Semi-Interpenetrating Polymer Networks Based on Hydroxy-Ethyl Methacrylate and Poly(4-vinylpyridine)/Polybetaines, as Supports for Sorption and Release of Tetracycline
Abstract
1. Introduction
- Their porous structure with interconnected pores and high specific surface area leading to an increased amount of immobilized drug and a controlled release profile [20].
- The improvement of their mechanical properties through introduction of a linear polymer into the three-dimensional network, compared with the classical hydrogel. The linear polymer can interact with the three-dimensional network through physical bonds such as: electrostatic interactions, hydrogen bonds, Van der Waals interactions, hydrophobic interactions or combinations thereof [21].
- The ability to combine the hydrophobic and hydrophilic polymers to shape the properties of the hydrogel [22].
2. Materials and Methods
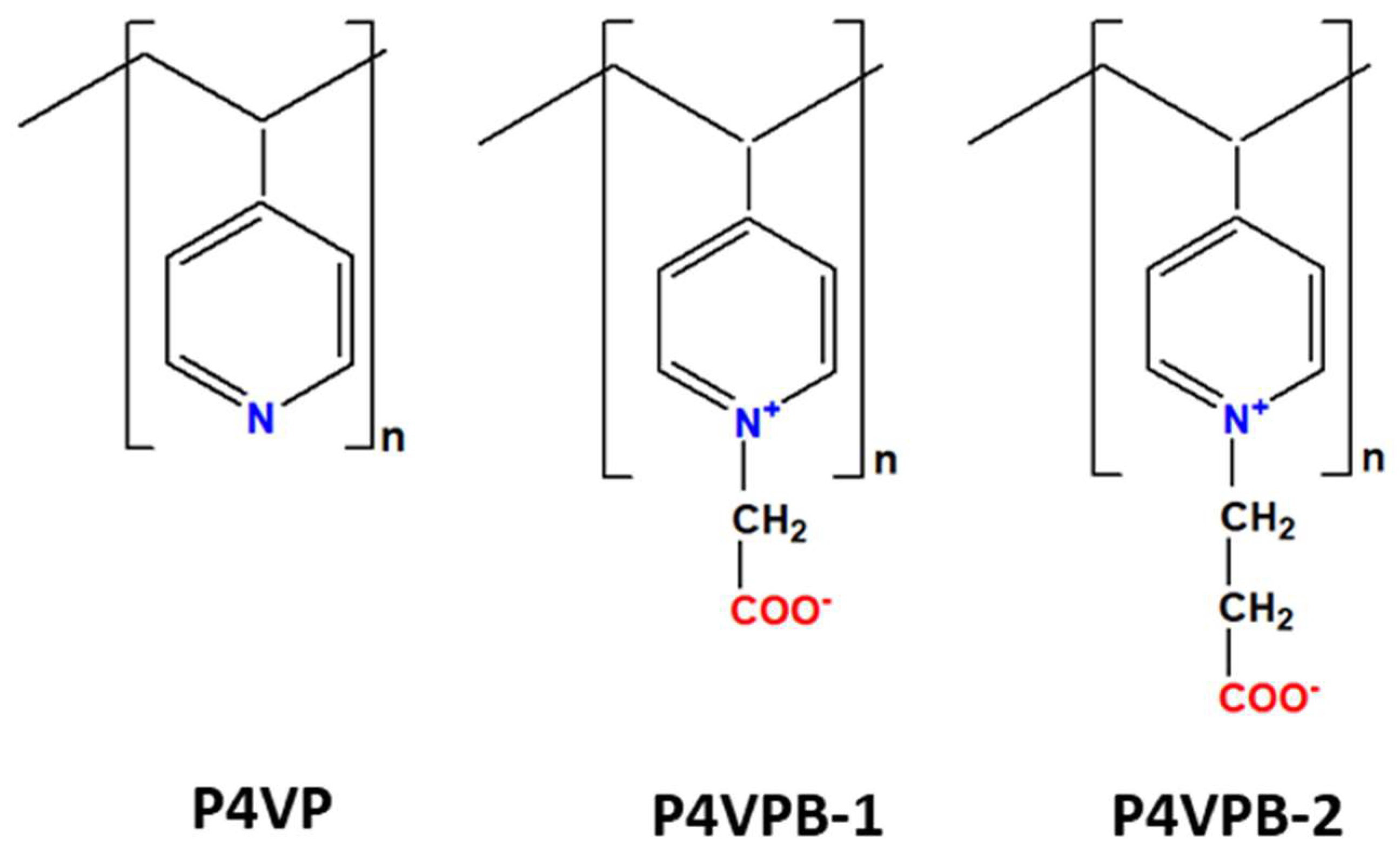
2.1. Materials
2.2. Synthesis of Semi-Interpenetrating Polymer Networks
- initial monomer concentration (HEMA + dimethacrylic monomers) of C0 = 10% was kept constant for all experiments;
- different molar ratios of the crosslinkers (EGDMA, DEGDMA or TEGDMA) to the monomer (HEMA) were as follows 1:20; 1:40; 1:50; 1:67 and 1:80 (mol/mol);
- different gravimetric ratios of HEMA to the linear polymers (P4VP or PB) were as follows 1:0.2; 1:0.3; 1:0.4; 1:0.5 (g/g);
- the redox initiator concentration was Ci = 2 g (APS + TEMED)/100 g monomers.
