Research Progress of Self-Healing Polymer for Ultraviolet-Curing Three-Dimensional Printing
Abstract
:1. Introduction

| Name | Operation Principle | UV-Curing Mechanism | Advantage | Disadvantage | Application |
|---|---|---|---|---|---|
| SLA | Laser beam single-point printing | Free radical and hybrid curing | Mature technology, form large size device | Slow curing speed | Dentistry, mold, automobile |
| DLP | Projection printing | Free radical curing | Fast curing rate, high precision | Form small size device | Medical care, jewelry, education |
| CLIP | Projective continuous printing | Free radical and thermocuring | Extremely fast curing speed | Expensive resin and equipment | Sports, cars |
| MJP 1 | Multi-nozzle printing | Free radical and hybrid curing | High precision, colorfulness | Expensive equipment | Consumer goods, medical care jewelry |
| TPP | Dual Laser Beam Printing | Free radical curing | Extremely high precision | Expensive equipment, complex process | Microelectronics, art, scientific research |
| LCD | Liquid crystal imaging printing | Free radical curing | Fast curing speed, low cost | Short service life | Jewelry, mold manufacturing |
2. Dynamic Covalent Self-Healing Polymers for UV-Curing 3D Printing
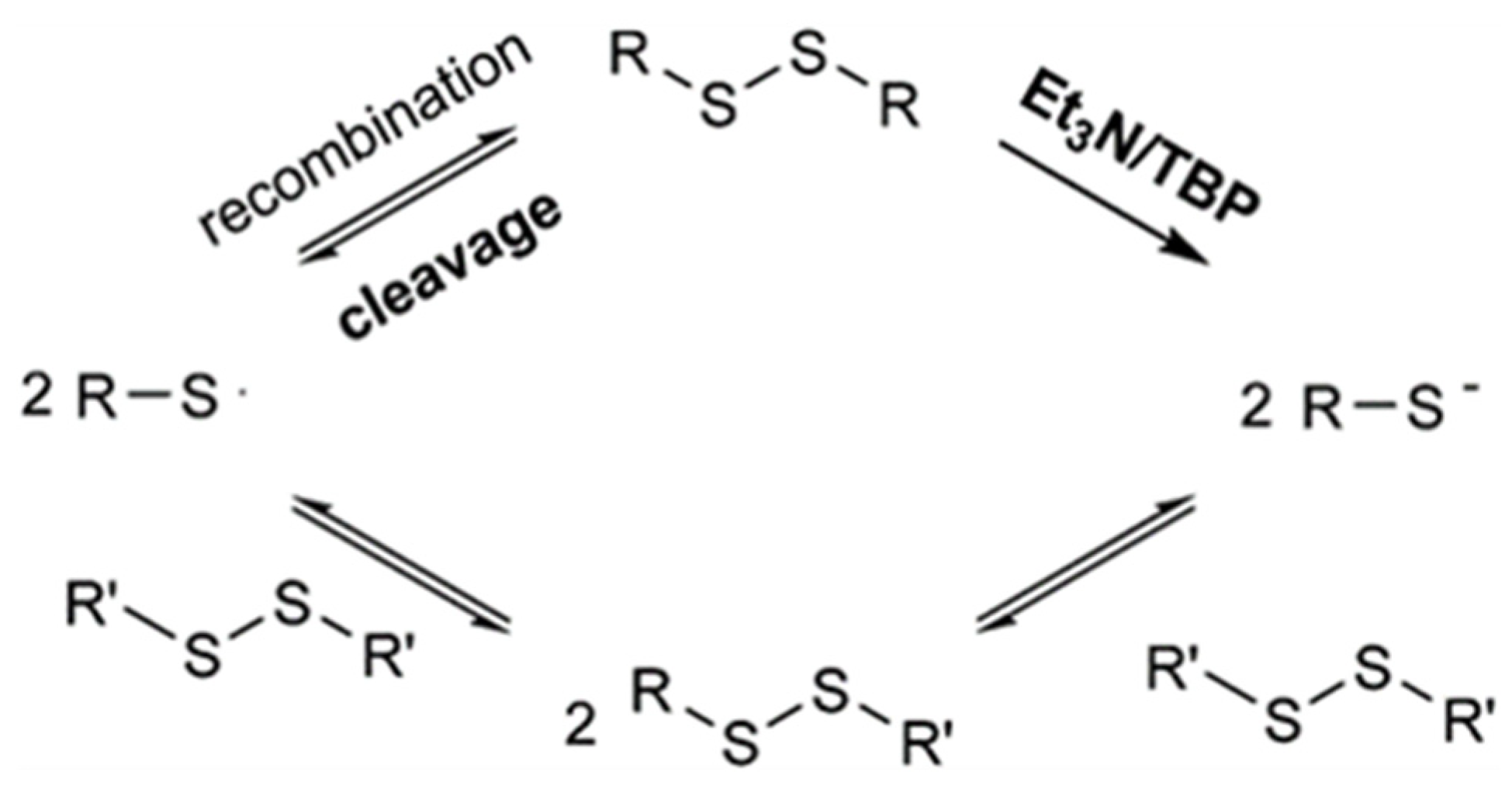
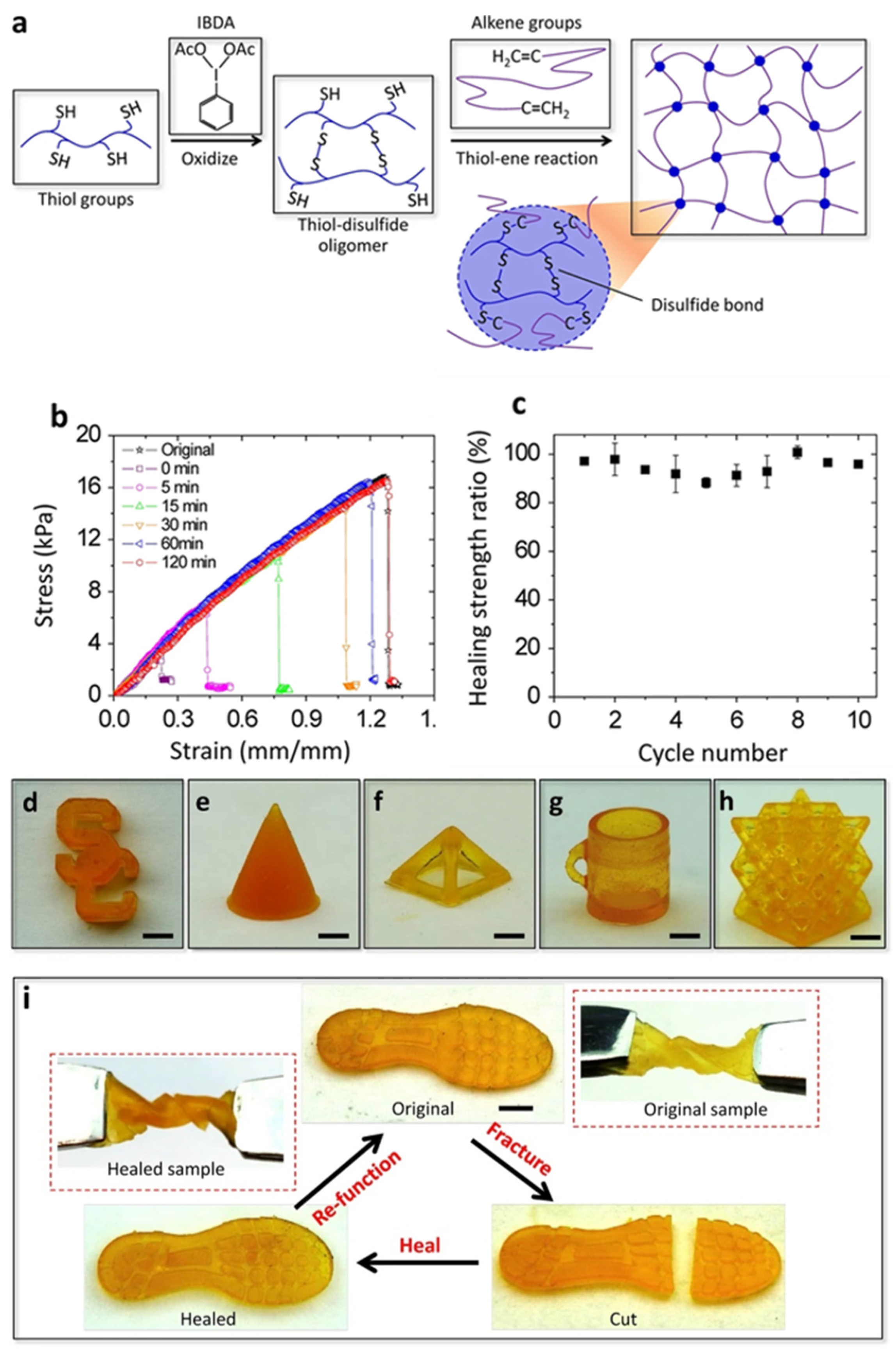
2.1. Dynamic Disulfide-Bond
2.2. Transesterified
2.3. Imine Bond
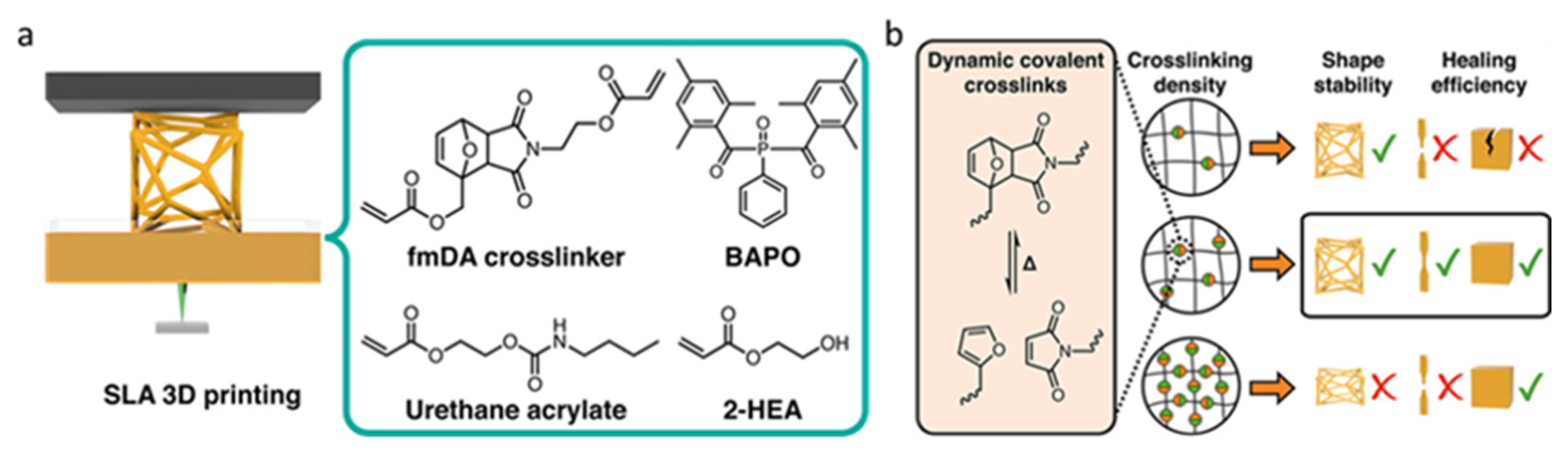
2.4. Diels–Alder Bond
3. Dynamic Non-Covalent Self-Healing Polymers for UV-Curing 3D Printing
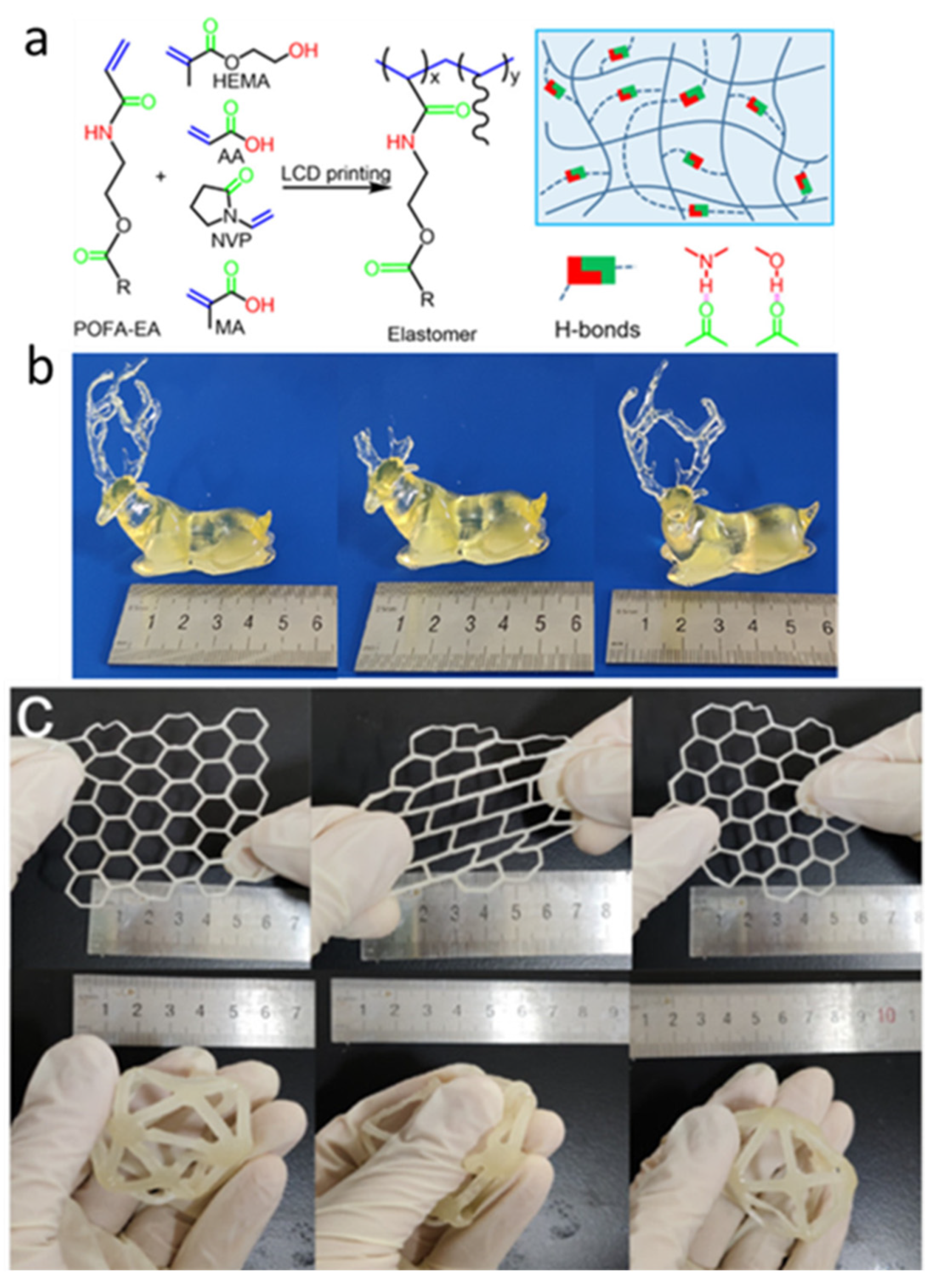
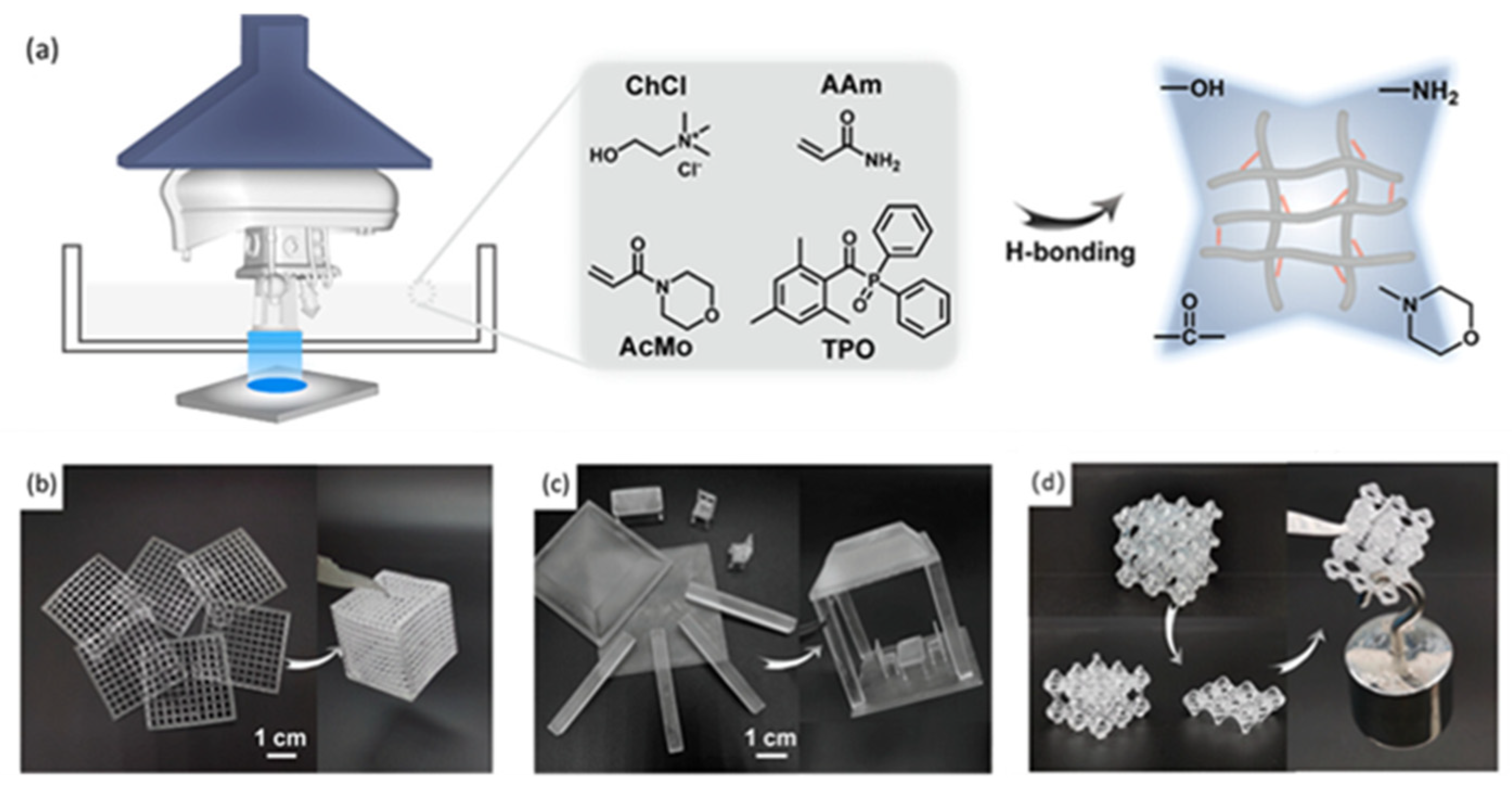
3.1. Hydrogen Bond
3.2. Crystallization
3.3. Host-Guest Interaction
4. Challenges and Prospects
- (1)
- Photocurable 3D printing technology requires photosensitive resin with a low viscosity, but the molecular weight of low-viscosity resin is small, which will make the cross-linking density of the cured material high, causing the material to become hard and brittle. If the molecular weight of the resin is large, a large amount of monomer dilution is required, which will cause the resin to lose its original performance. The contradiction between resin viscosity and performance needs to be solved urgently;
- (2)
- Balancing the mechanical performance and self-healing function of UV-curable self-healing polymers is the main goal. To achieve high mechanical performance, dynamic bonds with high bond energy are required, which decreases the self-healing efficiency of the polymer. At present, dual dynamic network structures [103,104,105] and multi-phase design [106,107] have been introduced into polymers and have achieved certain results, but new methods still need to be explored;
- (3)
- UV-curable, self-healing polymers for 3D printing require external stimuli to activate damage healing; however, the stimulation intensity required for healing cannot be easily provided in practical applications. Developing polymers that can self-heal at room temperature or lower is more valuable for practical applications;
- (4)
- Currently, the preparation of self-healing photosensitive resins is generally complicated and requires cumbersome steps. Therefore, simplification of the synthesis process, improvement of the yield, and the reduction of waste are necessary;
- (5)
- Photocuring printing equipment is usually expensive and mainly used for printing small devices. Using dynamic interaction, it is undoubtedly convenient to construct large-size devices through module assembly.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, J.; Jiang, M.; Chen, J.; Liang, C. Automobile Body Integration Manufacturing and Thickness Optimization for Stereo Lithography 3D Printing. J. Beijing Univ. Technol. 2017, 43, 551–556. [Google Scholar]
- Mohanavel, V.; Ali, K.S.A.; Ranganathan, K.; Jeffrey, J.A.; Ravikumar, M.M. The roles and applications of additive manufacturing in the aerospace and automobile sector. In Proceedings of the 12th National Web Conference on Recent Advancements in Biomedical Engineering (NCRABE), Electr Network, Online, 3 December 2021; pp. 405–409. [Google Scholar]
- Srinivasan, D.; Meignanamoorthy, M.; Ravichandran, M.; Mohanavel, V.; Alagarsamy, S.V.; Chanakyan, C.; Sakthivelu, S.; Karthick, A.; Prabhu, T.R.; Rajkumar, S. 3D Printing Manufacturing Techniques, Materials, and Applications: An Overview. Adv. Mater. Sci. Eng. 2021, 2021, 5756563. [Google Scholar] [CrossRef]
- Moroni, L.; Boland, T.; Burdick, J.A.; De Maria, C.; Derby, B.; Forgacs, G.; Groll, J.; Li, Q.; Malda, J.; Mironov, V.A.; et al. Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 2018, 36, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Muelhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Qin, Q.; Wang, J. A Review of Stereolithography: Processes and Systems. Processes 2020, 8, 1138. [Google Scholar] [CrossRef]
- Fang, H.-B.; Chen, J.-M. 3D printing based on digital light processing technology. J. Beijing Univ. Technol. 2015, 41, 1775–1782. [Google Scholar] [CrossRef]
- Saitta, L.; Tosto, C.; Pergolizzi, E.; Patti, A.; Cicala, G. Liquid Crystal Display (LCD) Printing: A Novel System for Polymer Hybrids Printing. In Proceedings of the 4th International Conference on Progress on Polymers and Composites Products and Manufacturing Technologies (POLCOM), Electr Network, Bucharest, Romania, 26–28 November 2020. [Google Scholar]
- Braga, R.R.; Ballester, R.Y.; Ferracane, J.L. Factors involved in the development of polymerization shrinkage stress in resin-composites: A systematic review. Dent. Mater. 2005, 21, 962–970. [Google Scholar] [CrossRef]
- Hayashi, J.; Espigares, J.; Takagaki, T.; Shimada, Y.; Tagami, J.; Numata, T.; Chan, D.; Sadr, A. Real-time in-depth imaging of gap formation in bulk-fill resin composites. Dent. Mater. 2019, 35, 585–596. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, S.; Gao, Y.; Nie, J.; Sun, F. Design of a disulfide bond-containing photoresist with extremely low volume shrinkage and excellent degradation ability for UV-nanoimprinting lithography. Chem. Eng. J. 2020, 390, 124625. [Google Scholar] [CrossRef]
- Zhang, Y.; Ju, X.; Gao, Y.; Sun, F. Design of a polymerizable dithioaniline derivative with double functions of reducing volume shrinkage and initiating polymerization for LED photopolymerization. Eur. Polym. J. 2022, 179, 111534. [Google Scholar] [CrossRef]
- Lee, Y.S.; Park, W. Current challenges and future directions for bacterial self-healing concrete. Appl. Microbiol. Biotechnol. 2018, 102, 3059–3070. [Google Scholar] [CrossRef] [PubMed]
- Sovljanski, O.; Tomic, A.; Markov, S. Relationship between Bacterial Contribution and Self-Healing Effect of Cement-Based Materials. Microorganisms 2022, 10, 1399. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, B.; Tasan, C.C. Self-Healing Metals. In Self-Healing Materials; Advances in Polymer Science; Hager, M.D., VanDerZwaag, S., Schubert, U.S., Eds.; Springer: Cham, Switzerland, 2016; Volume 273, pp. 387–407. [Google Scholar]
- Li, Y.; Zhang, L.; Zhang, J.; Wang, X.; Gu, C.; Xia, X.; Tu, J. Self-Healing Properties of Alkali Metals under “High-Energy Conditions” in Batteries. Adv. Energy Mater. 2021, 11, 2100470. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, T.; Niu, X.; Du, Y. Research progress in extrinsic self-healing polymer composites. Fiber Reinf. Plast. Compos. 2015, 92–96+63. [Google Scholar]
- Wang, J.; Jia, H.; Fang, E.; Jiang, J.; Jiang, Q. Progress in self-healing of polymer composites. China Synth. Rubber Ind. 2012, 35, 247–253. [Google Scholar]
- Wang, X.-X.; Yao, S.; Zhou, C.-J.; Wu, J.-L. Application and potential future directions of self-healing polymers in dentistry. Huaxi Kouqiang Yixue Zazhi West China J. Stomatol. 2020, 38, 75–79. [Google Scholar] [CrossRef]
- Ye, B.-h.; Meng, L.; Li, L.-h.; Li, N.; Li, Z.-w. Self-healing Hydrogels Based on Constitutional Dynamic Chemistry and Their Potential Biomedical Applications. Acta Polym. Sin. 2016, 2, 134–148. [Google Scholar]
- Li, S.; Yan, X.; Ma, S.; Liu, M.; Liu, J. Progress in the Application of Self-Healing Polymers in Leather Coatings. Polym. Mater. Sci. Eng. 2022, 38, 166–173. [Google Scholar]
- Zhang, M.; Zhang, B.; Li, X.; Jin, J.; Wang, J. Progress in Research of Self-Healing Superhydrophobic Coatings. Polym. Mater. Sci. Eng. 2022, 38, 168–175. [Google Scholar]
- Huynh, T.-P.; Sonar, P.; Haick, H. Advanced Materials for Use in Soft Self-Healing Devices. Adv. Mater. 2017, 29, 1604973. [Google Scholar] [CrossRef]
- Lin, W.; Wang, H.; Zhang, H.; Tang, J.; Wang, Y. Advance in extrinsic polymer self-healing composite. New Chem. Mater. 2020, 48, 50–53. [Google Scholar]
- Wang, X.; Cheng, B.; Liang, D.; Jia, D. Progress of Intrinsic Self-Healing Polymer Materials. Polym. Mater. Sci. Eng. 2019, 35, 183–190. [Google Scholar] [CrossRef]
- He, Z.; Zhang, W.; Ma, W.; Yu, H.; Zhao, Y. Research progress of microcapsule intelligent self-healing material. New Chem. Mater. 2018, 46, 25–29. [Google Scholar]
- Zhang, H.; Li, M.; Feng, X.; Han, X.; Fan, Z. Review of the Latest Research on Extrinsic Microcapsule Self-healing Materials. Eng. Plast. Appl. 2019, 47, 134–137. [Google Scholar]
- Shinde, V.V.; Celestine, A.-D.; Beckingham, L.E.; Beckingham, B.S. Stereolithography 3D Printing of Microcapsule Catalyst-Based Self-Healing Composites. ACS Appl. Polym. Mater. 2020, 2, 5048–5057. [Google Scholar] [CrossRef]
- Sanders, P.; Young, A.J.; Qin, Y.; Fancey, K.S.; Reithofer, M.R.; Guillet-Nicolas, R.; Kleitz, F.; Pamme, N.; Chin, J.M. Stereolithographic 3D printing of extrinsically self-healing composites. Sci. Rep. 2019, 9, 388. [Google Scholar] [CrossRef]
- Roy, N.; Bruchmann, B.; Lehn, J.-M. DYNAMERS: Dynamic polymers as self-healing materials. Chem. Soc. Rev. 2015, 44, 3786–3807. [Google Scholar] [CrossRef] [PubMed]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, J.; Singh, V.; Xu, L.; Kabi, P.; Bele, E.; Tiwari, M.K. Digital light 3D printing of a polymer composite featuring robustness, self-healing, recyclability and tailorable mechanical properties. Addit. Manuf. 2023, 61, 103343. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, J.; Huang, J.; Yu, X.; Cheng, J.; Shang, Q.; Hu, Y.; Liu, C.; Zhang, M.; Hu, L.; et al. Self-Healing, Antibacterial, and 3D-Printable Polymerizable Deep Eutectic Solvents Derived from Tannic Acid. ACS Sustain. Chem. Eng. 2022, 10, 7954–7964. [Google Scholar] [CrossRef]
- Wang, Z.; An, G.; Zhu, Y.; Liu, X.; Chen, Y.; Wu, H.; Wang, Y.; Shi, X.; Mao, C. 3D-printable self-healing and mechanically reinforced hydrogels with host-guest non-covalent interactions integrated into covalently linked networks. Mater. Horiz. 2019, 6, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Durand-Silva, A.; Cortés-Guzmán, K.P.; Johnson, R.M.; Perera, S.D.; Diwakara, S.D.; Smaldone, R.A. Balancing Self-Healing and Shape Stability in Dynamic Covalent Photoresins for Stereolithography 3D Printing. ACS Macro Lett. 2021, 10, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X. Research Progress in Self-Healing Materials Based on Diels-Alder Reaction. J. Funct. Polym. 2014, 27, 453–463. [Google Scholar]
- Grauzeliene, S.; Kazlauskaite, B.; Skliutas, E.; Malinauskas, M.; Ostrauskaite, J. Photocuring and digital light processing 3D printing of vitrimer composed of 2-hydroxy-2-phenoxypropyl acrylate and acrylated epoxidized soybean oil. Express Polym. Lett. 2023, 17, 54–68. [Google Scholar] [CrossRef]
- Zhang, M.; Tao, X.; Yu, R.; He, Y.; Li, X.; Chen, X.; Huang, W. Self-healing, mechanically robust, 3D printable ionogel for highly sensitive and long-term reliable ionotronics. J. Mater. Chem. A 2022, 10, 12005–12015. [Google Scholar] [CrossRef]
- Cortés-Guzmán, K.P.; Parikh, A.R.; Sparacin, M.L.; Remy, A.K.; Adegoke, L.; Chitrakar, C.; Ecker, M.; Voit, W.E.; Smaldone, R.A. Recyclable, Biobased Photoresins for 3D Printing Through Dynamic Imine Exchange. ACS Sustain. Chem. Eng. 2022, 10, 13091–13099. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Polymer engineering based on reversible covalent chemistry: A promising innovative pathway towards new materials and new functionalities. Prog. Polym. Sci. 2018, 80, 39–93. [Google Scholar] [CrossRef]
- Chakma, P.; Konkolewicz, D. Dynamic Covalent Bonds in Polymeric Materials. Angew. Chem. Int. Ed. 2019, 58, 9682–9695. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef]
- Xenikakis, I.; Tsongas, K.; Tzimtzimis, E.K.; Zacharis, C.K.; Theodoroula, N.; Kalogianni, E.P.; Demiri, E.; Vizirianakis, I.S.; Tzetzis, D.; Fatouros, D.G. Fabrication of hollow microneedles using liquid crystal display (LCD) vat polymerization 3D printing technology for transdermal macromolecular delivery. Int. J. Pharm. 2021, 597, 120303. [Google Scholar] [CrossRef]
- Tumbleston, J.R.; Shirvanyants, D.; Ermoshkin, N.; Janusziewicz, R.; Johnson, A.R.; Kelly, D.; Chen, K.; Pinschmidt, R.; Rolland, J.P.; Ermoshkin, A.; et al. Continuous liquid interface production of 3D objects. Science 2015, 347, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, Y.; Wu, Y.; Xiang, H.; Liu, X. Development and Applications of UV-Curing 3D Printing and Photosensitive Resin. J. Funct. Polym. 2022, 35, 19–35. [Google Scholar]
- Šercer, M.; Godec, D.; Šantek, B.; Ludwig, R.; Andlar, M.; Rezić, I.; Ivušić, F.; Pilipović, A.; Oros, D.; Rezić, T. Microreactor Production by PolyJet Matrix 3D-Printing Technology: Hydrodynamic Characterization. Food Technol. Biotechnol. 2019, 57, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-M.; Yu, H.-Z.; Sun, X.-H.; Dang, Z.-M. Density functional theory calculations on S-S bond dissociation energies of disulfides. J. Phys. Org. Chem. 2016, 29, 6–13. [Google Scholar] [CrossRef]
- Rekondo, A.; Martin, R.; de Luzuriaga, A.R.; Cabañero, G.; Grande, H.J.; Odriozola, I. Catalyst-free room-temperature self-healing elastomers based on aromatic disulfide metathesis. Mater. Horiz. 2014, 1, 237–240. [Google Scholar] [CrossRef]
- Nevejans, S.; Ballard, N.; Miranda, J.I.; Reck, B.; Asua, J.M. The underlying mechanisms for self-healing of poly(disulfide)s. Phys. Chem. Chem. Phys. 2016, 18, 27577–27583. [Google Scholar] [CrossRef]
- Li, X.; Yu, R.; He, Y.; Zhang, Y.; Yang, X.; Zhao, X.; Huang, W. Self-Healing Polyurethane Elastomers Based on a Disulfide Bond by Digital Light Processing 3D Printing. ACS Macro Lett. 2019, 8, 1511–1516. [Google Scholar] [CrossRef]
- Decker, C.; Viet, T.N.T. Photocrosslinking of functionalized rubbers, 8. The thiol-polybutadiene system. Macromol. Chem. Phys. 1999, 200, 1965–1974. [Google Scholar] [CrossRef]
- Yu, K.; Xin, A.; Du, H.; Li, Y.; Wang, Q. Additive manufacturing of self-healing elastomers. NPG Asia Mater. 2019, 11, 7. [Google Scholar] [CrossRef]
- Xu, Y.; Xiong, X.; Cai, L.; Tang, Z.; Ye, Z. Thiol-Ene Click Chemistry. Prog. Chem. 2012, 24, 385–394. [Google Scholar]
- Yang, Z.; Chen, Q.; Zhou, D.; Bu, Y. Synthesis of Functional Polymer Materials via Thiol-Ene/Yne Click Chemistry. Prog. Chem. 2012, 24, 395–404. [Google Scholar]
- Rahman, S.S.; Arshad, M.; Qureshi, A.; Ullah, A. Fabrication of a Self-Healing, 3D Printable, and Reprocessable Biobased Elastomer. ACS Appl. Mater. Interfaces 2020, 12, 51927–51939. [Google Scholar] [CrossRef]
- Rattanangkool, E.; Krailat, W.; Vilaivan, T.; Phuwapraisirisan, P.; Sukwattanasinitt, M.; Wacharasindhu, S. Hypervalent Iodine(III)-Promoted Metal-Free S-H Activation: An Approach for the Construction of S-S, S-N, and S-C Bonds. Eur. J. Org. Chem. 2014, 2014, 4795–4804. [Google Scholar] [CrossRef]
- Sijbesma, R.P.; Beijer, F.H.; Brunsveld, L.; Folmer, B.J.B.; Hirschberg, J.H.K.K.; Lange, R.F.M.; Lowe, J.K.L.; Meijer, E.W. Reversible polymers formed from self-complementary monomers using quadruple hydrogen bonding. Science 1997, 278, 1601–1604. [Google Scholar] [CrossRef]
- Cordier, P.; Tournilhac, F.; Soulie-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-H.; Wang, C.; Keplinger, C.; Zuo, J.-L.; Jin, L.; Sun, Y.; Zheng, P.; Cao, Y.; Lissel, F.; Linder, C.; et al. A highly stretchable autonomous self-healing elastomer. Nat. Chem. 2016, 8, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.F.; Wanasinghe, S.V.; Flynn, A.E.; Dodo, O.J.; Sparks, J.L.; Baldwin, L.A.; Tabor, C.E.; Durstock, M.F.; Konkolewicz, D.; Thrasher, C.J. 3D-Printed Self-Healing Elastomers for Modular Soft Robotics. ACS Appl. Mater. Interfaces 2021, 13, 28870–28877. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Feng, H.; Yan, Z.; Hao, Q.; Wang, L. Preparation and properties of carbon fiber reinforced unsaturated polyester self-healing composites. Mod. Chem. Ind. 2017, 37, 120–123,125. [Google Scholar]
- Lei, R.; Ma, Y.; Yang, X. Self-healing performance of epoxy coating containing microencapsulated alkyd resin. Chem. Ind. Eng. Prog. 2020, 39, 2782–2787. [Google Scholar]
- Huang, J.; Zhang, J.; Zhu, G.; Yu, X.; Hu, Y.; Shang, Q.; Chen, J.; Hu, L.; Zhou, Y.; Liu, C. Self-healing, high-performance, and high-biobased-content UV-curable coatings derived from rubber seed oil and itaconic acid. Prog. Org. Coat. 2021, 159, 106391. [Google Scholar] [CrossRef]
- Grauzeliene, S.; Kastanauskas, M.; Talacka, V.; Ostrauskaite, J. Photocurable Glycerol- and Vanillin-Based Resins for the Synthesis of Vitrimers. ACS Appl. Polym. Mater. 2022, 4, 6103–6110. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yang, J.; Wu, Q.; Wu, M.; Zhang, J.a. Preparation and properties of room temperature self-healing waterborne polyurethane based on imine bond and disulfide bond. Fine Chem. 2022, 39, 2449–2455,2466. [Google Scholar]
- Pan, Z.; Zhao, Q.; Xue, Y.; Bo, C.; Zhang, M. Preparation and Properties of Bio-Based Self-Healing and Recyclable Polyurethane Elastomer Based on Dynamic Iimine Bonds. Polym. Mater. Sci. Eng. 2022, 38, 11–18. [Google Scholar]
- Min, J.; Zhou, Z.; Wang, H.; Chen, Q.; Hong, M.; Fu, H. Room temperature self-healing and recyclable conductive composites for flexible electronic devices based on imine reversible covalent bond. J. Alloys Compd. 2022, 894, 162433. [Google Scholar] [CrossRef]
- Liguori, A.; Subramaniyan, S.; Yao, J.G.; Hakkarainen, M. Photocurable extended vanillin-based resin for mechanically and chemically recyclable, self-healable and digital light processing 3D printable thermosets. Eur. Polym. J. 2022, 178, 111489. [Google Scholar] [CrossRef]
- Miao, J.-T.; Ge, M.; Peng, S.; Zhong, J.; Li, Y.; Weng, Z.; Wu, L.; Zheng, L. Dynamic Imine Bond-Based Shape Memory Polymers with Permanent Shape Reconfigurability for 4D Printing. ACS Appl. Mater. Interfaces 2019, 11, 40642–40651. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin Production from Lignin and Its Use as a Renewable Chemical. ACS Sustain. Chem. Eng. 2016, 4, 35–46. [Google Scholar] [CrossRef]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A thermally re-mendable cross-linked polymeric material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef]
- Turkenburg, D.H.; Fischer, H.R. Diels-Alder based, thermo-reversible cross-linked epoxies for use in self-healing composites. Polymer 2015, 79, 187–194. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Chuo, T.-W. Self-healing polymers based on thermally reversible Diels-Alder chemistry. Polym. Chem. 2013, 4, 2194–2205. [Google Scholar] [CrossRef]
- Li, Q.-T.; Jiang, M.-J.; Wu, G.; Chen, L.; Chen, S.-C.; Cao, Y.-X.; Wang, Y.-Z. Photothermal Conversion Triggered Precisely Targeted Healing of Epoxy Resin Based on Thermoreversible Diels-Alder Network and Amino-Functionalized Carbon Nanotubes. ACS Appl. Mater. Interfaces 2017, 9, 20797–20807. [Google Scholar] [CrossRef] [PubMed]
- Turkenburg, D.H.; Durant, Y.; Fischer, H.R. Bio-based self-healing coatings based on thermo-reversible Diels-Alder reaction. Prog. Org. Coat. 2017, 111, 38–46. [Google Scholar] [CrossRef]
- Liu, M.; Zhong, J.; Li, Z.; Rong, J.; Yang, K.; Zhou, J.; Shen, L.; Gao, F.; Huang, X.; He, H. A high stiffness and self-healable polyurethane based on disulfide bonds and hydrogen bonding. Eur. Polym. J. 2020, 124, 109475. [Google Scholar] [CrossRef]
- Herbst, F.; Doehler, D.; Michael, P.; Binder, W.H. Self-Healing Polymers via Supramolecular Forces. Macromol. Rapid Commun. 2013, 34, 203–220. [Google Scholar] [CrossRef]
- Ma, S.; Qi, X.; Cao, Y.; Yang, S.; Xu, J. Hydrogen bond detachment in polymer complexes. Polymer 2013, 54, 5382–5390. [Google Scholar] [CrossRef]
- Wu, Y.; Fei, M.; Chen, T.; Li, C.; Wu, S.; Qiu, R.; Liu, W. Photocuring Three-Dimensional Printing of Thermoplastic Polymers Enabled by Hydrogen Bonds. ACS Appl. Mater. Interfaces 2021, 13, 22946–22954. [Google Scholar] [CrossRef]
- Wu, Y.; Fei, M.; Chen, T.; Li, C.; Fu, T.; Qiu, R.; Liu, W. H-bonds and metal-ligand coordination-enabled manufacture of palm oil-based thermoplastic elastomers by photocuring 3D printing. Addit. Manuf. 2021, 47, 102268. [Google Scholar] [CrossRef]
- Li, R.; Fan, T.; Chen, G.; Xie, H.; Su, B.; He, M. Highly transparent, self-healing conductive elastomers enabled by synergistic hydrogen bonding interactions. Chem. Eng. J. 2020, 393, 124685. [Google Scholar] [CrossRef]
- Lai, C.-W.; Yu, S.-S. 3D Printable Strain Sensors from Deep Eutectic Solvents and Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2020, 12, 34235–34244. [Google Scholar] [CrossRef]
- Cai, L.; Chen, G.; Su, B.; He, M. 3D printing of ultra-tough, self-healing transparent conductive elastomeric sensors. Chem. Eng. J. 2021, 426, 130545. [Google Scholar] [CrossRef]
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a sustainable raw material for green chemistry: A review. Ind. Crops Prod. 2018, 126, 316–332. [Google Scholar] [CrossRef]
- Lim, B.-S.; Lee, Y.-K.; 신현철; 최재윤. The Effect of Weight fraction of Photo Initiator and Inhibitor in the Expreimental Resins on the Dgree of Polymerization. Korean J. Dent. Mater. 2004, 31, 299–306. [Google Scholar]
- Bennett, J. Measuring UV curing parameters of commercial photopolymers used in additive manufacturing. Addit. Manuf. 2017, 18, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kankala, R.K.; Wu, L.; Chen, A.-Z.; Wang, S.-B. 3D-Printed Photocurable Resin with Synergistic Hydrogen Bonding Based on Deep Eutectic Solvent. ACS Appl. Polym. Mater. 2023, 5, 991–1001. [Google Scholar] [CrossRef]
- Zhu, G.; Hou, Y.; Xiang, J.; Xu, J.; Zhao, N. Digital Light Processing 3D Printing of Healable and Recyclable Polymers with Tailorable Mechanical Properties. ACS Appl. Mater. Interfaces 2021, 13, 34954–34961. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Han, S.; Zhu, J.; Chen, A.; Zhang, J.; Yan, Z.; Liu, J.; Huang, J.; Yang, X.; Guan, L. Stretchable and self-healing ionic conductive elastomer for multifunctional 3D printable sensor. Chem. Eng. J. 2023, 454, 140328. [Google Scholar] [CrossRef]
- Invernizzi, M.; Turri, S.; Levi, M.; Suriano, R. Processability of 4D printable modified polycaprolactone with self-healing abilities. In Proceedings of the 1st International Conference on Materials, Mimicking, Manufacturing from and for Bio Application (BioM&M), Milan, Italy, 27–29 June 2019; pp. 508–515. [Google Scholar]
- Suriano, R.; Bernasconi, R.; Magagnin, L.; Levi, M. 4D Printing of Smart Stimuli-Responsive Polymers. J. Electrochem. Soc. 2019, 166, B3274–B3281. [Google Scholar] [CrossRef]
- Durand-Silva, A.; Perera, S.D.; Remy, A.K.; Peng, H.-C.; Hargrove, J.A.; Ferneyhough, Z.D.; Landaverde, P.M.L.; Stelling, A.L.; Smaldone, R.A. Infrared Spectroscopic and Mechanical Analysis of Supramolecular Self-Healing in 3D Printable Urea Photoresins. ACS Appl. Polym. Mater. 2022, 4, 8825–8832. [Google Scholar] [CrossRef]
- Chen, X.; Zawaski, C.E.; Spiering, G.A.; Liu, B.; Orsino, C.M.; Moore, R.B.; Williams, C.B.; Long, T.E. Quadruple Hydrogen Bonding Supramolecular Elastomers for Melt Extrusion Additive Manufacturing. ACS Appl. Mater. Interfaces 2020, 12, 32006–32016. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, Y.; Chen, Y.; Li, C.; Qiu, R.; Liu, W. Photocurable 3D Printing of High Toughness and Self-Healing Hydrogels for Customized Wearable Flexible Sensors. Adv. Funct. Mater. 2021, 31, 2107202. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Yu, J.; Zhu, J.; Hu, Z. Effect on Performance of Reversible Covalent Self-healing Materials of Sacrificial Bonds. J. Donghua Univ. Nat. Sci. Ed. 2018, 44, 45–52. [Google Scholar]
- Flow-Induced Crystallization in Polymer Systems. 1979, p. x+370. Available online: https://sci-hub.yt/10.1016/0022-2860(80)85207-0 (accessed on 24 September 2023).
- Movsisyan, K.A.; Gasparyan, R.A.; Ovsepyan, A.M. Crystallization kinetics of crosslinked polymers. Sov. J. Contemp. Phys. 1990, 25, 51–53. [Google Scholar]
- Zhang, B.; Zhang, W.; Zhang, Z.; Zhang, Y.-F.; Hingorani, H.; Liu, Z.; Liu, J.; Ge, Q. Self-Healing Four-Dimensional Printing with an Ultraviolet Curable Double-Network Shape Memory Polymer System. ACS Appl. Mater. Interfaces 2019, 11, 10328–10336. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Chen, T.; Wang, J.; Tuo, X.; Gong, Y.; Guo, J. Study on the healing performance of poly(epsilon-caprolactone) filled ultraviolet-curable 3D printed cyclic trimethylolpropane formal acrylate shape memory polymers. J. Appl. Polym. Sci. 2022, 139, e53085. [Google Scholar] [CrossRef]
- Abdullah, T.; Okay, O. 4D Printing of Body Temperature-Responsive Hydrogels Based on Poly(acrylic acid) with Shape-Memory and Self-Healing Abilities. ACS Appl. Bio Mater. 2023, 6, 703–711. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, M. Cyclodextrin-based inclusion complexation bridging supramolecular chemistry and macromolecular self-assembly. Chem. Soc. Rev. 2011, 40, 2254–2266. [Google Scholar] [CrossRef]
- An, S.Y.; Noh, S.M.; Oh, J.K. Multiblock Copolymer-Based Dual Dynamic Disulfide and Supramolecular Crosslinked Self-Healing Networks. Macromol. Rapid Commun. 2017, 38, 1600777. [Google Scholar] [CrossRef]
- Cheng, B.; Lu, X.; Zhou, J.; Qin, R.; Yang, Y. Dual Cross-Linked Self-Healing and Recyclable Epoxidized Natural Rubber Based on Multiple Reversible Effects. ACS Sustain. Chem. Eng. 2019, 7, 4443–4455. [Google Scholar] [CrossRef]
- Zhang, Q.; Niu, S.; Wang, L.; Lopez, J.; Chen, S.; Cai, Y.; Du, R.; Liu, Y.; Lai, J.C.; Liu, L.; et al. An Elastic Autonomous Self-Healing Capacitive Sensor Based on a Dynamic Dual Crosslinked Chemical System. Adv. Mater. 2018, 30, 1801435. [Google Scholar] [CrossRef]
- Tolvanen, J.; Nelo, M.; Alasmäki, H.; Siponkoski, T.; Mäkelä, P.; Vahera, T.; Hannu, J.; Juuti, J.; Jantunen, H. Ultraelastic and High-Conductivity Multiphase Conductor with Universally Autonomous Self-Healing. Adv. Sci. 2022, 9, 2205485. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, P.; Wu, J.; Hu, P.; Fu, Y.; Jiang, W.; Fu, J. Notch-Insensitive, Ultrastretchable, Efficient Self-Healing Supramolecular Polymers Constructed from Multiphase Active Hydrogen Bonds for Electronic Applications. Chem. Mater. 2019, 31, 7951–7961. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Sun, Z.; Ren, H.; Wen, X.; Wang, W.; Zhang, T.; Xiao, L.; Zhang, G. Research Progress of Self-Healing Polymer for Ultraviolet-Curing Three-Dimensional Printing. Polymers 2023, 15, 4646. https://doi.org/10.3390/polym15244646
Liu W, Sun Z, Ren H, Wen X, Wang W, Zhang T, Xiao L, Zhang G. Research Progress of Self-Healing Polymer for Ultraviolet-Curing Three-Dimensional Printing. Polymers. 2023; 15(24):4646. https://doi.org/10.3390/polym15244646
Chicago/Turabian StyleLiu, Wenhao, Zhe Sun, Hao Ren, Xiaomu Wen, Wei Wang, Tianfu Zhang, Lei Xiao, and Guangpu Zhang. 2023. "Research Progress of Self-Healing Polymer for Ultraviolet-Curing Three-Dimensional Printing" Polymers 15, no. 24: 4646. https://doi.org/10.3390/polym15244646
APA StyleLiu, W., Sun, Z., Ren, H., Wen, X., Wang, W., Zhang, T., Xiao, L., & Zhang, G. (2023). Research Progress of Self-Healing Polymer for Ultraviolet-Curing Three-Dimensional Printing. Polymers, 15(24), 4646. https://doi.org/10.3390/polym15244646







