Poly(ethylene Glycol) Methyl Ether Methacrylate-Based Injectable Hydrogels: Swelling, Rheological, and In Vitro Biocompatibility Properties with ATDC5 Chondrogenic Lineage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of the Hydrogels
2.3. 1H Nuclear Magnetic Resonance (1H NMR)
2.4. Hydrogel Swelling Behavior
2.5. Rheological Properties of the Hydrogels
2.6. Biocompatibility Assays
2.6.1. Cell Culture
2.6.2. MTT Viability Assay
2.6.3. Nitrite Assay
2.6.4. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of the Hydrogel Network
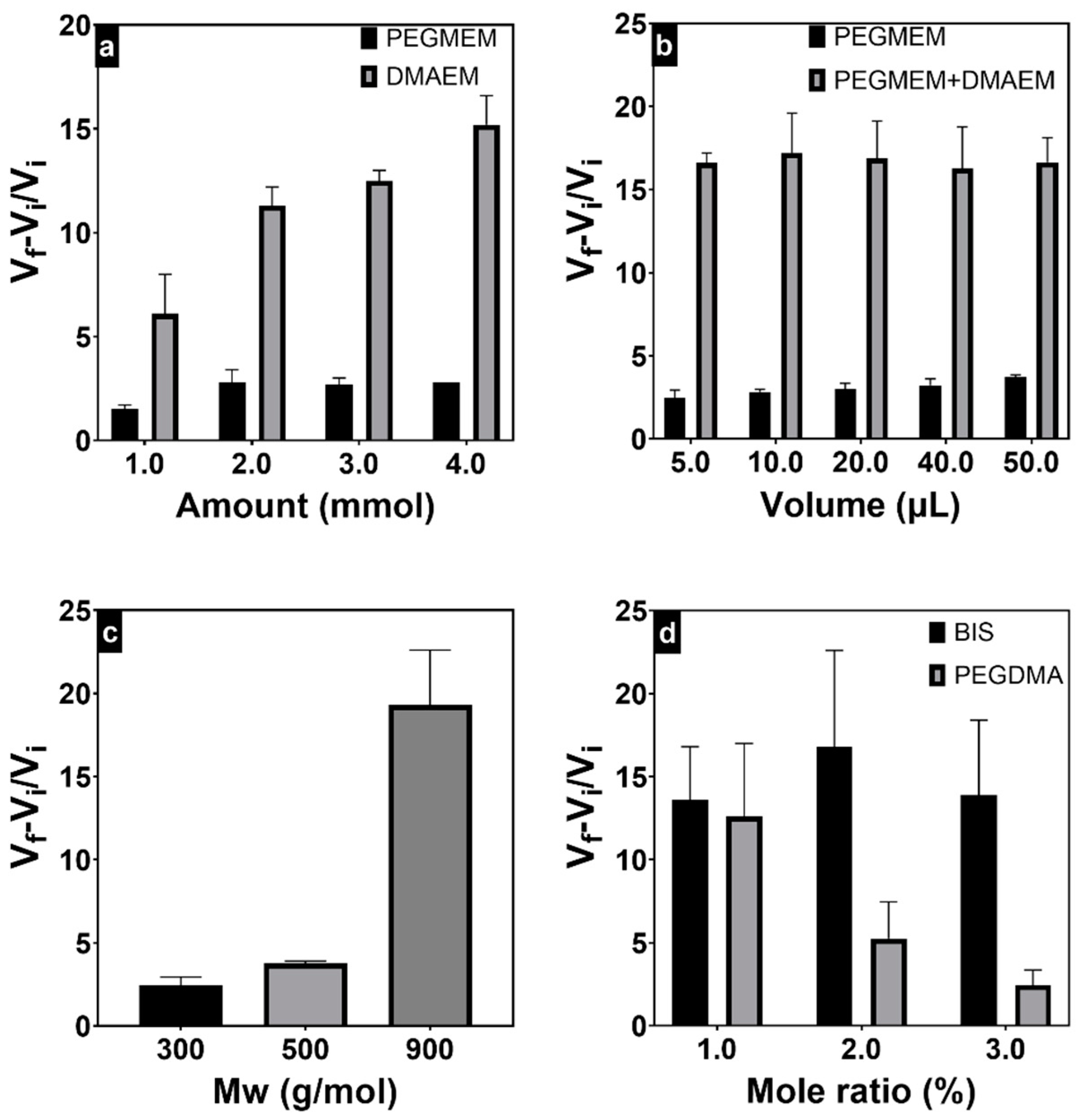
3.2. Swelling Behavior
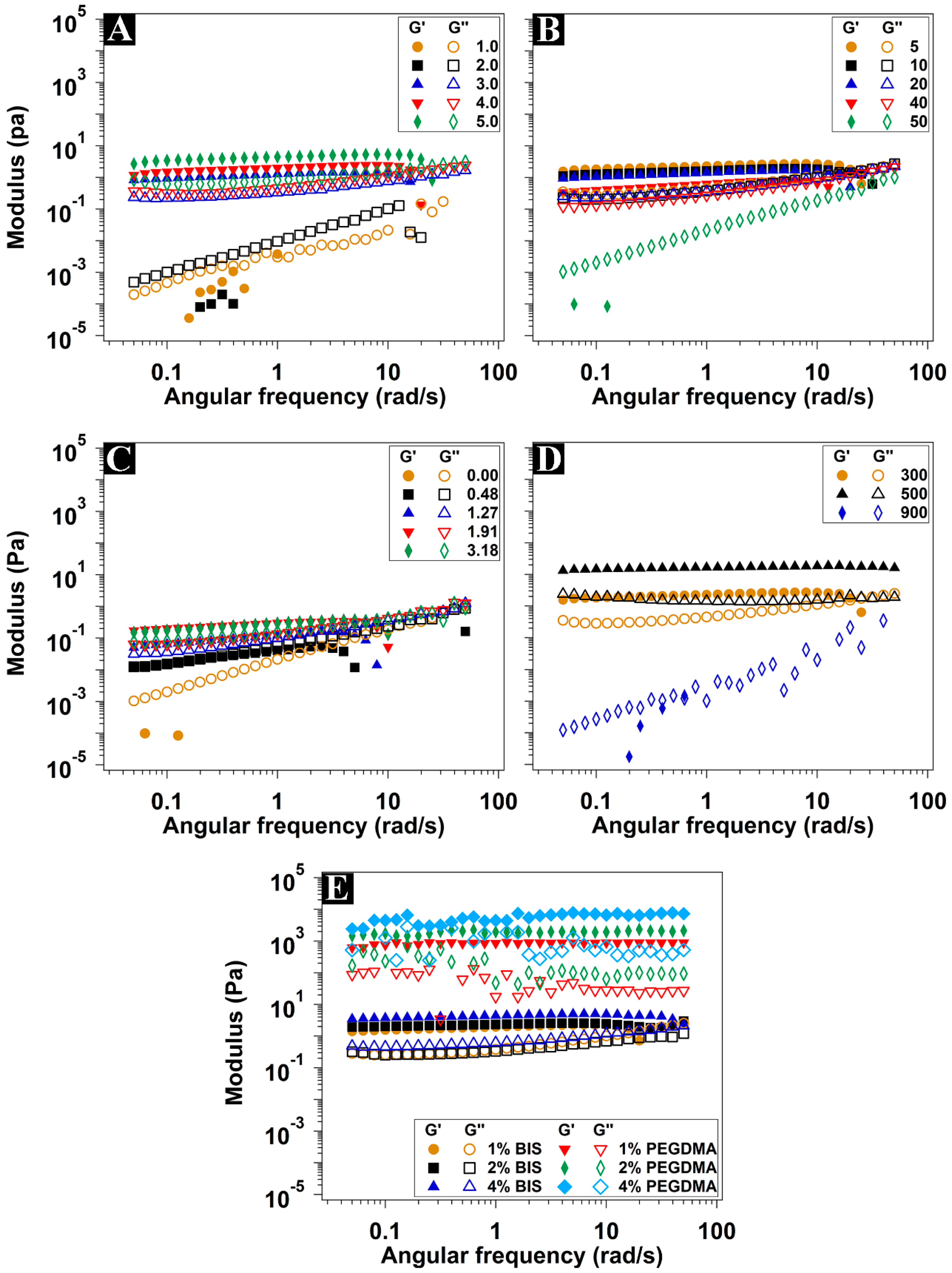
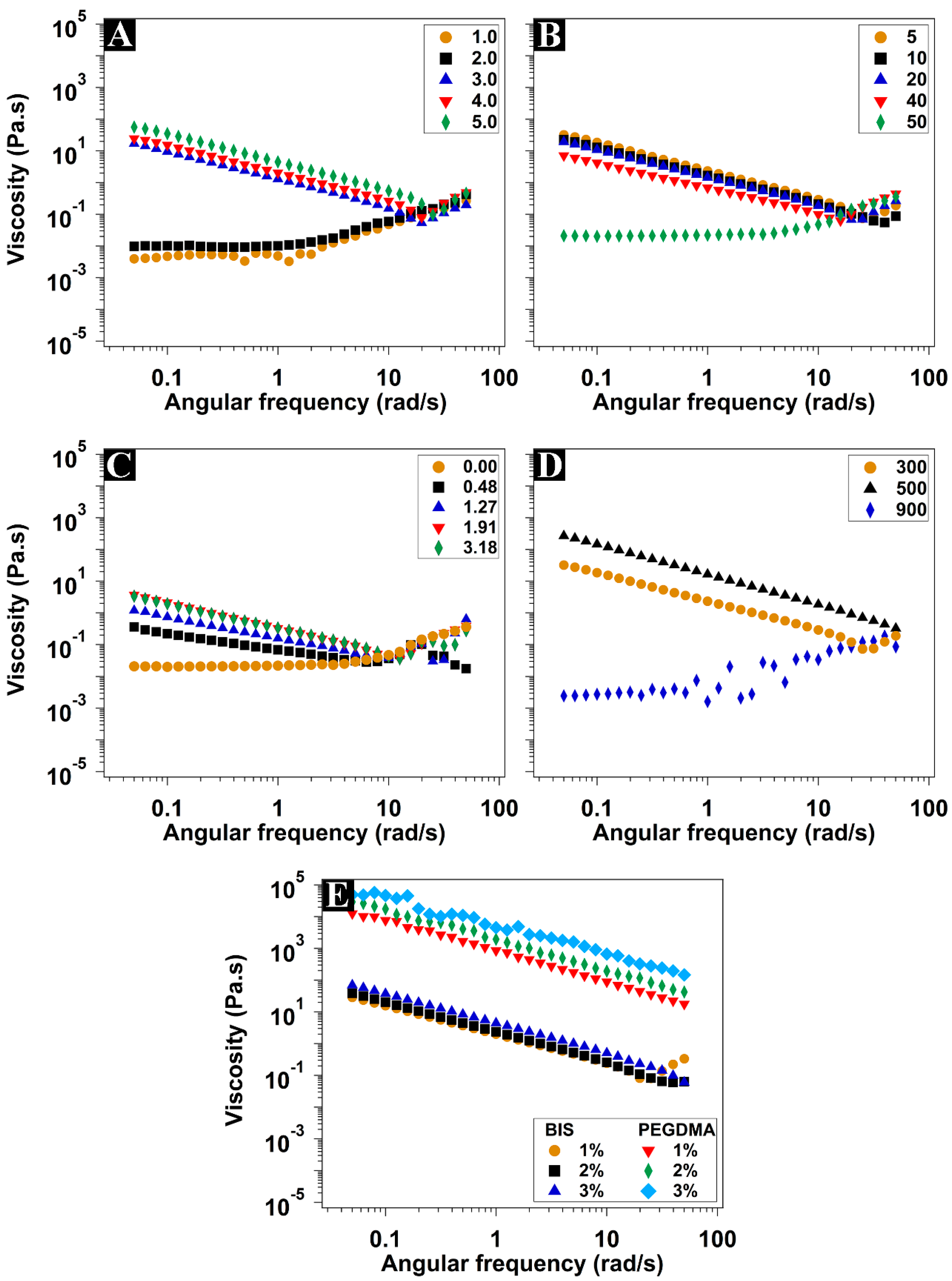
3.3. Rheological Characterization
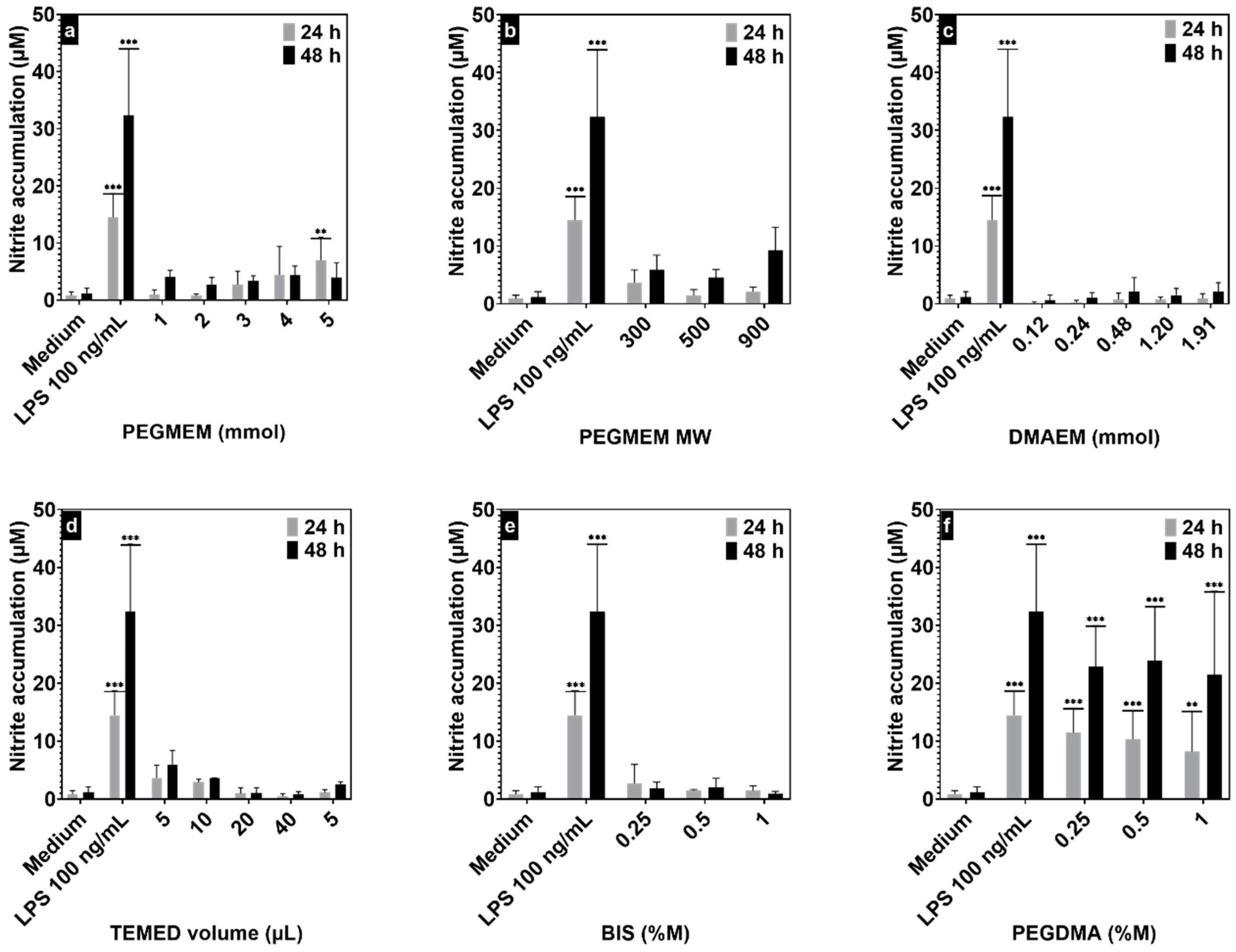
3.4. In Vitro Biocompatibility Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Peppas, N.A.; Slaughter, B.V.; Kanzelberger, M.A. Hydrogels. In Polymer Science: A Comprehensive Reference, 10 Volume Set; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 9, pp. 385–395. ISBN 9780080878621. [Google Scholar]
- Mohanty, A.R.; Ravikumar, A.; Peppas, N.A. Recent Advances in Glucose Responsive Insulin Delivery Systems: Novel Hydrogels and Future Applications. Regen. Biomater. 2022, 9, rbac056. [Google Scholar] [CrossRef] [PubMed]
- Ide, W.; Farrag, Y. Natural Polymeric Materials as a Vehicle for Antibiotics. In Antibiotic Materials in Healthcare; Kokkarachedu, V., Kanikireddy, V., Sadiku, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 51–64. ISBN 9780128200544. [Google Scholar]
- Seidlits, S.K.; Gower, R.M.; Shepard, J.A.; Shea, L.D. Hydrogels for Lentiviral Gene Delivery. Expert Opin. Drug Deliv. 2013, 10, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.P.; Uthaman, S.; Cho, K.H.; Cho, C.S.; Park, I.K. Injectable Hydrogels for Delivering Biotherapeutic Molecules. Int. J. Biol. Macromol. 2018, 110, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yao, H.; Li, X.; Chang, L.; Wang, Z.; Zhu, W.; Su, Y.; Qin, L.; Xu, J. Advanced Hydrogel Systems for Mandibular Reconstruction. Bioact. Mater. 2023, 21, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.M.; Kuhn, A.I.; Hentschel, G.; Glasmacher, B. A Review on Novel Channel Materials for Particle Image Velocimetry Measurements—Usability of Hydrogels in Cardiovascular Applications. Gels 2022, 8, 502. [Google Scholar] [CrossRef] [PubMed]
- Pettinelli, N.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Feijoo-Bandín, S.; Lago, F. Carrageenan-Based Physically Crosslinked Injectable Hydrogel for Wound Healing and Tissue Repairing Applications. Int. J. Pharm. 2020, 589, 119828. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, B.; Barbu, A.; Negrea, M.O.; Berghea-Neamțu, C.Ș.; Popescu, D.; Zăhan, M.; Mireșan, V. Carrageenan-Based Compounds as Wound Healing Materials. Int. J. Mol. Sci. 2022, 23, 9117. [Google Scholar] [CrossRef]
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic Hydrogels to Promote Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 718377. [Google Scholar] [CrossRef]
- Pereira-da-Mota, A.F.; Vivero-Lopez, M.; Serramito, M.; Diaz-Gomez, L.; Serro, A.P.; Carracedo, G.; Huete-Toral, F.; Concheiro, A.; Alvarez-Lorenzo, C. Contact Lenses for Pravastatin Delivery to Eye Segments: Design and in Vitro-in Vivo Correlations. J. Control. Release 2022, 348, 431–443. [Google Scholar] [CrossRef]
- Ma, X.; Ahadian, S.; Liu, S.; Zhang, J.; Liu, S.; Cao, T.; Lin, W.; Wu, D.; de Barros, N.R.; Zare, M.R.; et al. Smart Contact Lenses for Biosensing Applications. Adv. Intell. Syst. 2021, 3, 2000263. [Google Scholar] [CrossRef]
- Alonso, J.M.; Del Olmo, J.A.; Gonzalez, R.P.; Saez-martinez, V. Injectable Hydrogels: From Laboratory to Industrialization. Polymers 2021, 13, 650. [Google Scholar] [CrossRef] [PubMed]
- Uman, S.; Dhand, A.; Burdick, J.A. Recent Advances in Shear-Thinning and Self-Healing Hydrogels for Biomedical Applications. J. Appl. Polym. Sci. 2020, 137, 48668. [Google Scholar] [CrossRef]
- Bae, K.H.; Wang, L.S.; Kurisawa, M. Injectable Biodegradable Hydrogels: Progress and Challenges. J. Mater. Chem. B 2013, 1, 5371–5388. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Tomé, V.; García-Otero, X.; Varela-Fernández, R.; Martín-Pastor, M.; Conde-Penedo, A.; Aguiar, P.; González-Barcia, M.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. In Situ Forming and Mucoadhesive Ophthalmic Voriconazole/HPβCD Hydrogels for the Treatment of Fungal Keratitis. Int. J. Pharm. 2021, 597, 120318. [Google Scholar] [CrossRef]
- Thorpe, A.A.; Freeman, C.; Farthing, P.; Callaghan, J.; Hatton, P.V.; Brook, I.M.; Sammon, C.; Le Maitre, C.L. In Vivo Safety and Efficacy Testing of a Thermally Triggered Injectable Hydrogel Scaffold for Bone Regeneration and Augmentation in a Rat Model. Oncotarget 2018, 9, 18277–18295. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Pedrares, N.; Fuentes-Boquete, I.; Díaz-Prado, S.; Rey-Rico, A. Hydrogel-Based Localized Nonviral Gene Delivery in Regenerative Medicine Approaches—An Overview. Pharmaceutics 2020, 12, 752. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Salgado, C.; Pontes-Quero, G.M.; García-Fernández, L.; Aguilar, M.R.; de Wit, K.; Vázquez-Lasa, B.; Rojo, L.; Abradelo, C. The Role of Polymeric Biomaterials in the Treatment of Articular Osteoarthritis. Pharmaceutics 2022, 14, 1644. [Google Scholar] [CrossRef]
- Amiryaghoubi, N.; Fathi, M.; Barar, J.; Omidi, Y. Hydrogel-Based Scaffolds for Bone and Cartilage Tissue Engineering and Regeneration. React. Funct. Polym. 2022, 177, 105313. [Google Scholar] [CrossRef]
- Naghizadeh, Z.; Karkhaneh, A.; Nokhbatolfoghahaei, H.; Farzad-Mohajeri, S.; Rezai-Rad, M.; Dehghan, M.M.; Aminishakib, P.; Khojasteh, A. Cartilage Regeneration with Dual-Drug-Releasing Injectable Hydrogel/Microparticle System: In Vitro and in Vivo Study. J. Cell. Physiol. 2021, 236, 2194–2204. [Google Scholar] [CrossRef]
- Bączkowicz, D.; Skiba, G.; Szmajda, M.; Vařeka, I.; Falkowski, K.; Laudner, K. Effects of Viscosupplementation on Quality of Knee Joint Arthrokinematic Motion Analyzed by Vibroarthrography. Cartilage 2021, 12, 438–447. [Google Scholar] [CrossRef]
- Velasco-Rodriguez, B.; Diaz-vidal, T.; Rosales-rivera, L.C.; García-gonzález, C.A.; Alvarez-lorenzo, C.; Al-modlej, A.; Domínguez-arca, V.; Prieto, G.; Barbosa, S.; Soltero Martínez, J.F.A.; et al. Hybrid Methacrylated Gelatin and Hyaluronic Acid Hydrogel Scaffolds. Preparation and Systematic Characterization for Prospective Tissue Engineering Applications. Int. J. Mol. Sci. 2021, 22, 6758. [Google Scholar] [CrossRef] [PubMed]
- İlhan, G.T.; Irmak, G.; Gümüşderelioğlu, M. Microwave Assisted Methacrylation of Kappa Carrageenan: A Bioink for Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2020, 164, 3523–3534. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Garrido, C.; Rodriguez-Fontan, F.; Aisenbrey, E.A.; Payne, K.A.; Chahla, J.; Goodrich, L.R.; Bryant, S.J. Current and Novel Injectable Hydrogels to Treat Focal Chondral Lesions: Properties and Applicability. J. Orthop. Res. 2018, 36, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, Y.; González-Sánchez, M.I.; Hawkins, K.; Rubio-Retama, J.; Valero, E.; Perni, S.; Prokopovich, P.; López-Cabarcos, E. Obtaining New Composite Biomaterials by Means of Mineralization of Methacrylate Hydrogels Using the Reaction-Diffusion Method. Mater. Sci. Eng. C 2014, 42, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Pettinelli, N.; Rodríguez-Llamazares, S.; Abella, V.; Barral, L.; Bouza, R.; Farrag, Y.; Lago, F. Entrapment of Chitosan, Pectin or κ-Carrageenan within Methacrylate Based Hydrogels: Effect on Swelling and Mechanical Properties. Mater. Sci. Eng. C 2019, 96, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, A.; Selianitis, D.; Sory, D.R.; Rankin, S.M.; Jones, J.R.; Pispas, S. Poly(2-(Dimethylamino) Ethyl Methacrylate)-b-Poly(Lauryl Methacrylate)-b-Poly(Oligo Ethylene Glycol Methacrylate) Triblock Terpolymer Micelles as Drug Delivery Carriers for Curcumin. J. Appl. Polym. Sci. 2022, 139, e52899. [Google Scholar] [CrossRef]
- Balafouti, A.; Pispas, S. Hyperbranched Polyelectrolyte Copolymers as Novel Candidate Delivery Systems for Bio-Relevant Compounds. Materials 2023, 16, 1045. [Google Scholar] [CrossRef]
- Gerardos, A.M.; Balafouti, A.; Pispas, S. Mixed Hyperbranched/Triblock Copolymer Micelle Assemblies: Physicochemical Properties and Potential for Drug Encapsulation. Macromol. Chem. Phys. 2023, 224, 2300109. [Google Scholar] [CrossRef]
- Tommasi, G.; Perni, S.; Prokopovich, P. An Injectable Hydrogel as Bone Graft Material with Added Antimicrobial Properties. Tissue Eng. Part A 2016, 22, 862–872. [Google Scholar] [CrossRef]
- Raafat, A.I.; Kamal, H.; Sharada, H.M.; Abd elhalim, S.A.; Mohamed, R.D. Radiation Development of Gastroretentive Amoxicillin Trihydrate Floating-Alginate Based Beads for the Treatment of Helicobacter Pylori. Radiat. Phys. Chem. 2021, 179, 109268. [Google Scholar] [CrossRef]
- Tai, H.; Howard, D.; Takae, S.; Wang, W.; Vermonden, T.; Hennink, W.E.; Stayton, P.S.; Hoffman, A.S.; Endruweit, A.; Alexander, C.; et al. Photo-Cross-Linked Hydrogels from Thermoresponsive PEGMEMA-PPGMA-EGDMA Copolymers Containing Multiple Methacrylate Groups: Mechanical Property, Swelling, Protein Release, and Cytotoxicity. Biomacromolecules 2009, 10, 2895–2903. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Conde, J.; Scotece, M.; Abella, V.; López, V.; Pino, J.; Gómez, R.; Gómez-Reino, J.J.; Gualillo, O. Choosing the Right Chondrocyte Cell Line: Focus on Nitric Oxide. J. Orthop. Res. 2015, 33, 1784–1788. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, Y. ATDC5: An Excellent in Vitro Model Cell Line for Skeletal Development. J. Cell. Biochem. 2013, 114, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Strachota, B.; Matějka, L.; Zhigunov, A.; Konefał, R.; Spěváček, J.; Dybal, J.; Puffr, R. Poly(N-Isopropylacrylamide)-Clay Based Hydrogels Controlled by the Initiating Conditions: Evolution of Structure and Gel Formation. Soft Matter 2015, 11, 9291–9306. [Google Scholar] [CrossRef]
- Shirangi, M.; Sastre Toraño, J.; Sellergren, B.; Hennink, W.E.; Somsen, G.W.; Van Nostrum, C.F. Methyleneation of Peptides by N, N, N, N-Tetramethylethylenediamine (TEMED) under Conditions Used for Free Radical Polymerization: A Mechanistic Study. Bioconjug. Chem. 2015, 26, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Okeke, U.C.; Snyder, C.R.; Frukhtbeyn, S.A. Synthesis, Purification and Characterization of Polymerizable Multifunctional Quaternary Ammonium Compounds. Molecules 2019, 24, 1464. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hoffman, A.S. Hydrogels. In Biomaterials Science; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 153–166. [Google Scholar]
- Peppas, N.A.; Merrill, E.W. Crosslinked Poly(Vinyl Alcohol) Hydrogels as Swollen Elastic Networks. J. Appl. Polym. Sci. 1977, 21, 1763–1770. [Google Scholar] [CrossRef]
- Cursaru, B.; Stǎnescu, P.O.; Teodorescu, M. The States of Water in Hydrogels Synthesized from Diepoxy-Terminated Poly(Ethylene Glycol)s and Aliphatic Polyamines. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2010, 72, 99–114. [Google Scholar]
- Holback, H.; Yeo, Y.; Park, K. Hydrogel Swelling Behavior and Its Biomedical Applications; Woodhead Publishing Limited: Sawston, UK, 2011. [Google Scholar]
- Yan, C.; Pochan, D.J. Rheological Properties of Peptide-Based Hydrogels for Biomedical and Other Applications. Chem. Soc. Rev. 2010, 39, 3528–3540. [Google Scholar] [CrossRef]
- Chen, M.H.; Wang, L.L.; Chung, J.J.; Kim, Y.H.; Atluri, P.; Burdick, J.A. Methods to Assess Shear-Thinning Hydrogels for Application As Injectable Biomaterials. ACS Biomater. Sci. Eng. 2017, 3, 3146–3160. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Staropoli, M.; Appavou, M.S.; Wyss, H.M.; Pyckhout-Hintzen, W.; Sijbesma, R.P. Tough Supramolecular Hydrogel Based on Strong Hydrophobic Interactions in a Multiblock Segmented Copolymer. Macromolecules 2017, 50, 3333–3346. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Chun, C.; Lee, D.Y.; Kim, J.T.; Kwon, M.K.; Kim, Y.Z.; Kim, S.S. Effect of Molecular Weight of Hyaluronic Acid (HA) on Viscoelasticity and Particle Texturing Feel of HA Dermal Biphasic Fillers. Biomater. Res. 2016, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Guaresti, O.; García–Astrain, C.; Palomares, T.; Alonso–Varona, A.; Eceiza, A.; Gabilondo, N. Synthesis and Characterization of a Biocompatible Chitosan–Based Hydrogel Cross–Linked via ‘Click’ Chemistry for Controlled Drug Release. Int. J. Biol. Macromol. 2017, 102, 1–9. [Google Scholar] [CrossRef] [PubMed]


| Hydrogel ID | Water | PEGMEM MW and Amount | DMAEM Amount | Crosslinker | TMED Amount | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (mL) | MW | (mL) | (mmol) | (mL) | (mmol) | Type | Molar Ratio (M %) | (mg) | (µL) | |
| P1 | 4 | 300 | 0.3 | 1.00 | -- | -- | -- | 0.00 | 0.00 | 20.0 |
| P2 | 0.6 | 2.00 | 20.0 | |||||||
| P3 | 0.9 | 3.00 | 20.0 | |||||||
| P4 | 1.2 | 4.00 | 20.0 | |||||||
| P5 | 1.5 | 5.00 | 20.0 | |||||||
| PTEM5 | 1 | 3.33 | 5.0 | |||||||
| PTEM10 | 1 | 3.33 | 10.0 | |||||||
| PTEM20 | 1 | 3.33 | 20.0 | |||||||
| PTEM40 | 1 | 3.33 | 40.0 | |||||||
| PTEM50 | 1 | 3.33 | 50.0 | |||||||
| P3MW500 | 3.33 | 500 | 1.67 | 3.34 | 5.0 | |||||
| P3MW900 | 1 | 900 | 3 | 3.33 | 5.0 | |||||
| P3BIS025 | 4 | 300 | 1 | 3.33 | BIS | 0.25 | 1.3 | 5.0 | ||
| P3BIS05 | 1 | 3.33 | 0.5 | 2.6 | 5.0 | |||||
| P3BIS1 | 1 | 3.33 | 1 | 5.1 | 5.0 | |||||
| P3PDM025 | 1 | 3.33 | PEGDMA | 0.25 | 4.6 | 5.0 | ||||
| P3PDM05 | 1 | 3.33 | 0.5 | 9.2 | 5.0 | |||||
| P3PDM1 | 1 | 3.33 | 1 | 18.3 | 5.0 | |||||
| P3D024 | 1 | 3.33 | 0.038 | 0.24 | -- | 0.00 | 0.00 | 50.0 | ||
| P3D048 | 1 | 3.33 | 0.075 | 0.48 | 50.0 | |||||
| P3D127 | 1 | 3.33 | 0.2 | 1.27 | 50.0 | |||||
| P3D191 | 1 | 3.33 | 0.3 | 1.91 | 50.0 | |||||
| P13D3TEM5 | 1 | 3.33 | 0.5 | 3.18 | 5.0 | |||||
| P3D3TEM10 | 1 | 3.33 | 0.5 | 3.18 | 10.0 | |||||
| P3D3TEM20 | 1 | 3.33 | 0.5 | 3.18 | 20.0 | |||||
| P3D3TEM40 | 1 | 3.33 | 0.5 | 3.18 | 40.0 | |||||
| P3D3TEM50 | 1 | 3.33 | 0.5 | 3.18 | 50.0 | |||||
| P2.5D2.4BIS1 | 0.75 | 2.50 | 0.375 | 2.39 | BIS | 1 | 7.5 | -- | ||
| P2.5D2.4BIS2 | 0.75 | 2.50 | 0.375 | 2.39 | 2 | 15.1 | ||||
| P2.5D2.4BIS4 | 0.75 | 2.50 | 0.375 | 2.39 | 4 | 30.1 | ||||
| P2.5D2.4PDM1 | 0.75 | 2.50 | 0.375 | 2.39 | PEGDMA | 1 | 26.9 | |||
| P2.5D2.4PDM2 | 0.75 | 2.50 | 0.375 | 2.39 | 2 | 53.7 | ||||
| P2.5D2.4PDM4 | 0.75 | 2.50 | 0.375 | 2.39 | 4 | 107.5 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrag, Y.; Ait Eldjoudi, D.; Farrag, M.; González-Rodríguez, M.; Ruiz-Fernández, C.; Cordero, A.; Varela-García, M.; Torrijos Pulpón, C.; Bouza, R.; Lago, F.; et al. Poly(ethylene Glycol) Methyl Ether Methacrylate-Based Injectable Hydrogels: Swelling, Rheological, and In Vitro Biocompatibility Properties with ATDC5 Chondrogenic Lineage. Polymers 2023, 15, 4635. https://doi.org/10.3390/polym15244635
Farrag Y, Ait Eldjoudi D, Farrag M, González-Rodríguez M, Ruiz-Fernández C, Cordero A, Varela-García M, Torrijos Pulpón C, Bouza R, Lago F, et al. Poly(ethylene Glycol) Methyl Ether Methacrylate-Based Injectable Hydrogels: Swelling, Rheological, and In Vitro Biocompatibility Properties with ATDC5 Chondrogenic Lineage. Polymers. 2023; 15(24):4635. https://doi.org/10.3390/polym15244635
Chicago/Turabian StyleFarrag, Yousof, Djedjiga Ait Eldjoudi, Mariam Farrag, María González-Rodríguez, Clara Ruiz-Fernández, Alfonso Cordero, María Varela-García, Carlos Torrijos Pulpón, Rebeca Bouza, Francisca Lago, and et al. 2023. "Poly(ethylene Glycol) Methyl Ether Methacrylate-Based Injectable Hydrogels: Swelling, Rheological, and In Vitro Biocompatibility Properties with ATDC5 Chondrogenic Lineage" Polymers 15, no. 24: 4635. https://doi.org/10.3390/polym15244635
APA StyleFarrag, Y., Ait Eldjoudi, D., Farrag, M., González-Rodríguez, M., Ruiz-Fernández, C., Cordero, A., Varela-García, M., Torrijos Pulpón, C., Bouza, R., Lago, F., Pino, J., Alvarez-Lorenzo, C., & Gualillo, O. (2023). Poly(ethylene Glycol) Methyl Ether Methacrylate-Based Injectable Hydrogels: Swelling, Rheological, and In Vitro Biocompatibility Properties with ATDC5 Chondrogenic Lineage. Polymers, 15(24), 4635. https://doi.org/10.3390/polym15244635










