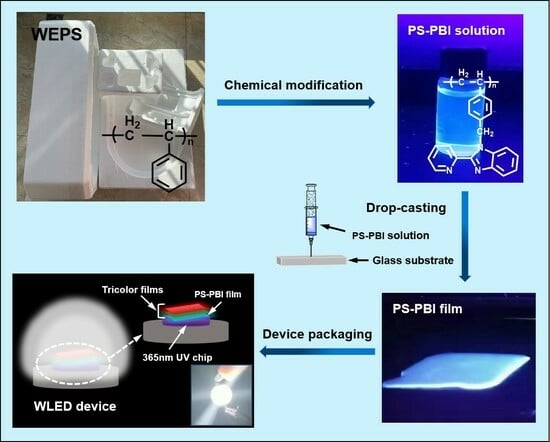
Conversion of Waste Expanded Polystyrene into Blue-Emitting Polymer Film for Light-Emitting Diode Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
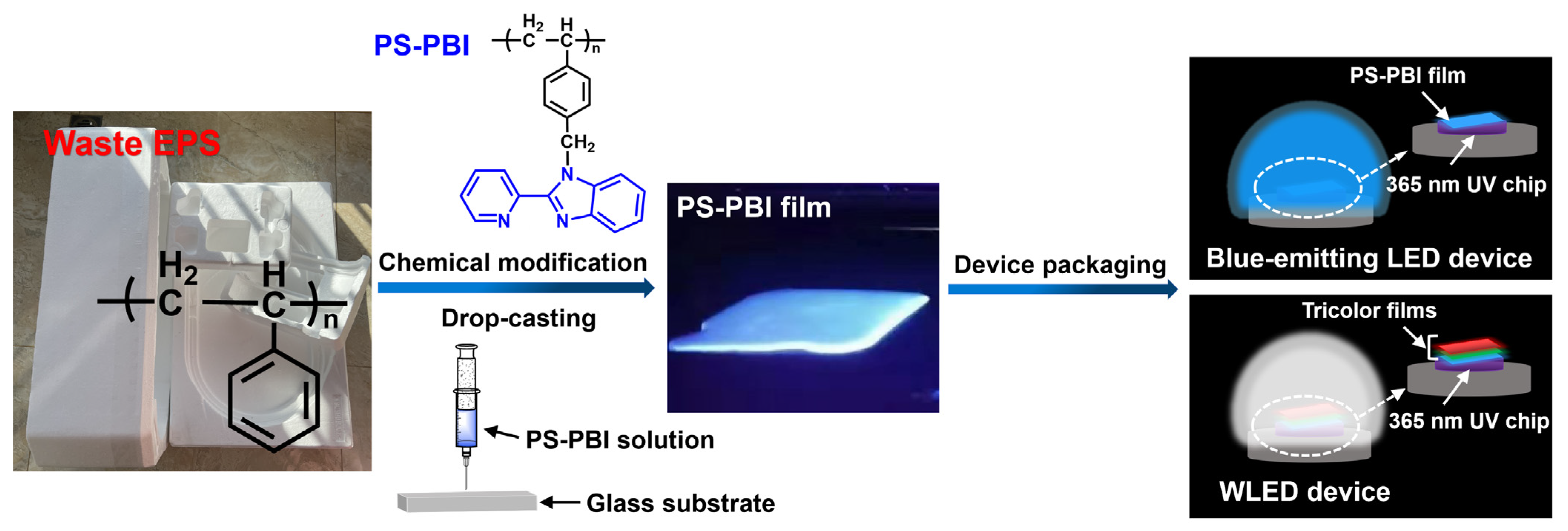
2.2. Recycling and Disposal of Waste EPS
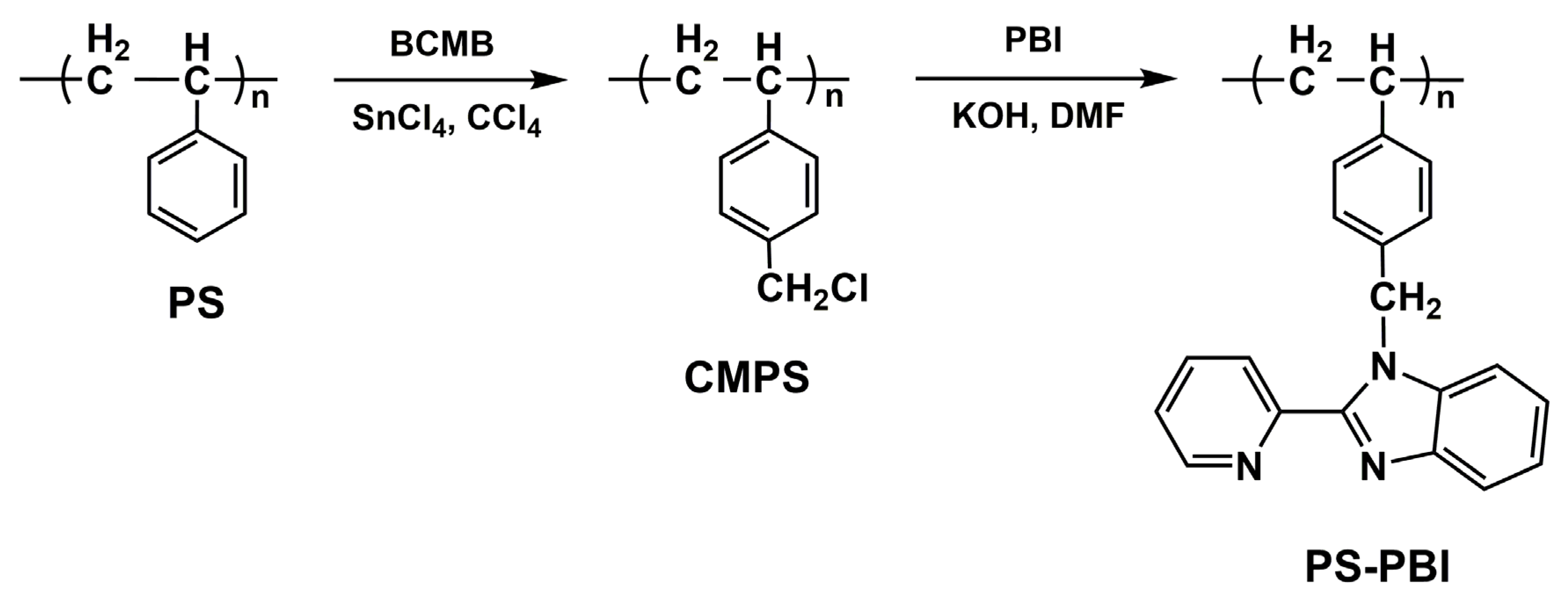
2.3. Preparation of PS-PBI
2.4. Preparation of PS-PBI Film
2.5. Preparation of Blue-Emitting LED Device
2.6. Preparation of WLED Device
2.7. Characterization
3. Results and Discussion
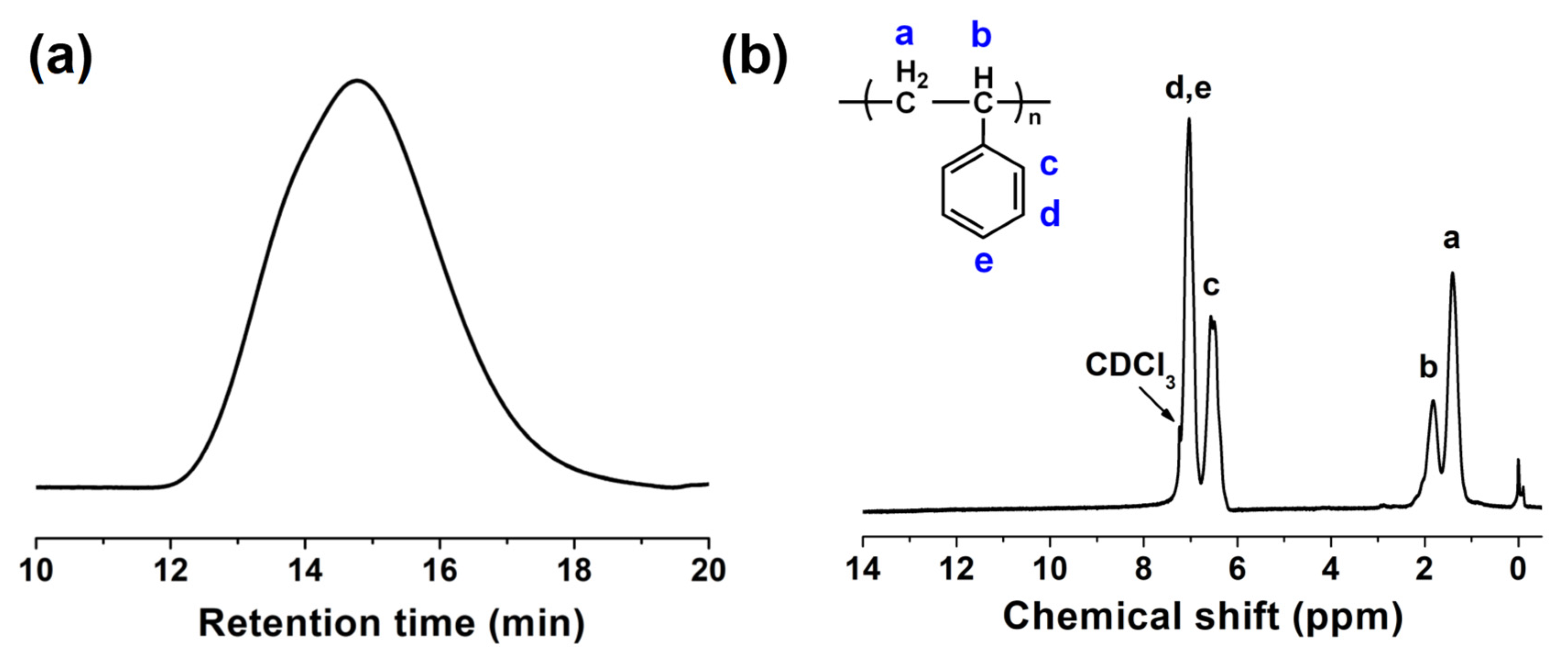
3.1. Purification of Waste EPS
3.2. Preparation and Characterization of PS-PBI
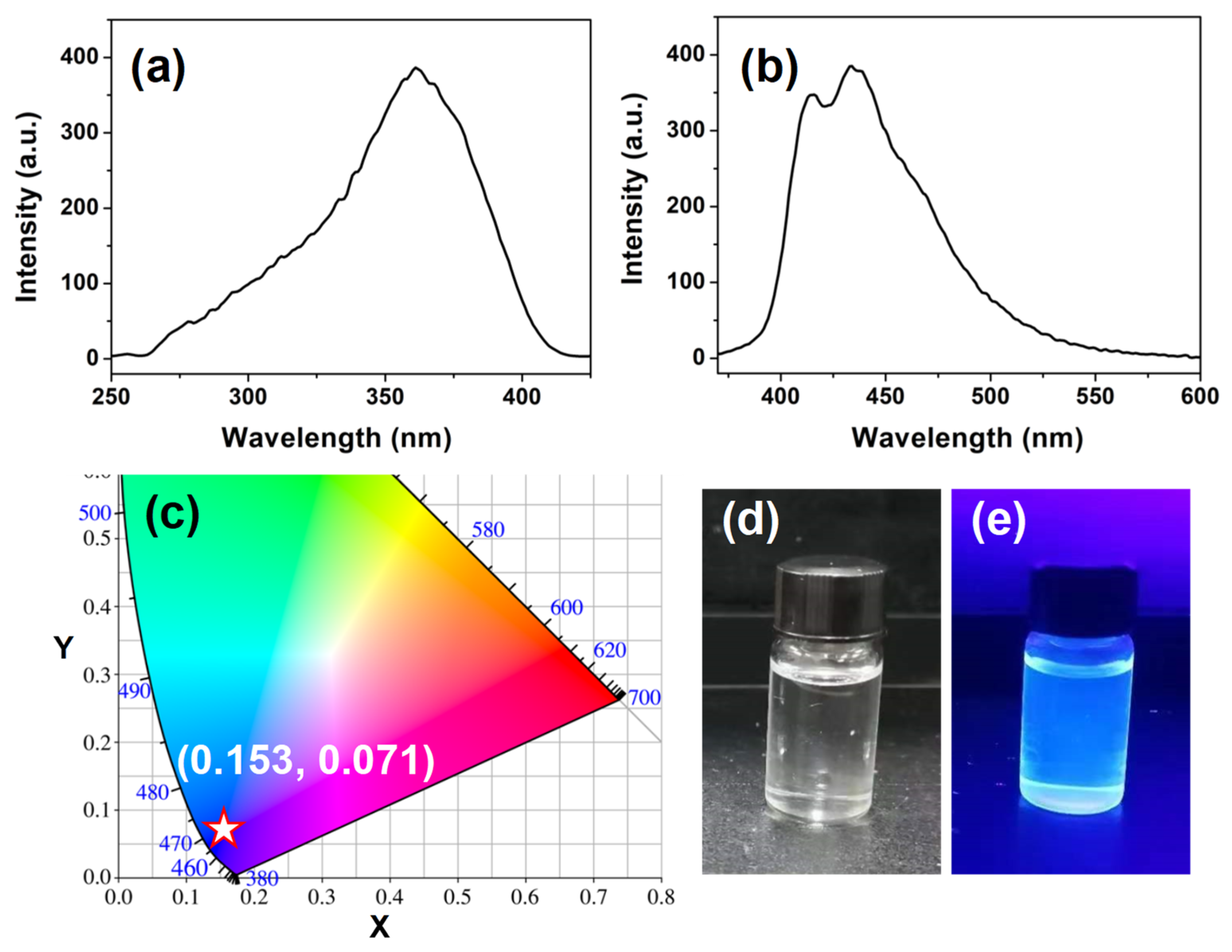
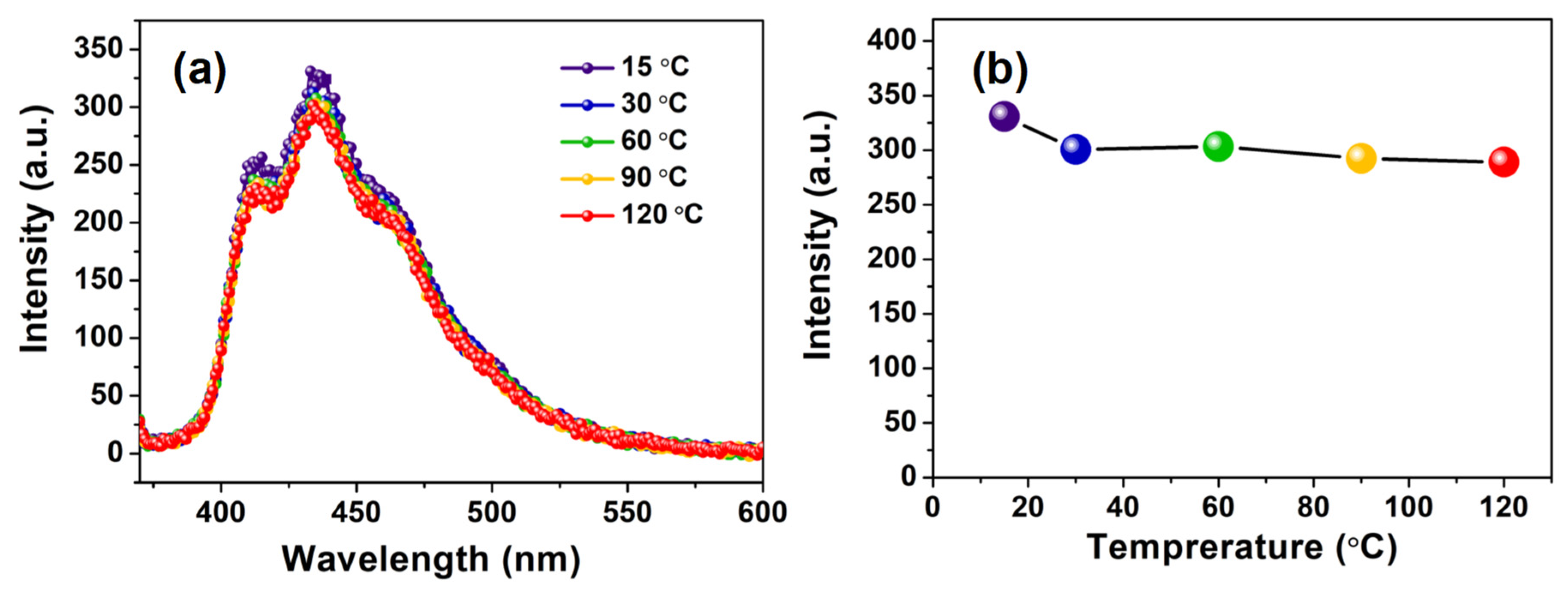
3.3. Luminescence Properties of PS-PBI
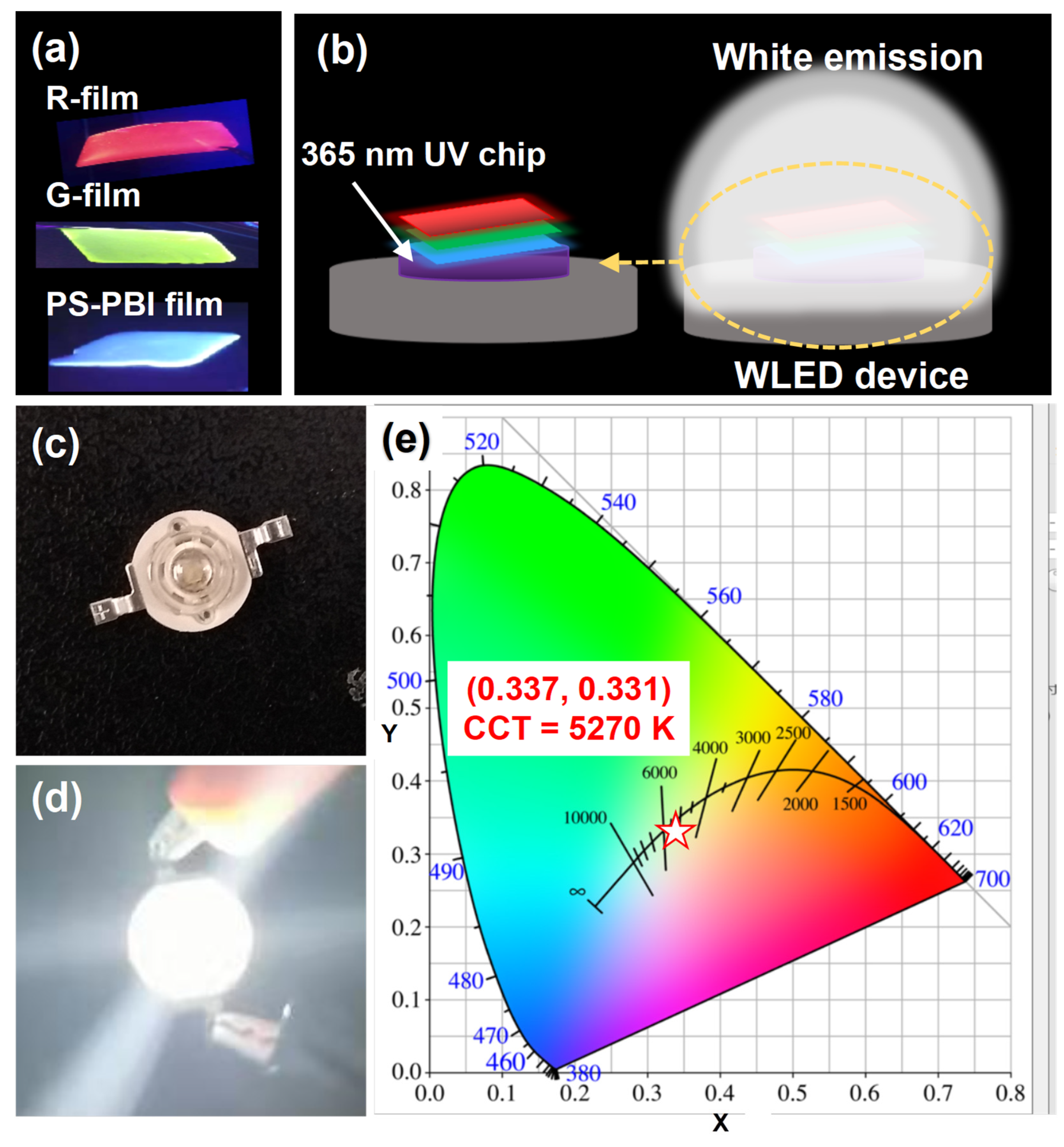
3.4. Luminescence Properties of PS-PBI Film and Its Application in LED Devices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Plastics Europe. Plastics—The Fast Facts 2023. Available online: https://plasticseurope.org/wp-content/uploads/2023/10/Plasticsthefastfacts2023-1.pdf (accessed on 3 November 2023).
- Wilts, C.H.; Schinkel, J.; Feder, L. Prevention of Plastic Waste in Production and Consumption by Multi-Actor Partnerships. Available online: https://prevent-waste.net/wp-content/uploads/2020/07/Prevention_of_plastic_waste_in_production_and_consumption_final.pdf (accessed on 3 November 2023).
- Walker, T.R. (Micro) plastics and the UN Sustainable Development Goals. Curr. Opin. Green Sust. 2021, 30, 100497. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. Plastics—The Facts 2019: An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2019/ (accessed on 3 November 2023).
- Prabhakar, R.P.; Sanket, S.S.; Rauphunnisa, F.I.; Rahul, B.P. Impacts of thermocol waste on marine life: A review. Int. Mult. Res. J. 2016, 3, 60–68. [Google Scholar]
- Prasittisopin, L.; Termkhajornkit, P.; Kim, Y.H. Review of concrete with expanded polystyrene (EPS): Performance and environmental aspects. J. Clean. Prod. 2022, 366, 132919. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.Q.; Yu, J.T.; Wang, X.M.; Wang, L.J.; Zhao, B.; Hao, L.; Liu, W.H.; Wang, Z.; Chen, H.; et al. Converting waste expanded polystyrene into higher-value-added hyper-crosslinked porous polymer for rapid and high-efficient adsorption of aflatoxins. J. Clean. Prod. 2023, 408, 137102. [Google Scholar] [CrossRef]
- Gonzalez-Aguilar, A.M.; Pérez-García, V.; Riesco-Ávila, J.M. A Thermo-Catalytic Pyrolysis of Polystyrene Waste Review: A Systematic, Statistical, and Bibliometric Approach. Polymers 2023, 15, 1582. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.Y.; Xie, Z.F.; Chen, D.; Long, J.; Wen, Y.P.; Shi, W. Porous Adsorbents Cross-Linked with Waste Polystyrene Foam and 3,3′,4,4′-Biphenyltetracarboxylic Acid Dianhydride for the Effective Removal and Enrichment of Cationic Dyes. ACS Appl. Polym. Mater. 2023, 5, 8108–8120. [Google Scholar] [CrossRef]
- Nady, N.; Rehim, M.H.A.; Badawy, A.A. Dye removal membrane from electrospun nanofibers of blended polybutylenesuccinate and sulphonated expanded polystyrene waste. Sci. Rep. 2023, 13, 15455. [Google Scholar] [CrossRef]
- Ozdemir, N.C.; Yel, E. Synthesis of a New Flocculant from Waste Polystyrene: Plastic Recycling Industry Wastewater Treatability. Water Air Soil Pollut. 2023, 234, 88. [Google Scholar] [CrossRef]
- Srinivasan, V.; Sumalatha, V.; Prasannan, A.; Govindarajan, S. Utilization of SulfonatedWaste Polystyrene-Based Cobalt Ferrite Magnetic Nanocomposites for Efficient Degradation of Calcon Dye. Polymers 2022, 14, 2909. [Google Scholar] [CrossRef]
- Inagaki, Y.; Kuromiya, M.; Noguchi, T.; Watanabe, H. Reclamation of Waste Polystyrene by Sulfonation. Langmuir 1999, 15, 4171–4175. [Google Scholar] [CrossRef]
- Li, W.; Xie, Z.F.; Xue, S.S.; Ye, H.; Liu, M.Y.; Shi, W.; Liu, Y.C. Studies on the adsorption of dyes, Methylene blue, Safranin T, and Malachite green onto Polystyrene foam. Sep. Purif. Technol. 2021, 276, 119435. [Google Scholar] [CrossRef]
- Ye, H.; Xie, Z.F.; Li, W.; Pu, Y.H.; Liu, M.Y.; Wen, Y.P.; Liu, Y.C. Converting waste polystyrene foam into new value-added materials: A large-capacity scavenger to remove cationic dyes and heavy metals. J. Appl. Polym. Sci. 2022, 139, e51868. [Google Scholar] [CrossRef]
- Liu, M.Y.; Xie, Z.F.; Ye, H.; Li, W.; Shi, W.; Liu, Y.C.; Zhang, Y. Waste polystyrene foam-Chitosan composite materials as high-efficient scavenger for the anionic dyes. Colloid. Surface A 2021, 627, 127155. [Google Scholar] [CrossRef]
- Cho, J.; Park, J.H.; Kim, J.K.; Schubert, E.F. White light-emitting diodes: History, progress, and future. Laser Photonics Rev. 2017, 11, 1600147. [Google Scholar] [CrossRef]
- Hua, Y.B.; Wang, T.; Yu, J.S. Synthesis and emission enhancement of intrinsic green-emitting materials for versatile applications. Ceram. Int. 2023, 49, 2689–2697. [Google Scholar] [CrossRef]
- Anand, V.; Mishra, R.; Barot, Y. Recent advances in the development of pure organic white light emitters. Dyes Pigment. 2021, 191, 109390. [Google Scholar] [CrossRef]
- Zhang, W.B.; Shi, C.; Ye, S.S.; Zhou, J.C.; Wu, D.W.; Li, Y.W.; Chen, M.T.; Ding, J.Y.; Wu, Q.S. A novel narrow band blue-emitting phosphor Rb2ZrSi3O9:Eu2+ with low thermal quenching and high quantum efficiency. Ceram. Int. 2021, 47, 22786–22793. [Google Scholar] [CrossRef]
- Geng, X.; Xie, Y.; Chen, S.S.; Luo, J.M.; Li, S.C.; Wang, T.; Zhao, S.C.; Wang, H.; Deng, B.; Yu, R.J.; et al. Enhanced local symmetry achieved zero-thermal-quenching luminescence characteristic in the Ca2InSbO6:Sm3+ phosphors for w-LEDs. Chem. Eng. J. 2021, 410, 128396. [Google Scholar] [CrossRef]
- Zhou, J.B.; Wang, Y.F.; Chen, Y.Y.; Zhou, Y.Y.; Milićević, B.; Zhou, L.; Yan, J.; Shi, J.X.; Liu, R.-S.; Wu, M.M. Single-Crystal Red Phosphors and Their Core-Shell Structure for Improved Water-Resistance for Laser Diodes Applications. Angew. Chem. Int. Ed. 2021, 60, 3940–3945. [Google Scholar] [CrossRef]
- Duan, Y.T.; Zhao, C.Y.; Lin, H.; Hong, R.J.; Tao, C.X.; Han, Z.X.; Zhang, D.W.; Zhou, S.M. Photoluminescence properties of Tb3Al5O12:Ce3+, Mn2+ phosphor ceramics for high color rendering index warm white LEDs. Opt. Mater. 2021, 111, 110670. [Google Scholar] [CrossRef]
- Behar-Cohen, F.; Martinsons, C.; Viénot, F.; Zissis, G.; Barlier-Salsi, A.; Cesarini, J.P.; Enouf, O.; Garcia, M.; Picaud, S.; Attia, D. Light-emitting diodes (LED) for domestic lighting: Any risks for the eye? Prog. Retin. Eye Res. 2011, 30, 239–257. [Google Scholar] [CrossRef]
- Bullough, J.D.; Bierman, A.; Rea, M.S. Evaluating the blue-light hazard from solid state lighting. Int. J. Occup. Saf. Ergon. 2019, 25, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Tan, T.; Wang, S.W.; Zhang, S.; Pang, R.; Li, D.; Jiang, L.H.; Li, H.M.; Li, C.Y.; Zhang, H.J. Low-concentration Ce3+-activated ScCaO(BO3) blue-cyan phosphor with high efficiency toward full-spectrum white LED applications. Mater. Today Chem. 2022, 26, 101030. [Google Scholar] [CrossRef]
- Leite Silva, C.M.B.; Bispo-Jr, A.G.; Lima, S.A.M.; Pires, A.M. Eu3+ complex/polymer films for light-emitting diode applications. Opt. Mater. 2019, 96, 109323. [Google Scholar] [CrossRef]
- Bai, H.; Wu, G.D.; Qing, Q.; Hou, J.Y.; Liu, J.H.; Song, F.; Tang, Z.B.; Leng, Z.H. Novel near-ultraviolet-excited and thermally-stable blue-emitting phosphor for healthy WLED lighting. J. Lumin. 2022, 252, 119346. [Google Scholar] [CrossRef]
- Xia, Z.G.; Liu, Q.L. Progress in discovery and structural design of color conversion phosphors for LEDs. Prog. Mater. Sci. 2016, 84, 59–117. [Google Scholar] [CrossRef]
- Ye, S.; Xiao, F.; Pan, Y.X.; Ma, Y.Y.; Zhang, Q.Y. Phosphors in phosphor-converted white light-emitting diodes: Recent advances in materials, techniques and properties. Mater. Sci. Eng. R 2010, 71, 1–34. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Li, T.G.; Lei, M.M.; Huang, X.Q.; Wang, T.L. Efficient One-Pot Synthesis of Bright Blue-Emitting Ce3+-Based Phosphor: Application for the Construction of Warm White-Light-Emitting Diodes and Anticounterfeiting. ACS Appl. Electron. Mater. 2022, 4, 3575–3582. [Google Scholar] [CrossRef]
- Liu, Y.K.; Liu, Y.-G.; Yu, H.J.; Yang, J.Y.; Mi, R.Y.; Chen, J. Co-solvent-assisted sintering and thermal stability investment of BaMgAl10O17:Eu2+ by cationic substitution. Opt. Mater. 2021, 122, 111717. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Li, Y.Y.; Wu, H.Y.; Li, X.; Yu, S.S.; Wu, J.X.; Wang, W.J.; Zhao, L. Insight into the crystal structure and photoluminescence properties of an extremely broad-band yellow-emitting phosphor Sr8MgCe(PO4)7:Eu2+. Dalton Trans. 2021, 50, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Lou, Q.; Ni, Q.C.; Niu, C.Y.; Wei, J.Y.; Zhang, Z.F.; Shen, W.X.; Shen, C.L.; Qin, C.C.; Zheng, G.S.; Liu, K.K.; et al. Carbon Nanodots with Nearly Unity Fluorescent EfficiencyRealized via Localized Excitons. Adv. Sci. 2022, 30, 2203622. [Google Scholar] [CrossRef]
- Yue, S.-M.; Xu, H.-B.; Ma, J.-F.; Su, Z.-M.; Kan, Y.-H.; Zhang, H.-J. Design and syntheses of blue luminescent zinc(II) and cadmium(II) complexes with bidentate or tridentate pyridyl-imidazole ligands. Polyhedron 2006, 25, 635–644. [Google Scholar] [CrossRef]
- Fan, Y.J.; Su, H.Y.; Li, P.F.; Lin, M.M.; Liu, D.; Pei, K.M.; Cao, X.B. Upcycling waste expanded polystyrene into UVexcited dual-mode multicolor luminescent electrospun fiber membranes for advanced anticounterfeitin. RSC Adv. 2023, 13, 10123–10134. [Google Scholar] [CrossRef] [PubMed]
- Burlov, A.S.; Antsyshkina, A.S.; Sadkov, G.G.; Chesnokov, V.V.; Koshchienko, Y.V.; Garnovskii, D.A.; Vasil’chenko, I.S.; Uraev, A.I.; Borodkin, G.S.; Sergienko, V.S.; et al. Coordination Compounds of Ambidentate 1-(H)Alkyl-2 -(2-pyridyl)benzimidazoles. Synthesis and Crystal Structure. Russ. J. Coord. Chem. 2010, 36, 906–912. [Google Scholar] [CrossRef]
- Liu, Q.-D.; Jia, W.-L.; Wang, S.N. Blue Luminescent 2-(2′-Pyridyl)benzimidazole Derivative Ligands and Their Orange Luminescent Mononuclear and Polynuclear Organoplatinum(II) Complexes. Inorg. Chem. 2005, 44, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-G.; Wang, F.; Wang, H.-Y.; Si, G.; Tung, C.-H.; Wu, L.-Z. Photocatalytic Hydrogen Evolution by [FeFe] Hydrogenase Mimics in Homogeneous Solution. Chem. Asian J. 2010, 5, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; Shen, K.S.; Mao, S.S.; Shi, X.K.; Wu, H.L.; Fan, X.Y. Mono- and tetranuclear copper(I) complexes with N-heterocyclic chelating and triphenylphosphine ligands: Crystal structures, luminescent and heterogeneous catalytic properties. Appl. Organometal. Chem. 2018, 32, e4041. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, M.-H.; Zhao, X.-H.; Liang, H. Supramolecular networks of hexanuclear cadmium (II): Synthesis, crystal structure and emission property. Inorg. Chim. Acta 2009, 362, 3065–3068. [Google Scholar] [CrossRef]
- Hou, T.T.; Yue, S.M.; Yue, X.R.; Ma, J.F. Syntheses, crystal structures, and properties of nickel and cadmium complexes containing imidazole derivatives. J. Coord. Chem. 2012, 65, 3895–3902. [Google Scholar] [CrossRef]
- Korkmaz, U.; Özlem, B.; Erol, E.; Alas, M.Ö.; Altürk, R.G.; Ersundu, M.Ç.; Ersundu, A.E. The coupling of blue emitting carbon dots with Eu3+/Tb3+ co-doped luminescent glasses for utilization in white light emitting diodes. Phys. Chem. Chem. Phys. 2023, 25, 11452. [Google Scholar] [CrossRef]
- Kong, H.X.; Jia, G.; Li, H.L.; Meng, Z.X.; Zhang, N.; Zhang, C.M. Deep blue, cyan, orange-red, and white multicolor emissions generated by Bi3+/Eu3+ activated KBaYSi2O7 luminescent materials for white light-emitting diodes. Ceram. Int. 2023, 49, 15320–15332. [Google Scholar] [CrossRef]
- Yao, Z.C.; Dai, W.B.; Luo, J.; Nie, K.; Xu, M. Study on the stable and efficient white-emitting Bi/Tb/Eu tridoped single-phase borate Sr3YB3O9 phosphors. J. Alloys Compd. 2023, 968, 172140. [Google Scholar] [CrossRef]
- Li, W.; Ma, N.; Devakumar, B.; Huang, X.Y. High-color-quality blue-light-pumped full-spectrum white-light-emitting diodes realized by efficient green-emitting CaY2HfScAl3O12:Ce3+ phosphors. J. Lumin. 2023, 264, 120183. [Google Scholar] [CrossRef]
- Mi, R.Y.; Liu, Y.G.; Mei, L.F.; Min, X.; Fang, M.H.; Wu, X.W.; Huang, Z.H.; Chen, C.J. Highly-efficient cyan-emitting phosphor enabling high-color-quality lighting and transparent anticounterfeiting. Chem. Eng. J. 2023, 457, 141377. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, H.; Lin, H.; Li, P.; Li, B.; Xu, X.; Li, J.; Wu, Y.; Hui, J.; Liu, D. Conversion of Waste Expanded Polystyrene into Blue-Emitting Polymer Film for Light-Emitting Diode Applications. Polymers 2023, 15, 4693. https://doi.org/10.3390/polym15244693
Su H, Lin H, Li P, Li B, Xu X, Li J, Wu Y, Hui J, Liu D. Conversion of Waste Expanded Polystyrene into Blue-Emitting Polymer Film for Light-Emitting Diode Applications. Polymers. 2023; 15(24):4693. https://doi.org/10.3390/polym15244693
Chicago/Turabian StyleSu, Huanyou, Hua Lin, Pengfei Li, Bowen Li, Xiaodong Xu, Jiacheng Li, Yuanquan Wu, Jiaqi Hui, and Dan Liu. 2023. "Conversion of Waste Expanded Polystyrene into Blue-Emitting Polymer Film for Light-Emitting Diode Applications" Polymers 15, no. 24: 4693. https://doi.org/10.3390/polym15244693
APA StyleSu, H., Lin, H., Li, P., Li, B., Xu, X., Li, J., Wu, Y., Hui, J., & Liu, D. (2023). Conversion of Waste Expanded Polystyrene into Blue-Emitting Polymer Film for Light-Emitting Diode Applications. Polymers, 15(24), 4693. https://doi.org/10.3390/polym15244693