2.3. Physico-Chemical Characterization of Semi-IPNs
2.4. Sorption Batch Experiments
2.5. In Vitro Release Studies
2.6. Antimicrobial Activity Tests
3. Results and Discussion
3.1. Synthesis and Optimized Semi-IPNs
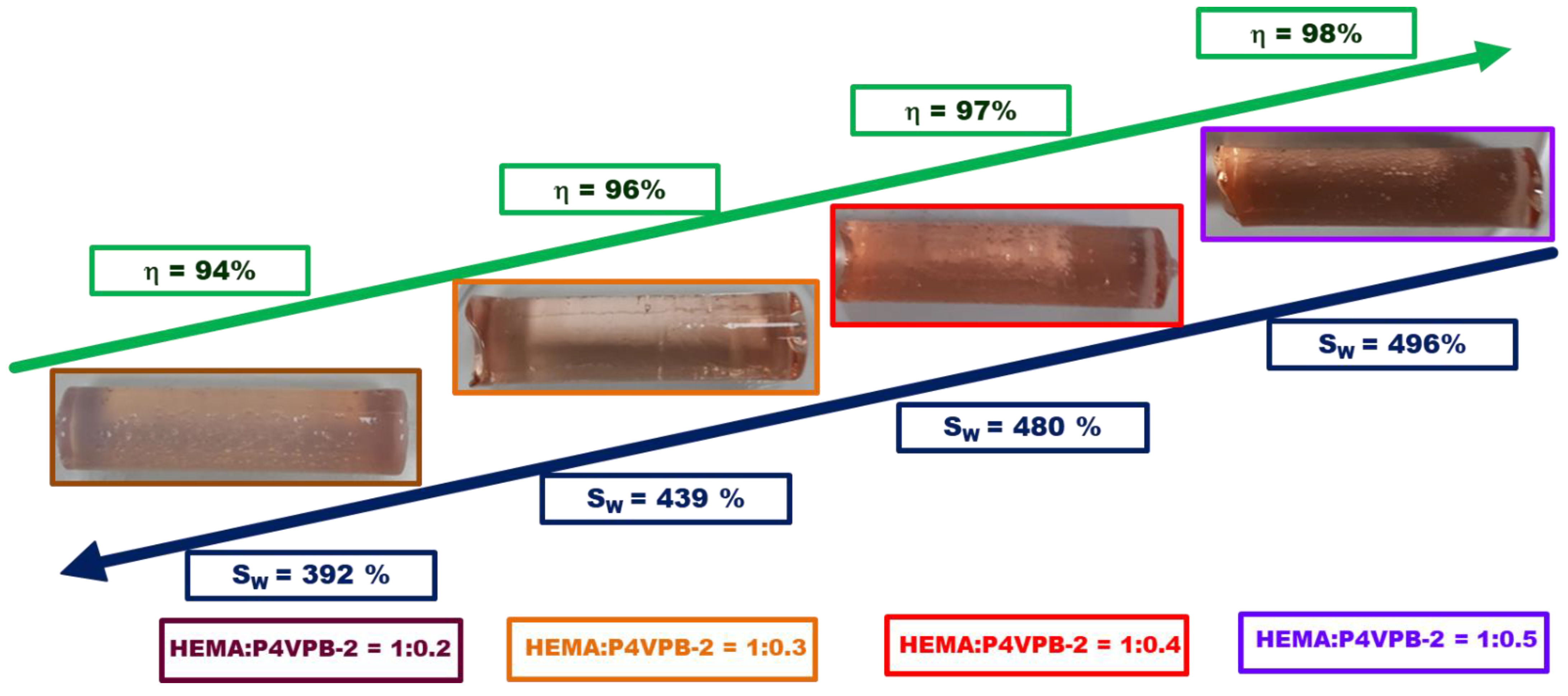
- the color of PHEMA/P4VPB-2 semi-IPNs changes from pale pink to reddish brown, a sign that polybetaine is retained in higher amounts within the polymer networks when HEMA:P4VPB-2 is 1:0.5, g/g;
- η values increase slightly from 94% to 98% with increasing the amount of linear polymer retained;
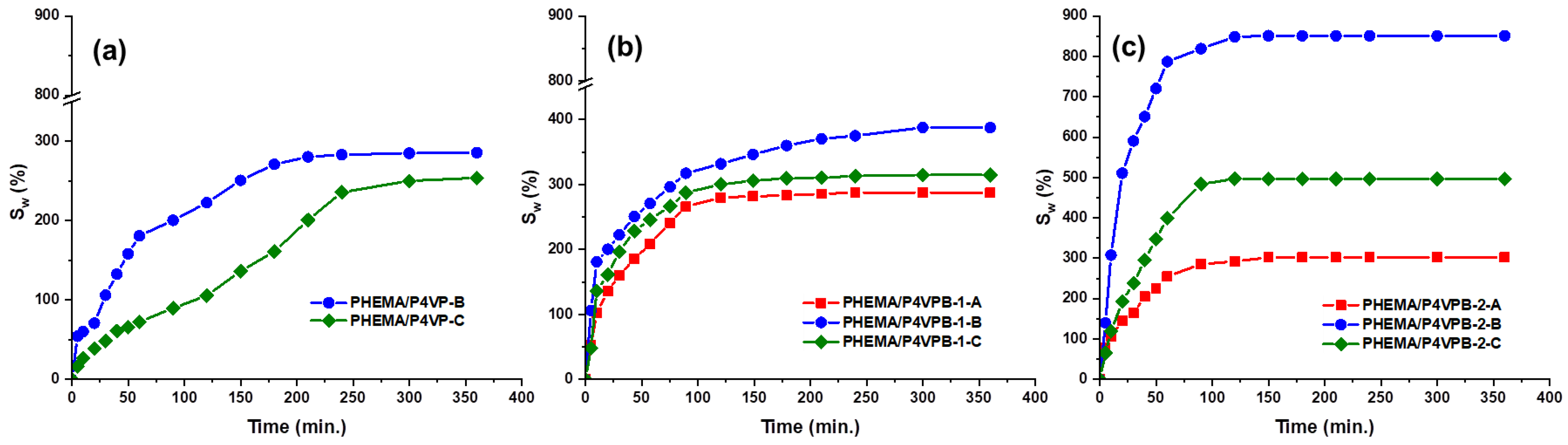
- the values of the maximum swelling degree increase with the increasing amount of polybetaine, because P4VPB-2 is a hydrophilic polymer and its retention in the structure of semi-IPN leads to the formation of polymeric materials with a high water swelling capacity.
- 3443 cm−1, attributed to the valence vibration of OH (νO-H) groups involved in hydrogen bond formation;
- 2952 and 2886 cm−1, which are characteristic of the valence vibrations of the symmetric and asymmetric -CH2- group;
- 1730 cm−1, the stretching vibration of the >C=O bond belonging to the ester group located in the cross-linker molecule.
