solution. Colloids Surf. B Biointerfaces 2005, 43, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Verduzco, R.; Li, X.; Pesek, S.L.; Stein, G.E. Structure, function, self-assembly, and applications of bottlebrush copolymers. Chem. Soc. Rev. 2015, 44, 2405–2420. [Google Scholar] [CrossRef]
- Munro, N.H.; Hanton, L.R.; Moratti, S.C.; Robinson, B.H. Synthesis and characterisation of chitosan-graft-poly(OEGMA) copolymers prepared by ATRP. Carbohydr. Polym. 2009, 77, 496–505. [Google Scholar] [CrossRef]
- Li, X.; Han, Y.; Zhong, H.; Ye, W.; Liu, B.; Wang, X.; Sun, R. Preparation, Characterization and Antibacterial Activity of Quaternized Carboxymethyl Chitosan/Organic Rectorite Nanocomposites. Curr. Nanosci. 2013, 9, 278–282. [Google Scholar] [CrossRef]
- de Abreu, F.R.; Campana-Filho, S.P. Preparation and characterization of carboxymethylchitosan. Polimeros 2005, 15, 79–83. [Google Scholar] [CrossRef]
- Gou, S.; Li, S.; Feng, M.; Zhang, Q.; Pan, Q.; Wen, J.; Wu, Y.; Guo, Q. Novel biodegradable graftmodified water-soluble copolymer using acrylamide and konjac glucomannan for enhanced oil recovery. Ind. Eng. Chem. Res. 2017, 56, 942–951. [Google Scholar] [CrossRef]
- Novoa-Carballal, R.; Riguera, R.; Fernandez-Megia, E. Disclosing an NMRinvisible fraction in chitosan and PEGylated copolymers and its role on the determination of degrees of substitution. Mol. Pharm. 2013, 10, 3225–3231. [Google Scholar] [CrossRef]
- Scott, J.E.; Heatley, F.; Wood, B. Comparison of Secondary Structures in Water of Chondroitin-4-sulfate and Dermatan sulfate: Implications in the Formation of Tertiary Structures. Biochemistry 1995, 34, 15467–15474. [Google Scholar] [CrossRef]
- Scott, J.E.; Heatley, F. 1H nuclear-magnetic-resonance spectra of the methyl group of the acetamido moiety and the structure of acid glycosaminoglycans in solution. Biochem. J. 1979, 181, 445–449. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Q.; Chen, H.; Wang, X.; Shen, Z.; Shu, X.; Sun, R. Direct grafting modification of pulp in ionic liquids and self-assembly behavior of the graft copolymers. Cellulose 2013, 20, 873–884. [Google Scholar] [CrossRef]
- Wan, S.; Jiang, M.; Zhang, G. Dual temperature- and pH-dependent self-assembly of cellulose-based copolymer with a pair of complementary grafts. Macromolecules 2007, 40, 5552–5558. [Google Scholar] [CrossRef]
- Lutz, J.F. Polymerization of oligo(ethylene glycol) (meth)acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive polymers with lower critical solution temperature: From fundamental aspects and measuring techniques to recommended turbidimetry conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Lutz, J.F.; Both, A. Preparation of ideal PEG analogues with a tunable thermosensitivity by controlled radical copolymerization of 2-(2-methoxyethoxy)ethyl methacrylate and oligo(ethylene glycol) methacrylate. Macromolecules 2006, 39, 893–896. [Google Scholar] [CrossRef]
- Morimoto, N.; Qiu, X.P.; Winnik, F.M.; Akiyoshi, K. Dual stimuli-responsive nanogels by self-assembly of polysaccharides lightly grafted with thiolterminated poly(N-isopropylacrylamide) chains. Macromolecules 2008, 41, 5985–5987. [Google Scholar] [CrossRef]
- da Camara, P.C.F.; Madruga, L.Y.C.; Marques, N.D.N.; de Balaban, R.C. Evaluation of polymer/bentonite synergy on the properties of aqueous drilling fluids for hightemperature and high-pressure oil wells. J. Mol. Liq. 2020, 327, 114808. [Google Scholar] [CrossRef]
- Lei, M.; Huang, W.; Sun, J.; Shao, Z.; Duan, W.; Wu, T.; Wang, Y. Synthesis, characterization, and performance of carboxymethyl chitosan with different molecular weight as additive in water-based drilling fluid. J. Mol. Liq. 2020, 310, 113135. [Google Scholar] [CrossRef]
- Giles, C.H.; D’Silva, A.P.; Easton, I.A. A general treatment and classification of the solute adsorption isotherm part II. Experimental interpretation. J. Colloid. Interface Sci. 1974, 47, 766–778. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lee, D.S.; Lee, M.W.; Woo, S.H. Enhanced molar sorption ratio for naphthalene through the impregnation of surfactant into chitosan hydrogel beads. Bioresour. Technol. 2010, 101, 4315–4321. [Google Scholar] [CrossRef]
- Barreca, S.; Orecchio, S.; Pace, A. The effect of montmorillonite clay in alginate gel beads for polychlorinated biphenyl adsorption: Isothermal and kinetic studies. Appl. Clay Sci. 2014, 99, 220–228. [Google Scholar] [CrossRef]





| CMCS (g) | MAOEM (g) | AA (g) | MAOEM/AA (mol/mol) | APS (g) | GR (%) | GE (%) | |
|---|---|---|---|---|---|---|---|
| CCMMA-A | 1.20 | 6.00 | 0.00 | 10/0 | 0.06 | 360.67 | 72.11 |
| CCMMA-B | 1.20 | 5.90 | 0.10 | 9/1 | 0.06 | 380.46 | 76.09 |
| CCMMA-C | 1.20 | 5.78 | 0.22 | 8/2 | 0.06 | 390.46 | 78.13 |
| CCMMA-D | 1.20 | 5.63 | 0.37 | 7/3 | 0.06 | 401.27 | 80.27 |
| CCMMA-E | 1.20 | 5.45 | 0.55 | 6/4 | 0.06 | 398.00 | 79.56 |
| CCMMA-F | 1.20 | 5.21 | 0.79 | 5/5 | 0.06 | 419.22 | 83.84 |
| Test Samples | Test Conditions | YP (Pa) | PV (mPa·s) | AV (mPa·s) | Filtration Loss (mL) |
|---|---|---|---|---|---|
| Na-B dispersion | BHR | 6.51 | 2.79 | 3.72 | 22.79 |
| AHR at 100 °C | 13.49 | 9.30 | 4.19 | 27.53 | |
| AHR at 115 °C | 14.88 | 10.23 | 4.65 | 27.16 | |
| AHR at 130 °C | 6.74 | 6.05 | 0.70 | 26.78 | |
| Na-B dispersion + 2 wt% CCMMA | BHR | 14.88 | 13.02 | 1.86 | 22.13 |
| AHR at 100 °C | 12.56 | 10.23 | 2.33 | 25.48 | |
| AHR at 115 °C | 10.70 | 9.30 | 1.40 | 75.52 | |
| AHR at 130 °C | 14.42 | 12.09 | 2.33 | 14.51 | |
| Na-B dispersion + 4 wt% CCMMA | BHR | 13.25 | 10.70 | 2.56 | 18.60 |
| AHR at 100 °C | 10.23 | 8.37 | 1.86 | 58.59 | |
| AHR at 115 °C | 19.53 | 17.67 | 1.86 | 8.93 | |
| AHR at 130 °C | 17.21 | 14.88 | 2.33 | 13.21 | |
| Na-B dispersion + 6 wt% CCMMA | BHR | 11.63 | 9.30 | 2.33 | 32.74 |
| AHR at 100 °C | 6.51 | 4.65 | 1.86 | 13.95 | |
| AHR at 115 °C | 6.98 | 5.58 | 1.40 | 15.81 | |
| AHR at 130 °C | 5.58 | 5.12 | 0.47 | 17.67 | |
| Na-B dispersion + 2 wt% CMCS | BHR | 6.51 | 2.79 | 3.72 | 22.79 |
| AHR at 100 °C | 13.49 | 9.30 | 4.19 | 27.53 | |
| AHR at 115 °C | 14.88 | 10.23 | 4.65 | 27.16 | |
| AHR at 130 °C | 6.74 | 6.05 | 0.70 | 26.78 |
| Fitting Equation | Fitting Parameters | T = 20 °C | T = 130 °C |
|---|---|---|---|
| Langmuir | qm (mg/g) | 338.32197 | 240.003535 |
| KL (L/mg) | 0.00063 | 0.000665 | |
| R2 | 0.988365 | 0.873905 | |
| Freundlich | KF (L/mg) | 1.14471 | 1.21334 |
| n | 1.557045 | 1.502995 | |
| R2 | 0.972195 | 0.85614 | |
| Chapman sigmoidal equation | a | 177.421335 | 134.679505 |
| b | 0.002625 | 0.002945 | |
| c | 2.66448 | 3.0001 | |
| R2 | 0.9994 | 0.949335 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Lou, Y.; Li, Z.; Zhai, X.; Gao, F. Development and Characterization of Thermoresponsive Smart Self-Adaptive Chitosan-Based Polymer for Wellbore Plugging. Polymers 2023, 15, 4632. https://doi.org/10.3390/polym15244632
Wu H, Lou Y, Li Z, Zhai X, Gao F. Development and Characterization of Thermoresponsive Smart Self-Adaptive Chitosan-Based Polymer for Wellbore Plugging. Polymers. 2023; 15(24):4632. https://doi.org/10.3390/polym15244632
Chicago/Turabian StyleWu, Huimei, Yishan Lou, Zhonghui Li, Xiaopeng Zhai, and Fei Gao. 2023. "Development and Characterization of Thermoresponsive Smart Self-Adaptive Chitosan-Based Polymer for Wellbore Plugging" Polymers 15, no. 24: 4632. https://doi.org/10.3390/polym15244632







