Epoxy (Meth)acrylate-Based Thermally and UV Initiated Curable Coating Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Epoxy (Meth)acrylate Resins
2.3. Preparation of Coating Formulations and Cured Films
2.4. Characterization Methods
3. Results
3.1. Synthesis Parameters and Properties of Obtained Epoxy (Meth)acrylates
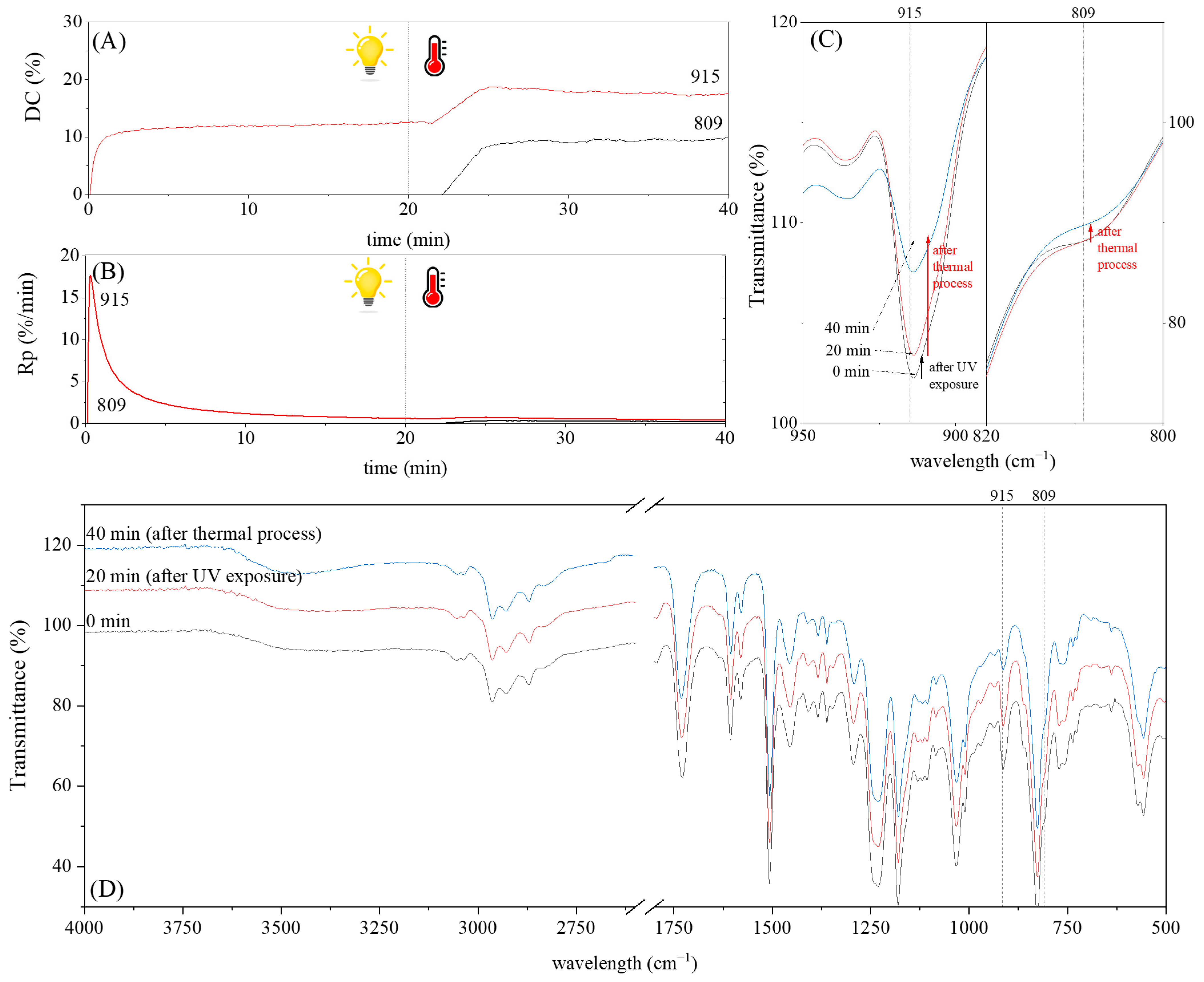
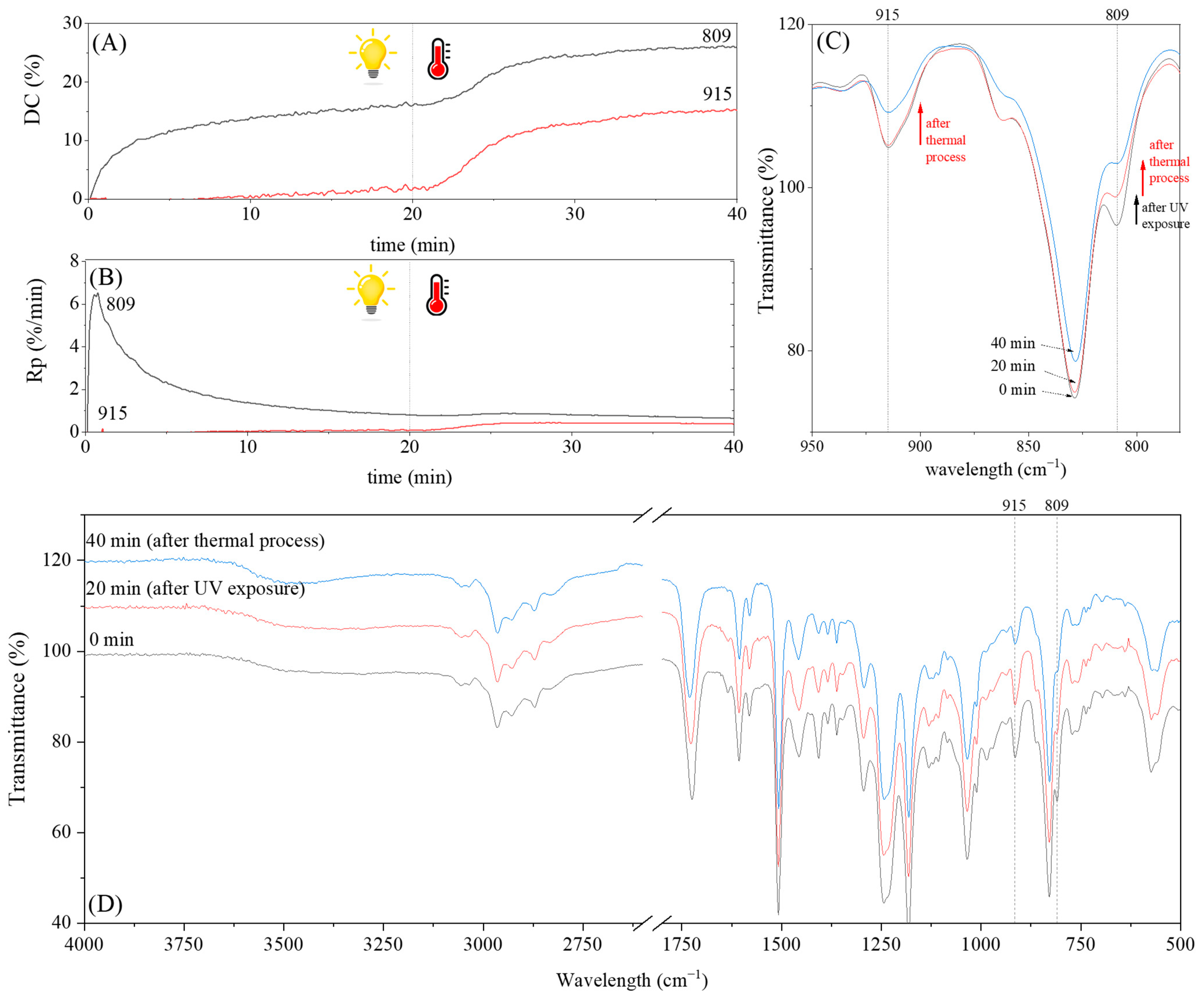
3.2. Characteristics of the Dual-Curing Process of the Epoxy (Meth)acrylates
3.3. The Thermal Properties of the Obtained EAs
3.4. Properties of Cured Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, N.; Xu, Y.; Zhao, Q.; Xie, T. Dynamic Covalent Polymer Networks: A Molecular Platform for Designing Functions beyond Chemical Recycling and Self-Healing. Chem. Rev. 2021, 121, 1716–1745. [Google Scholar] [CrossRef] [PubMed]
- Khudyakov, I.V. Fast Photopolymerization of Acrylate Coatings: Achievements and Problems. Prog. Org. Coat. 2018, 121, 151–159. [Google Scholar] [CrossRef]
- Song, W.; Li, X.; Zhao, Y.; Liu, C.; Xu, J.; Wang, H.; Zhang, T. Functional, UV-Curable Coating for the Capture of Circulating Tumor Cells. Biomater. Sci. 2019, 7, 2383–2393. [Google Scholar] [CrossRef] [PubMed]
- Pierau, L.; Elian, C.; Akimoto, J.; Ito, Y.; Caillol, S.; Versace, D.-L. Bio-Sourced Monomers and Cationic Photopolymerization–The Green Combination towards Eco-Friendly and Non-Toxic Materials. Prog. Polym. Sci. 2022, 127, 101517. [Google Scholar] [CrossRef]
- Balcerak, A.; Kabatc, J.; Czech, Z.; Nowak, M.; Mozelewska, K. High-Performance UV-Vis Light Induces Radical Photopolymerization Using Novel 2-Aminobenzothiazole-Based Photosensitizers. Materials 2021, 14, 7814. [Google Scholar] [CrossRef]
- An, R.; Zhang, X.; Han, L.; Wang, X.; Zhang, Y.; Shi, L.; Ran, R. Healing, Flexible, High Thermal Sensitive Dual-Network Ionic Conductive Hydrogels for 3D Linear Temperature Sensor. Mater. Sci. Eng. C 2020, 107, 110310. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Mozelewska, K.; Nowak, M.; Czech, Z. Photocurable Epoxy Acrylate Coatings Preparation by Dual Cationic and Radical Photocrosslinking. Materials 2021, 14, 4150. [Google Scholar] [CrossRef]
- Fouilloux, H.; Thomas, C.M. Production and Polymerization of Biobased Acrylates and Analogs. Macromol. Rapid Commun. 2021, 42, 2000530. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Patil, R.S.; John, J.; Patil, M. A Comprehensive Outlook of Scope within Exterior Automotive Plastic Substrates and Its Coatings. Coatings 2023, 13, 1569. [Google Scholar] [CrossRef]
- Sangermano, M. Advances in Cationic Photopolymerization. Pure Appl. Chem. 2012, 84, 2089–2101. [Google Scholar] [CrossRef]
- Sangermano, M.; Roppolo, I.; Chiappone, A. New Horizons in Cationic Photopolymerization. Polymers 2018, 10, 136. [Google Scholar] [CrossRef]
- Malik, M.S.; Schlögl, S.; Wolfahrt, M.; Sangermano, M. Review on UV-Induced Cationic Frontal Polymerization of Epoxy Monomers. Polymers 2020, 12, 2146. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Meng, X.; Li, Z.; Li, M.; Jin, M.; Liu, R.; Yagci, Y. Chemiluminescence Induced Cationic Photopolymerization Using Sulfonium Salt. ACS Macro Lett. 2020, 9, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Klikovits, N.; Knaack, P.; Bomze, D.; Krossing, I.; Liska, R. Novel Photoacid Generators for Cationic Photopolymerization. Polym. Chem. 2017, 8, 4414–4421. [Google Scholar] [CrossRef]
- Dietliker, K.; Hüsler, R.; Birbaum, J.-L.; Ilg, S.; Villeneuve, S.; Studer, K.; Jung, T.; Benkhoff, J.; Kura, H.; Matsumoto, A.; et al. Advancements in Photoinitiators—Opening up New Applications for Radiation Curing. Prog. Org. Coat. 2007, 58, 146–157. [Google Scholar] [CrossRef]
- Topa, M.; Ortyl, J. Moving Towards a Finer Way of Light-Cured Resin-Based Restorative Dental Materials: Recent Advances in Photoinitiating Systems Based on Iodonium Salts. Materials 2020, 13, 4093. [Google Scholar] [CrossRef]
- Decker, C.; Masson, F.; Schwalm, R. Dual-Curing of Waterborne Urethane-Acrylate Coatings by UV and Thermal Processing. Macromol. Mater. Eng. 2003, 288, 17–28. [Google Scholar] [CrossRef]
- Zhou, C.; Lu, X.; Xin, Z.; Liu, J.; Zhang, Y. Hydrophobic Benzoxazine-Cured Epoxy Coatings for Corrosion Protection. Prog. Org. Coat. 2013, 76, 1178–1183. [Google Scholar] [CrossRef]
- Czachor-Jadacka, D.; Pilch-Pitera, B. Progress in Development of UV Curable Powder Coatings. Prog. Org. Coat. 2021, 158, 106355. [Google Scholar] [CrossRef]
- Tran, A.D.; Koch, T.; Knaack, P.; Liska, R. Radical Induced Cationic Frontal Polymerization for Preparation of Epoxy Composites. Compos. Part A Appl. Sci. Manuf. 2020, 132, 105855. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Irska, I.; Gziut, K.; Ossowicz-Rupniewska, P. Novel Multifunctional Epoxy (Meth)Acrylate Resins and Coatings Preparation via Cationic and Free-Radical Photopolymerization. Polymers 2021, 13, 1718. [Google Scholar] [CrossRef]
- Vidil, T.; Tournilhac, F.; Musso, S.; Robisson, A.; Leibler, L. Control of Reactions and Network Structures of Epoxy Thermosets. Prog. Polym. Sci. 2016, 62, 126–179. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Panda, S.S.; Raju, K.V.S.N. Thermal and Mechanical Properties of Epoxy Acrylate/Methacrylates UV Cured Coatings. Prog. Org. Coat. 2005, 54, 10–19. [Google Scholar] [CrossRef]
- Jaswal, S.; Gaur, B. New Trends in Vinyl Ester Resins. Rev. Chem. Eng. 2014, 30, 567–581. [Google Scholar] [CrossRef]
- Yadav, S.K.; Schmalbach, K.M.; Kinaci, E.; Stanzione, J.F.; Palmese, G.R. Recent Advances in Plant-Based Vinyl Ester Resins and Reactive Diluents. Eur. Polym. J. 2018, 98, 199–215. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Irska, I.; Gziut, K.; Ossowicz-Rupniewska, P. Synthesis of Epoxy Methacrylate Resin and Coatings Preparation by Cationic and Radical Photocrosslinking. Molecules 2021, 26, 7663. [Google Scholar] [CrossRef]
- Tosto, C.; Pergolizzi, E.; Blanco, I.; Patti, A.; Holt, P.; Karmel, S.; Cicala, G. Epoxy Based Blends for Additive Manufacturing by Liquid Crystal Display (LCD) Printing: The Effect of Blending and Dual Curing on Daylight Curable Resins. Polymers 2020, 12, 1594. [Google Scholar] [CrossRef] [PubMed]
- Ligon, S.K.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Štaffová, M.; Ondreáš, F.; Svatík, J.; Zbončák, M.; Jančář, J.; Petr, L. 3D printing and post-curing optimization of photopolymerized structures: Basic concepts and effective tools for improved thermomechanical properties. Polym. Test. 2022, 108, 107499. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Irska, I.; Gziut, K.; Mozelewska, K.; Ossowicz-Rupniewska, P. Synthesis of Hybrid Epoxy Methacrylate Resin Based on Diglycidyl Ethers and Coatings Preparation via Cationic and Free-Radical Photopolymerization. Int. J. Mol. Sci. 2022, 23, 15592. [Google Scholar] [CrossRef]
- González, M.G.; Cabanelas, J.C.; Baselga, J. Applications of FTIR on Epoxy Resins—Identification, Monitoring the Curing Process, Phase Separation and Water Uptake. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Theophanides, T., Ed.; InTech: Orlando, FL, USA, 2012; ISBN 978-953-51-0537-4. [Google Scholar]
- Cañavate, J.; Colom, X.; Pagès, P.; Carrasco, F. Study of the curing process of an epoxy resin by FTIR spectroscopy. Polym.-Plast. Technol. Eng. 2000, 39, 937–943. [Google Scholar] [CrossRef]
- Sahmetlioglu, E.; Mart, H.; Yuruk, H.; Surme, Y. Synthesis and Characterization of Oligosalicylaldehyde-Based Epoxy Resins. Chem. Pap. 2006, 60, 65–68. [Google Scholar] [CrossRef]
- Feng, L.; Yang, H.; Dong, X.; Lei, H.; Chen, D. PH-sensitive Polymeric Particles as Smart Carriers for Rebar Inhibitors Delivery in Alkaline Condition. J. Appl. Polym. Sci. 2018, 135, 45886. [Google Scholar] [CrossRef]
- Sani Mamman, I.; Teo, Y.Y.; Misran, M. Synthesis, Characterization and Rheological Study of Arabic Gum-Grafted-Poly (Methacrylic Acid) Hydrogels. Polym. Bull. 2021, 78, 3399–3423. [Google Scholar] [CrossRef]
- Available online: https://www.ulprospector.com/En/La/Coatings/Detail/107930/80142/EBECRYL-860 (accessed on 1 September 2023).