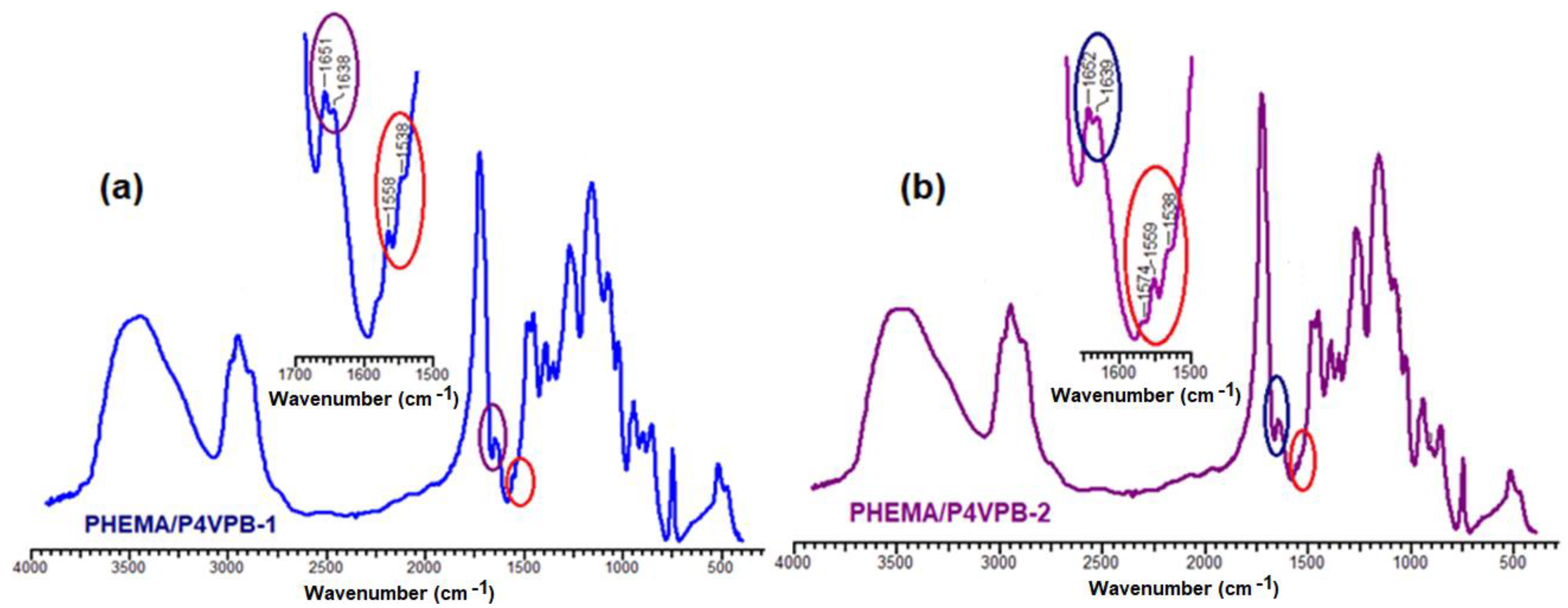
- 1604 and 1559 cm−1, attributed to the vibrations of the pyridine ring (C=C and C–N);
- 825 and 563 cm−1, specific to out-of-plane pyridine ring deformation vibrations.
3.2. Sorption Studies of Tetracycline, Selection of Optimized Formulations
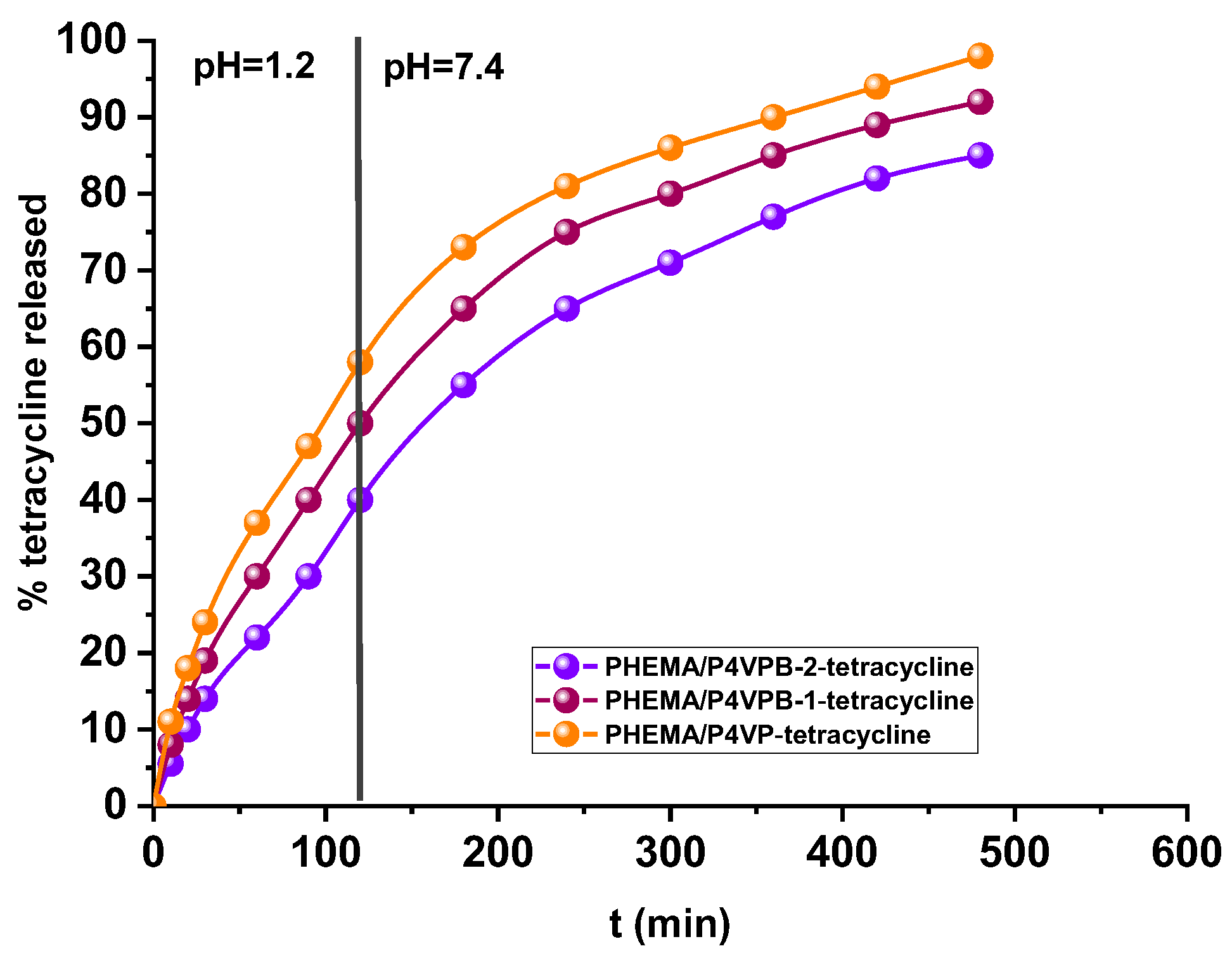
3.3. Release Studies of Tetracycline from Semi-IPN–Drug Systems
3.4. Antimicrobial Activity of Tetracycline-Loaded Semi-IPNs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rungrod, A.; Kapanya, A.; Punyodom, W.; Molloy, R.; Mahomed, A.; Somsunan, R. Synthesis and characterization of semi-IPN hydrogels composed of sodium 2- acrylamide-2-methylprpanesulfonateand poly(ε-caprolactone) diol for controlled drug delivery. Eur. Polym. J. 2022, 164, 110978. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 417–433. [Google Scholar]
- Hu, X.; Feng, L.; Wei, W.; Xie, A.; Wang, S.; Zhang, J.; Dong, W. Synthesis and characterization of a novel semi-IPN hydrogel based on Salecan and poly(N,N-dimethylacrylamide-co-2-hydroxyethyl methacrylate). Carbohydr. Polym. 2014, 105, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [PubMed]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends and applications. J. Appl. Polym. Sci. 2021, 138, e50376. [Google Scholar] [CrossRef]
- Cai, M.H.; Chen, X.Y.; Fu, L.Q.; Du, W.L.; Yang, X.; Mou, X.Z.; Hu, P.Y. Design and development of hybrid hydrogels for biomedical applications: Recent trends in anticancer drug delivery and tissue engineering. Front. Bioeng. Biotechnol. 2021, 9, 630943. [Google Scholar] [CrossRef]
- Chamkouri, H.; Chamkouri, M. A review of hydrogels, their properties and applications in medicine. Am. J. Biomed. Sci. Res. 2021, 11, 485–493. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 13–36. [Google Scholar]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioactive Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, K.P.; Zang, Q.; Shi, Q.; Wang, Y.; Xiao, Z.; Zhang, Q.; Wang, L. Anionic organo-hydrogel electrolyte with enhanced ionic conductivity and balanced mechanical properties for flexible supercapacitors. J. Mater. Chem. A 2022, 10, 11277–11287. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.; Cheng, S.; Li, R.; Pan, X.; Zhang, C.; Gu, H.; Xie, A.; Dong, W. Synthesis of cationic hydrogels with tunable physicochemical properties for antibacterial applications. Eur. Polym. J. 2022, 173, 111228. [Google Scholar] [CrossRef]
- Liu, S.; Tang, J.; Ji, F.; Lin, W.; Chen, S. Recent advances in zwitterionic hydrogels: Preparation, property and biomedical application. Gels 2022, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Okay, O. Semicrystalline physical hydrogels with shape-memory and self-healing properties. J. Mater. Chem. B 2019, 7, 1581–1596. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, C.; Dong, Y.; Liu, D. Supramolecular hydrogels: Mechanical strengthening with dynamics. Polymer 2020, 210, 122993. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Su, T.; Lu, F.; Chang, Q.; Gao, J. Recent advances in macroporous hydrogels for cell behavior and tissue engineering. Gels 2022, 8, 606. [Google Scholar] [CrossRef]
- Krstic, M.; Rogic Miladinovic, Z.; Barudzija, T.; Mladenovic, A.; Suljovrujic, E. Stimuli-responsive copolymeric hydrogels based on oligo(ethylene glycol) dimethacrylate for biomedical applications: An optimisation study of pH and thermoresponsive behavior. React. Funct. Polym. 2022, 170, 105140. [Google Scholar] [CrossRef]
- Liang, X.; Ding, H.; Wang, