Investigation of Crystallization, Morphology, and Mechanical Properties of Polypropylene/Polypropylene-Polyethylene Block Copolymer Blends
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Materials
2.2. Thermal Analysis (DSC)
2.3. Synchrotron Wide-Angle X-ray Diffraction (WAXD) Measurements
2.4. Small Angle X-ray Scattering (SAXS)
2.5. Mechanical Scattering
2.5.1. Tensile Testing
2.5.2. Impact Test
2.6. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Non-Isothermal Crystallization Kinetics and Thermodynamic Behavior
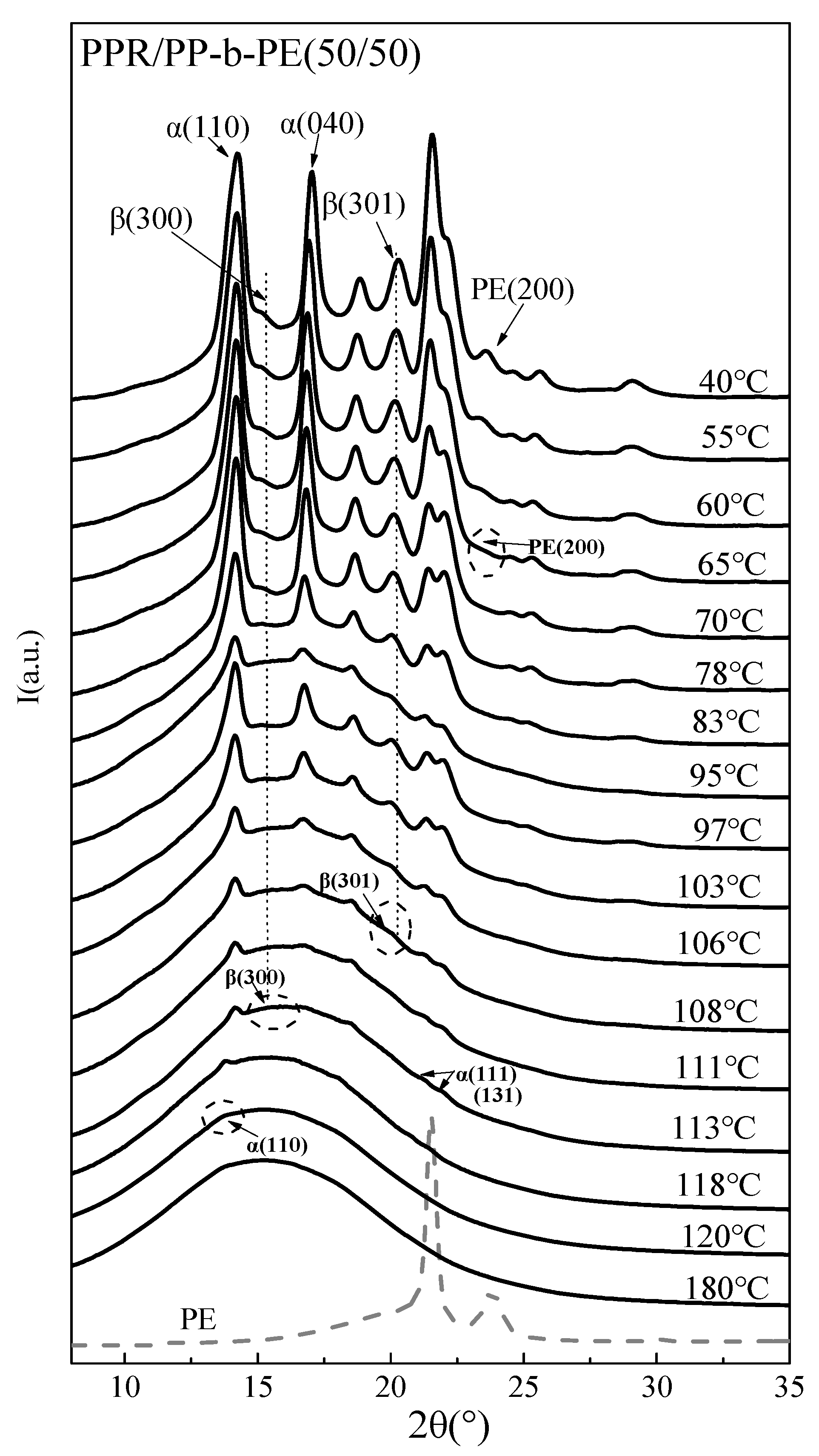
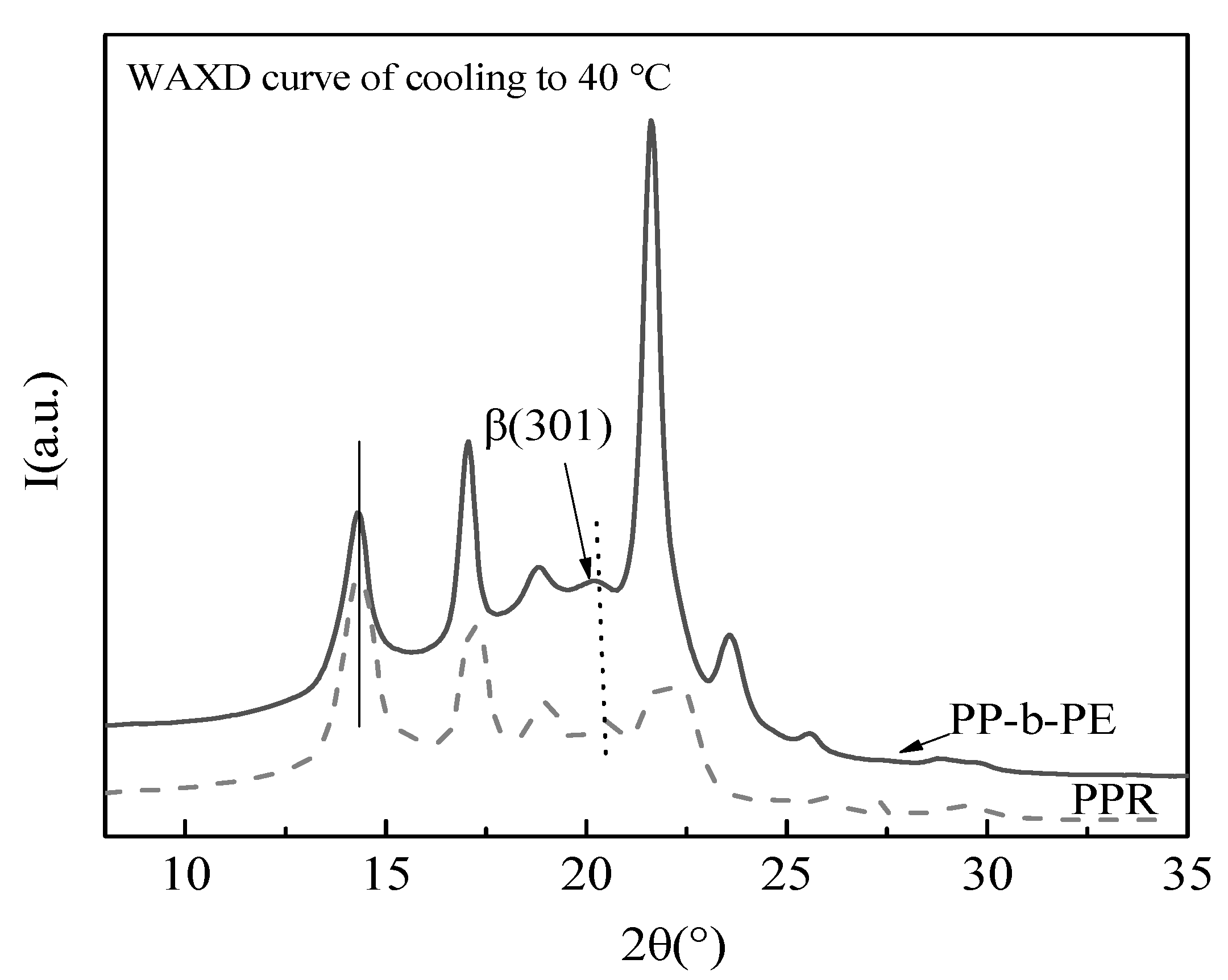
3.2. In Situ Non-Isothermal Crystal Structure Change
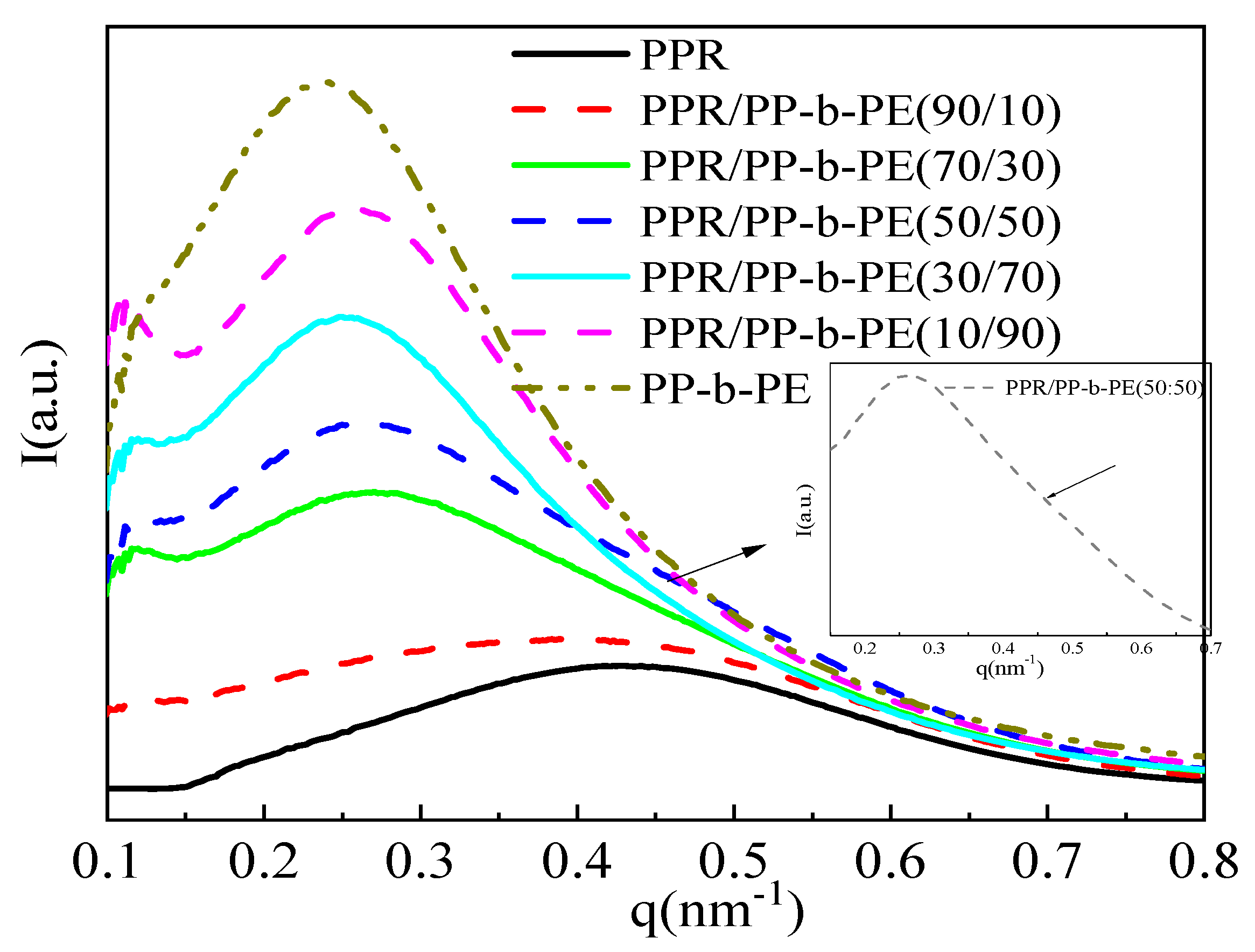
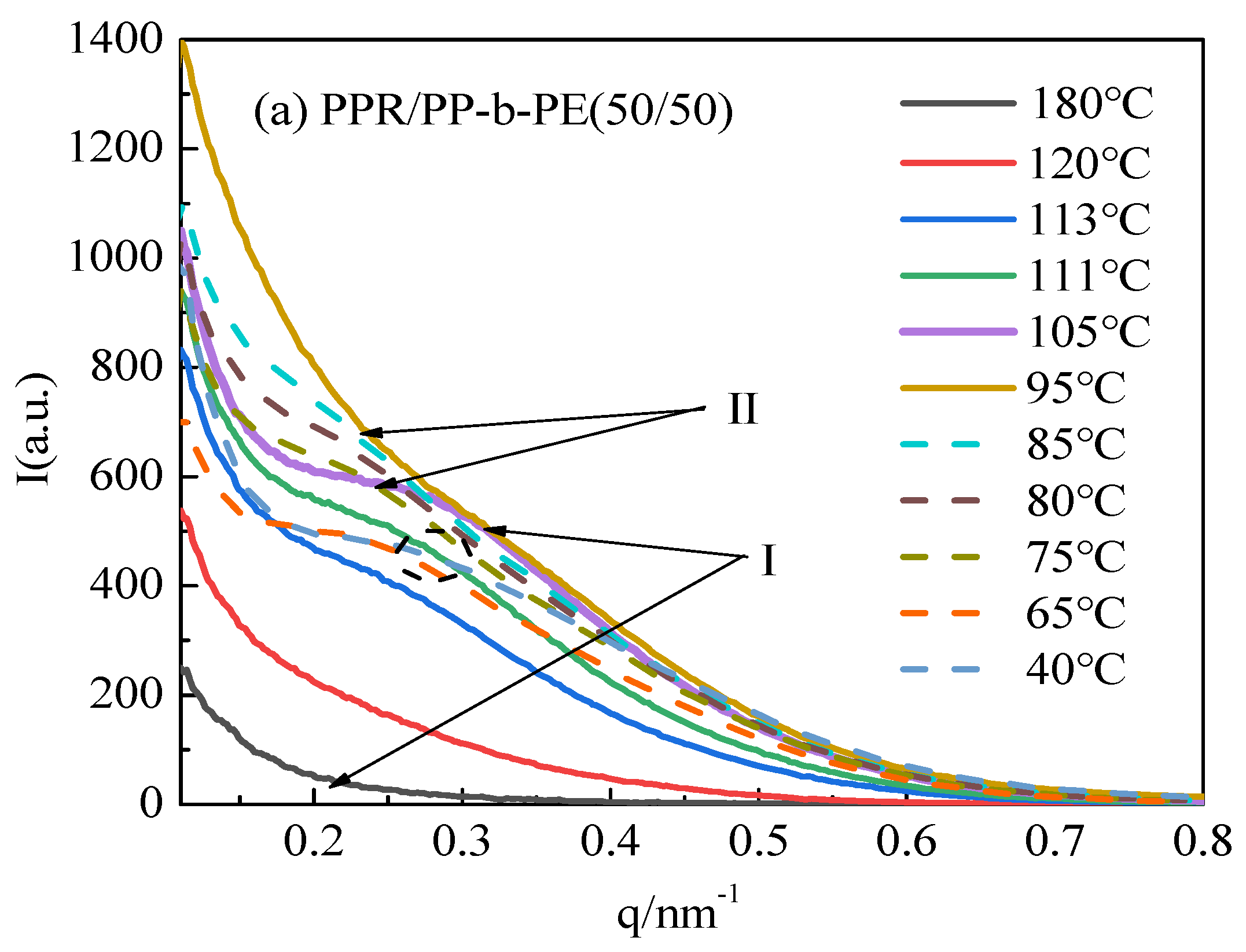
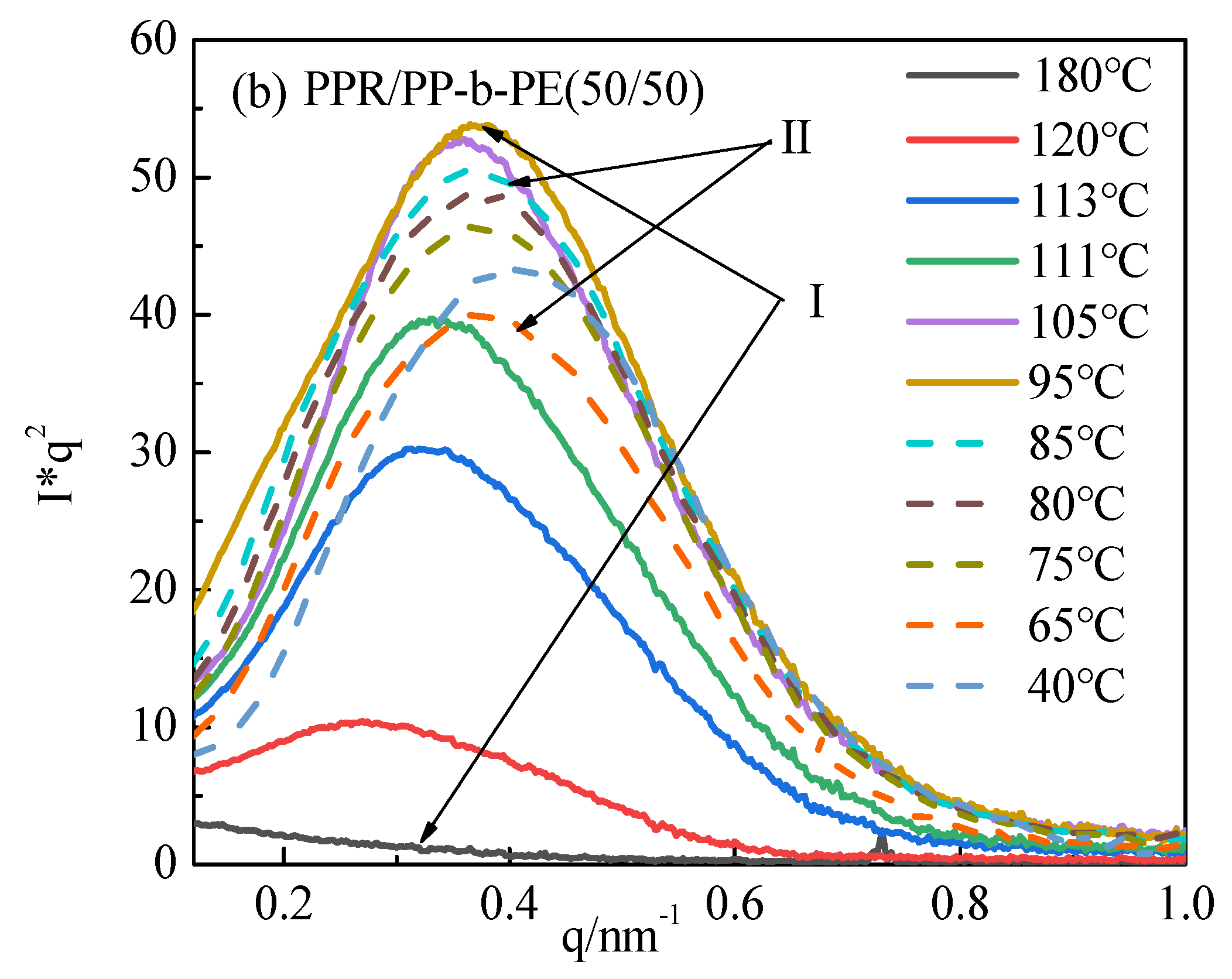
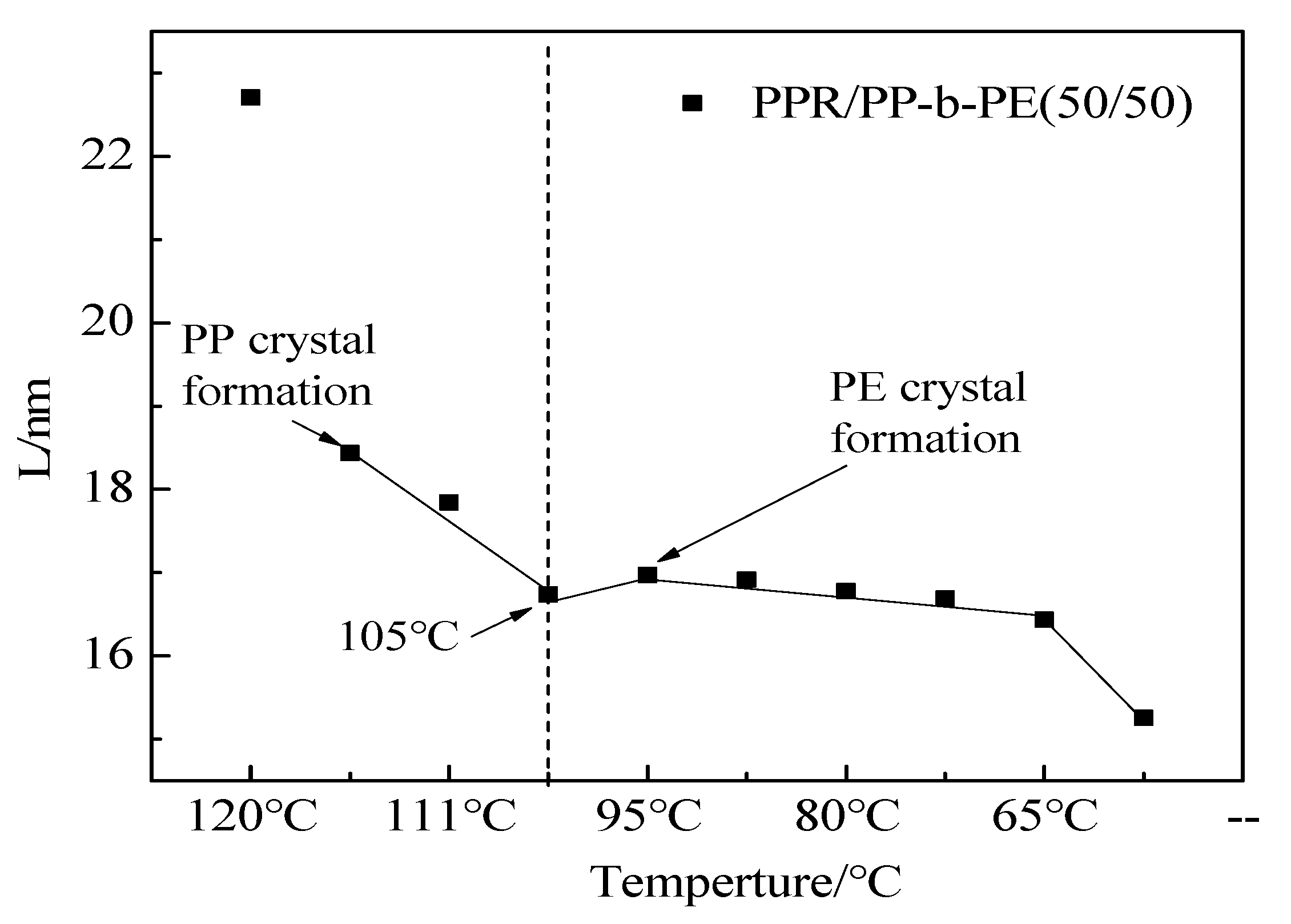
3.3. Microcrystals and Arrangement Structure of Different Blending Systems
3.4. Mechanical Behavior
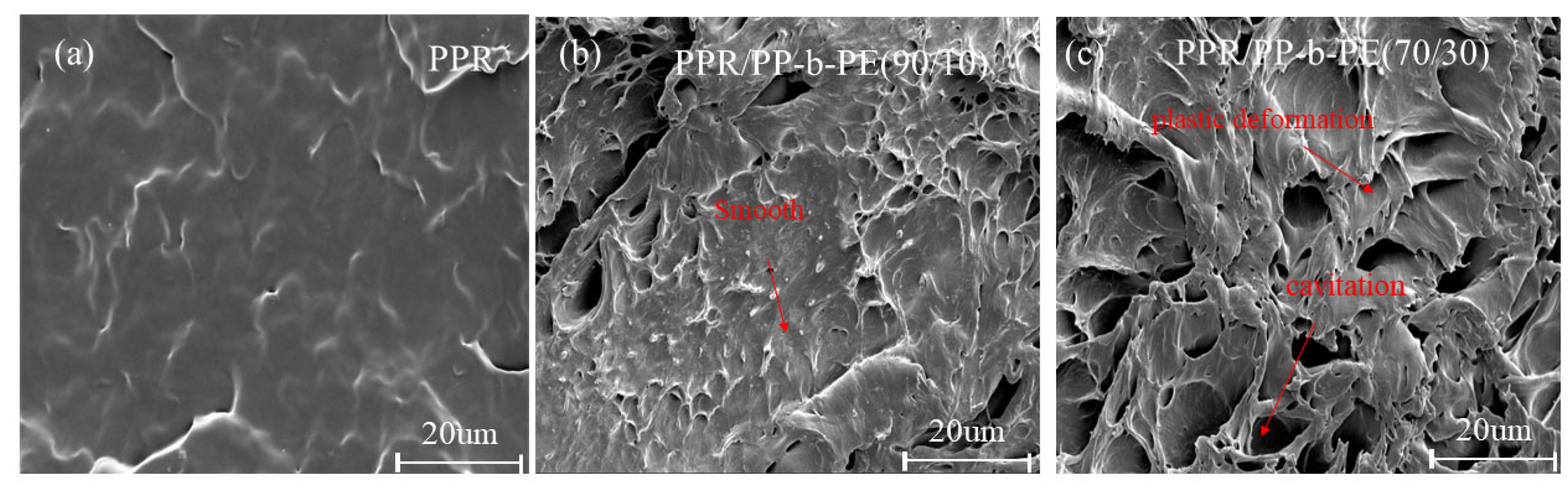
3.5. SEM
4. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nedkov, T.; Lednický, F. Morphologies of polyethylene-ethylene/propylene/diene monomer particles in polypropylene-rich polyolefin blends: Flake structure. J. Appl. Polym. Sci. 2010, 90, 3087–3092. [Google Scholar] [CrossRef]
- Nedkov, T.; Lednicky, F.; Mihailova, M. Compatibilization of PP/PE blends and scraps with Royalene: Mechanical properties, SAXS, and WAXS. J. Appl. Polym. Sci. 2010, 109, 226–233. [Google Scholar] [CrossRef]
- Heino, M.; Kirjava, J.; Hietaoja, P. Compatibilization of polyethylene terephthalate/polypropylene blends with styrene-ethylene/butylene-styrene (SEBS) block copolymers. J. Appl. Polym. Sci. 2015, 65, 241–249. [Google Scholar] [CrossRef]
- Nakamura, S.; Tokumitsu, K.; Kitamura, M.; Miyagawa, E.; Tanaka, A. A Study for Improvement of Mechanical Properties of Recycle PE/PP Blends. Resour. Process. 2007, 54, 167–174. [Google Scholar] [CrossRef][Green Version]
- Wu, Y.; Ge, Q.; Yang, F.; Wu, T.; Xiang, M. Improving the low-temperature toughness of PPR pipe by compounding with PERT. J. Polym. Res. 2021, 28, 143. [Google Scholar] [CrossRef]
- Kim, B.C.; Hwang, S.S.; Lim, K.Y.; Yoon, K.J. Toughening of PP/EPDM blend by compatibilization. J. Appl. Polym. Sci. 2000, 78, 1267–1274. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, F.; Zhou, N.; Zhang, H. Compatibilization by Olefin Block Copolymer (OBC) in Polypropylene/Ethylene-Propylene-Diene Terpolymer (PP/EPDM) Blends. J. Macromol. Sci. B 2015, 54, 159–176. [Google Scholar] [CrossRef]
- Ubonnut, L.; Thongyai, S.; Praserthdam, P. Interfacial adhesion enhancement of polyethylene–polypropylene mixtures by adding synthesized diisocyanate compatibilizers. J. Appl. Polym. Sci. 2010, 104, 3766–3773. [Google Scholar] [CrossRef]
- Silva-Vela, A.; Roudet, F.; Calderón, N.; Huanca-Zuñiga, P.; Tupayachy-Quispe, D.; Almirón, J. Study of the Mechanical Properties of Polymer Composites Based on Polyolefins with the Addition of Rice Husk and Compatibilizer. Mater. Sci. Forum 2022, 1053, 9–15. [Google Scholar] [CrossRef]
- Penava, N.V.; Rek, V.; Houra, I.F. Effect of EPDM as a compatibilizer on mechanical properties and morphology of PP/LDPE blends. J. Elastomers Plast. 2012, 45, 391–403. [Google Scholar] [CrossRef]
- Kotlar, H.K.; Gustafson, C.-G.; Børve, K.L. Polypropylene-phenol formaldehyde-based compatibilizers. II. Application in PP/PA6 75/25 (wt/wt) blends. J. Appl. Polym. Sci. 2000, 75, 355–360. [Google Scholar]
- Zhang, Y.; He, J.; Liu, F. Synthesis of novel polycarbonate-based thermoplastic polyurethane elastomers compatibilizers with octadecyl side chains and their application in PC/PP blends. Polym. Adv. Technol. 2021, 32, 3070–3081. [Google Scholar] [CrossRef]
- Lee, B.Y.; Lee, D.H.; Jang, K.S. Impact Modifiers and Compatibilizers for Versatile Epoxy-Based Adhesive Films with Curing and Deoxidizing Capabilities. Polymers 2021, 13, 1129. [Google Scholar] [CrossRef] [PubMed]
- Marić, M.; Macosko, C.W. Block copolymer compatibilizers for polystyrene/poly(dimethylsiloxane) blends. J. Polym. Sci. Polym. Phys. 2002, 40, 346–357. [Google Scholar] [CrossRef]
- Patel, R.; Park, J.T.; Hong, H.P.; Kim, J.H.; Min, B.R. Use of block copolymer as compatibilizer in polyimide/zeolite composite membranes. Polym. Adv. Technol. 2011, 22, 768–772. [Google Scholar] [CrossRef]
- Auriemma, F.; De Rosa, C.; Scoti, M.; Di Girolamo, R.; Malafronte, A.; D’alterio, M.C.; Boggioni, L.; Losio, S.; Boccia, A.C.; Tritto, I. Structure and Mechanical Properties of Ethylene/1-Octene Multiblock Copolymers from Chain Shuttling Technology. Macromolecules 2019, 52, 2669–2680. [Google Scholar] [CrossRef]
- Saito, M.; Ito, K.; Yokoyama, H. Mechanical Properties of Ultrathin Polystyrene-b-Polybutadiene-b-Polystyrene Block Copolymer Films: Film Thickness-Dependent Young’s Modulus. Macromolecules 2021, 54, 8538–8547. [Google Scholar] [CrossRef]
- Karaagac, E.; Koch, T.; Archodoulaki, V.-M. Choosing an Effective Compatibilizer for a Virgin HDPE Rich-HDPE/PP Model Blend. Polymers 2021, 13, 3567. [Google Scholar] [CrossRef]
- Wang, P.; Gao, S.; Chen, X.; Yang, L.; Cao, T.; Fan, B.; Liu, J.; Hu, X. Effect of PCL-b-PEG Oligomer Containing Ionic Elements on Phase Interfacial Properties and Aggregated Structure of PLA/PCL Blends. Macromol. Res. 2022, 30, 438–445. [Google Scholar] [CrossRef]
- Ma, Y.; He, A.; Liu, C. Crystallization kinetics, crystalline structures and properties of PB/PP blends regulated by poly(butene-block-propylene) copolymers. Polymer 2021, 228, 123901. [Google Scholar] [CrossRef]
- Sun, X.; Kharbas, H.; Peng, J.; Turng, L.-S. A novel method of producing lightweight microcellular injection molded parts with improved ductility and toughness. Polymer 2015, 56, 102–110. [Google Scholar] [CrossRef]
- Fortelný, I.; Michálková, D.; Kruliš, Z. An efficient method of material recycling of municipal plastic waste-ScienceDirect. Polym. Degrad. Stab. 2004, 85, 975–979. [Google Scholar] [CrossRef]
- Yang, F.; Pan, L.; Du, H.Z.; Ma, Z.; Li, Y.S. Effect of Olefin-based Compatibilizers on the Formation of continuous Structure in Immiscible HDPE/iPP Blends. Chin. J. Polym. Sci. 2020, 38, 1248–1257. [Google Scholar] [CrossRef]
- Eagan, J.M.; Xu, J.; Di Girolamo, R.; Thurber, C.M.; Macosko, C.W.; LaPointe, A.M.; Bates, F.S.; Coates, G.W. Combining polyethylene and polypropylene: Enhanced performance with PE/iPP multiblock polymers. Science 2017, 355, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yakovleva, V.; Chen, H.; Hiltner, A.; Baer, E. Comparison of olefin copolymers as compatibilizers for polypropylene and high-density polyethylene. J. Appl. Polym. Sci. 2010, 113, 1945–1952. [Google Scholar] [CrossRef]
- Souza, F.G., Jr.; Soares, B.G.; Manjunath, A.; Somashekar, R. Blends of styrene-butadiene-styrene tri-block copolymer/polyaniline-characterization by SAXS. Polymer 2006, 476, 240–247. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Tian, F.; Li, X.H.; Qiu, H.B.; Wang, J. Crystallization and Phase Behavior in Block Copolymer Solution: An in Situ Small Angle X-ray Scattering Study. Chin. J. Polym. Sci. 2019, 37, 1162–1168. [Google Scholar] [CrossRef]
- Li, S.; Lv, Y.; Sheng, J.; Tian, H.; Ning, N.; Zhang, L.; Wu, H.; Tian, M. Morphology development of POE/PP thermoplastic vulcanizates (TPVs) during dynamic vulcanization. Eur. Polym. J. 2017, 96, 590–601. [Google Scholar] [CrossRef]
- Jordan, A.M.; Kim, K.; Soetrisno, D.; Hannah, J.; Bates, F.S.; Jaffer, S.A.; Lhost, O.; Macosko, C.W. Role of Crystallization on Polyolefin Interfaces: An Improved Outlook for Polyolefin Blends. Macromolecules 2018, 51, 2506–2516. [Google Scholar] [CrossRef]
- Xu, J.; Eagan, J.M.; Kim, S.-S.; Pan, S.; Lee, B.; Klimovica, K.; Jin, K.; Lin, T.-W.; Howard, M.J.; Ellison, C.J.; et al. Compatibilization of Isotactic Polypropylene (iPP) and High-Density Polyethylene (HDPE) with iPP–PE Multiblock Copolymers. Macromolecules 2018, 51, 8585–8596. [Google Scholar] [CrossRef]
- Unger, T.; Klocke, L.; Herrington, K.; Miethlinger, J. Investigation of the rheological and mechanical behavior of Polypropylene/ultra-high molecular weight polyethylene compounds related to new online process control. Polym. Test. 2020, 86, 36–39. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, Q.; Wang, L.; Yi, J.; Feng, J. Synergistic Toughening Effect of Olefin Block Copolymer and Highly Effective β-Nucleating Agent on the Low-Temperature Toughness of Polypropylene Random Copolymer. Ind. Eng. Chem. Res. 2017, 56, 5277–5283. [Google Scholar] [CrossRef]
- Lin, Y.; Marchand, G.R.; Hiltner, A.; Baer, E. Adhesion of olefin block copolymers to polypropylene and high density polyethylene and their effectiveness as compatibilizers in blends. Polymer 2011, 52, 1635–1644. [Google Scholar] [CrossRef]
- Ren, Q.; Fan, J.; Zhang, Q.; Yi, J.; Feng, J. Toughened polypropylene random copolymer with olefin block copolymer. Mater. Des. 2016, 107, 295–301. [Google Scholar] [CrossRef]
- Karaagac, E.; Koch, T.; Archodoulaki, V.M. The effect of PP contamination in recycled high-density polyethylene (rPE-HD) from post-consumer bottle waste and their compatibilization with olefin block copolymer (OBC). Waste Manag. 2020, 119, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Cheng, J.; Wang, Y.; Liu, L.-Z.; Wang, Y.; Shi, Y. Crystallization, structure and properties of PP-b-PE block copolymer. Polymer 2023, 284, 126310. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J. Characterization of maleic anhydride/styrene melt-grafted random copolypropylene and its impact on crystallization and mechanical properties of isotactic polypropylene. Polym. Bull. 2019, 76, 4369–4387. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Shao, W.; Ren, Y.; Dong, W.; Zhang, F.; Liu, L.-Z. Crystallization, Structures, and Properties of Different Polyolefins with Similar Grafting Degree of Maleic Anhydride. Polymers 2020, 12, 675. [Google Scholar] [CrossRef]

| Sample | PPR | PP-b-PE | |
|---|---|---|---|
| 1 | PPR | 100 | 0 |
| 2 | PPR/PP-b-PE (90/10) | 90 | 10 |
| 3 | PPR/PP-b-PE (70/30) | 70 | 30 |
| 4 | PPR/PP-b-PE (50/50) | 50 | 50 |
| 5 | PPR/PP-b-PE (30/70) | 30 | 70 |
| 6 | PPR/PP-b-PE (10/90) | 10 | 90 |
| 7 | PP-b-PE | 0 | 100 |
| Sample | Tc/°C | Tm (PE)/°C | Tm (PP)/°C | Tg/°C |
|---|---|---|---|---|
| PP-b-PE | 86.9 | 108.5 | −17.5 | |
| PPR/PP-b-PE (10/90) | 90.6 | 109.3 | 136.5 | −17.5 |
| PPR/PP-b-PE (30/70) | 93.9 | 109.1 | 140.0 | −17.4 |
| PPR/PP-b-PE (50/50) | 99.6 | 109.1 | 142.8 | −15.7 |
| PPR/PP-b-PE (70/30) | 100.7 | 108.9 | 143.3 | −14.7 |
| PPR/PP-b-PE (90/10) | 102.1 | 107.8 | 143.4 | −14.1 |
| PPR | 102.3 | - | 143.8 | −13.8 |
| Sample | Yield Stress | Elongation at Break | Impact Strength (KJ/m2) | Breaking Strength | Modulus | |
|---|---|---|---|---|---|---|
| MPa | (%) | 25 °C | −5 °C | MPa | GPa | |
| PPR | 23.0 | 457.5 | 15.8 | 4.1 | 32.7 | 7.2 |
| PPR/PP-b-PE (90/10) | 21.1 | 467.1 | 22.3 | 8.1 | 31.3 | 6.5 |
| PPR/PP-b-PE (70/30) | 19.9 | 522.6 | 58.0 | 9.8 | 33.6 | 6.2 |
| PPR/PP-b-PE (50/50) | 16.5 | 535.1 | unbreak | 28.4 | 4.8 | |
| PPR/PP-b-PE (30/70) | 13.9 | 573.5 | unbreak | 26.0 | 3.9 | |
| PPR/PP-b-PE (10/90) | 12.5 | 694.4 | unbreak | 22.1 | 3.3 | |
| PP-b-PE | 13.1 | 739.5 | unbreak | 25.6 | 3.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, W.; Liu, L.-Z.; Wang, Y.; Wang, Y.; Shi, Y.; Song, L. Investigation of Crystallization, Morphology, and Mechanical Properties of Polypropylene/Polypropylene-Polyethylene Block Copolymer Blends. Polymers 2023, 15, 4680. https://doi.org/10.3390/polym15244680
Shao W, Liu L-Z, Wang Y, Wang Y, Shi Y, Song L. Investigation of Crystallization, Morphology, and Mechanical Properties of Polypropylene/Polypropylene-Polyethylene Block Copolymer Blends. Polymers. 2023; 15(24):4680. https://doi.org/10.3390/polym15244680
Chicago/Turabian StyleShao, Wenjun, Li-Zhi Liu, Ying Wang, Yuanxia Wang, Ying Shi, and Lixin Song. 2023. "Investigation of Crystallization, Morphology, and Mechanical Properties of Polypropylene/Polypropylene-Polyethylene Block Copolymer Blends" Polymers 15, no. 24: 4680. https://doi.org/10.3390/polym15244680
APA StyleShao, W., Liu, L.-Z., Wang, Y., Wang, Y., Shi, Y., & Song, L. (2023). Investigation of Crystallization, Morphology, and Mechanical Properties of Polypropylene/Polypropylene-Polyethylene Block Copolymer Blends. Polymers, 15(24), 4680. https://doi.org/10.3390/polym15244680