- Goiti, E.; Huglin, M.B.; Rego, J.M. Some Observations on the Copolymerization of Styrene with Furfuryl Methacrylate. Polymer 2001, 42, 10187–10193. [Google Scholar] [CrossRef]
- Danso, R.; Hoedebecke, B.; Whang, K.; Sarrami, S.; Johnston, A.; Flipse, S.; Wong, N.; Rawls, H.R. Development of an Oxirane/Acrylate Interpenetrating Polymer Network (IPN) Resin System. Dent. Mater. 2018, 34, 1459–1465. [Google Scholar] [CrossRef]

| Resin | Chemical Structure |
|---|---|
| Epidian 6® (Ep6) |  |
| Bisphenol A glycidyl ether (DGEBA) | 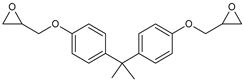 |
| Cyclohexanedimethanol diglycidyl ether (CHDMDE) | 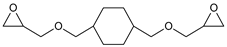 |
| Neopentyl glycol diglycidyl ether (NPDE) | 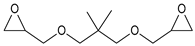 |
| AA | MAA | |
|---|---|---|
| Ep6 | 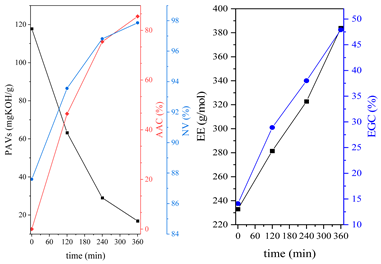 |  |
| DGEBA | 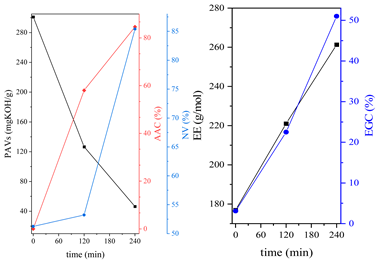 | 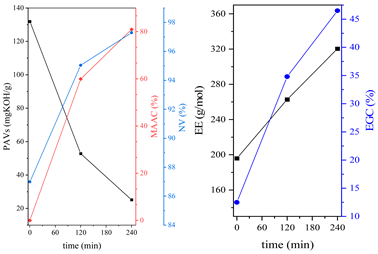 |
| NPDE |  | 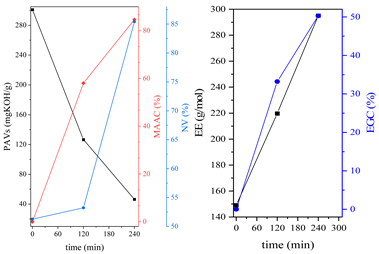 |
| CHDMDE | 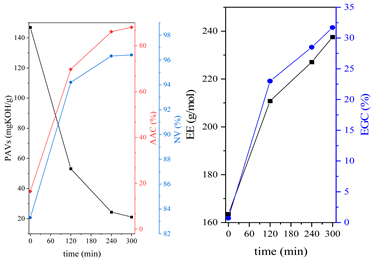 | 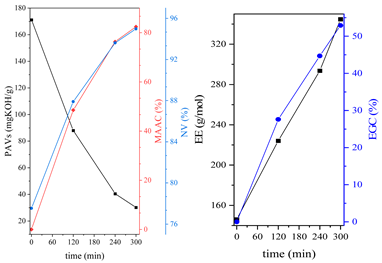 |
| AA | MAA | |
|---|---|---|
| Ep6 |  | 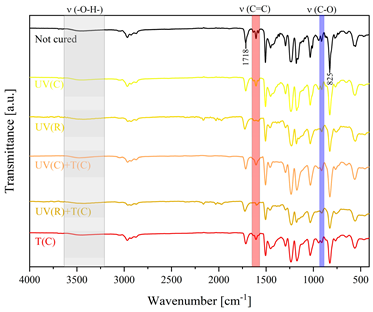 |
| DGEBA | 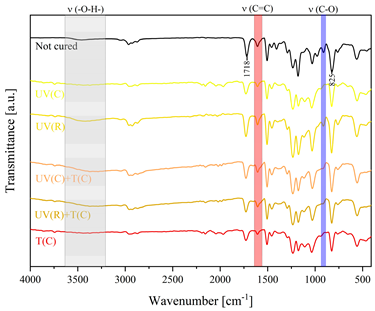 | 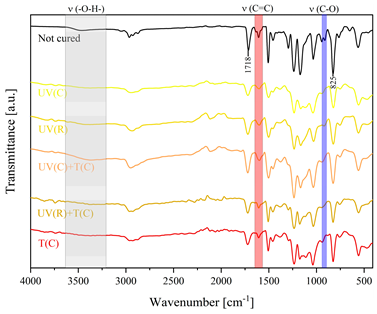 |
| NPDE |  | 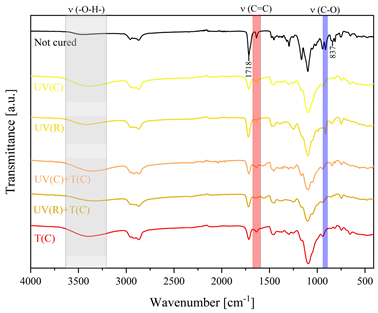 |
| CHDMDE |  | 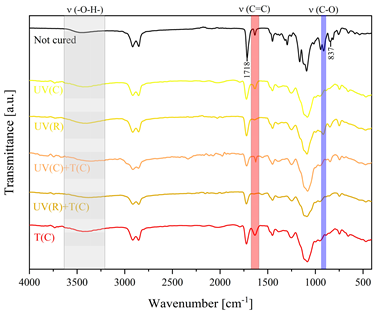 |
| Sample | No Cure | UV(C) | UV(R) | T(C) | UV(C) + T(C) | UV(R) + T(C) |
|---|---|---|---|---|---|---|
| Ep6-MMA | −12 | 58 | 37 | 53 | 63 | 62 |
| Ep6-AA | −17 | 59 | 19 | 52 | 57 | 56 |
| DGEBA-MAA | −15 | 53 | 39 | 41 | 60 | 58 |
| DGEBA-AA | −13 | 60 | 28 | 52 | 56 | 57 |
| NDPE MAA | −39 | 42 | 30 | - | 56 | 53 |
| NDPE AA | −45 | 11 | 9 | 10 | 19 | 21 |
| CHDMDE-MAA | −48 | 31 | 14 | - | 31 | 46 |
| CHDMDE-AA | −43 | 21 | 2 | 3 | 18 | 25 |
| Sample | Hardness | Scratchability (N) | Adhesion | Gloss (GU) | ||||
|---|---|---|---|---|---|---|---|---|
| UV curing | UV(C) | UV(R) | UV(C) | UV(R) | UV(C) | UV(R) | UV(C) | UV(R) |
| Ep6-MMA | 299 ± 3 | 117 ± 2 | 1 ± 0.5 | 1 ± 0.25 | 5 | 1 | 444 ± 6 | 168 ± 5 |
| Ep6-AA | 303 ± 3 | 58 ± 2 | 1 ± 0.5 | 0.2 ± 0.25 | 4 | 0 | 467 ± 5 | 113 ± 2 |
| DGEBA-MAA | 300 ± 3 | 138 ± 2 | 1 ± 0.25 | 1.5 ± 0.25 | 5 | 1 | 400 ± 5 | 145 ± 3 |
| DGEBA-AA | 278 ± 3 | 90 ± 2 | 1 ± 0.25 | 1 ± 0.25 | 5 | 1 | 450 ± 5 | 139 ± 5 |
| NDPE-MAA | 51 ± 2 | 42 ± 1 | 0.5 ± 0.25 | 0.2 ± 0.25 | 0 | 1 | 80 ± 2 | 144 ± 2 |
| NDPE AA | 26 ± 1 | 78 ± 2 | 0.5 ± 0.25 | 0.2 ± 0.25 | 0 | 1 | 18 ± 5 | 124 ± 2 |
| CHDMDE-MAA | 103 ± 2 | 43 ± 1 | 0.5 ± 0.25 | 1.5 ± 0.25 | 3 | 1 | 9 ± 3 | 122 ± 2 |
| CHDMDE-AA | 122 ± 2 | 64 ± 2 | 1.5 ± 0.25 | 0.5 ± 0.25 | 1 | 0 | 102 ± 2 | 122 ± 2 |
| Thermal curing | T(C) | T(R) | T(C) | T(R) | T(C) | T(R) | T(C) | T(R) |
| Ep6-MMA | 351 ± 3 | - | 1 ± 0.25 | - | 1 | - | 156 ± 3 | - |
| Ep6-AA | 359 ± 3 | - | 1 ± 0.25 | - | 1 | - | 205 ± 5 | - |
| DGEBA-MAA | 320 ± 3 | - | 1.5 ± 0.25 | - | 1.5 | - | 110 ± 3 | - |
| DGEBA-AA | 352 ± 3 | - | 1 ± 0.25 | - | 1 | - | 210 ± 3 | - |
| NDPE MAA | - | - | - | - | - | - | - | - |
| NDPE AA | 42 ± 2 | - | 0.5 ± 0.25 | - | 0.5 | - | 200 ± 3 | - |
| CHDMDE-MAA | - | - | - | - | - | - | - | - |
| CHDMDE-AA | 46 ± 2 | - | 1 ± 0.25 | - | 1 | - | 300 ± 3 | - |
| Dual curing | UV(C) + T(C) | UV(R) + T(C) | UV(C) + T(C) | UV(R) + T(C) | UV(C) + T(C) | UV(R) + T(C) | UV(C) + T(C) | UV(R) + T(C) |
| Ep6-MMA | 226 ± 3 | 266 ± 3 | 2.5 ± 0.5 | 2.5 ± 0.25 | 3 | 5 | 185 ± 4 | 208 ± 3 |
| Ep6-AA | 231 ± 3 | 277 ± 3 | 1.5 ± 0.25 | 1.5 ± 0.25 | 3 | 3 | 195 ± 3 | 211 ± 2 |
| DGEBA-MAA | 250 ± 3 | 334 ± 4 | 2.5 ± 0.5 | 5 ± 0.5 | 3 | 5 | 144 ± 3 | 145 ± 1 |
| DGEBA-AA | 238 ± 3 | 186 ± 2 | 2.5 ± 0.5 | 3.5 ± 0.5 | 0 | 0 | 193 ± 3 | 185 ± 4 |
| NDPE MAA | 219 ± 2 | 78 ± 2 | 6 ± 0.5 | 1.5 ± 0.25 | 3 | 3 | 103 ± 2 | 95 ± 2 |
| NDPE AA | 65 ± 2 | 31 ± 2 | 2.5 ± 0.25 | 1 ± 0.25 | 0 | 0 | 128 ± 3 | 173 ± 3 |
| CHDMDE-MAA | 88 ± 2 | 102 ± 3 | 4 ± 0.5 | 3.5 ± 0.25 | 4 | 4 | 100 ± 2 | 102 ± 2 |
| CHDMDE-AA | 50 ± 2 | 30 ± 1 | 2.5 ± 0.25 | 1.5 ± 0.25 | 0 | 0 | 105 ± 2 | 176 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarczyk, P.; Walkowiak, K.; Irska, I. Epoxy (Meth)acrylate-Based Thermally and UV Initiated Curable Coating Systems. Polymers 2023, 15, 4664. https://doi.org/10.3390/polym15244664
Bednarczyk P, Walkowiak K, Irska I. Epoxy (Meth)acrylate-Based Thermally and UV Initiated Curable Coating Systems. Polymers. 2023; 15(24):4664. https://doi.org/10.3390/polym15244664
Chicago/Turabian StyleBednarczyk, Paulina, Konrad Walkowiak, and Izabela Irska. 2023. "Epoxy (Meth)acrylate-Based Thermally and UV Initiated Curable Coating Systems" Polymers 15, no. 24: 4664. https://doi.org/10.3390/polym15244664
APA StyleBednarczyk, P., Walkowiak, K., & Irska, I. (2023). Epoxy (Meth)acrylate-Based Thermally and UV Initiated Curable Coating Systems. Polymers, 15(24), 4664. https://doi.org/10.3390/polym15244664








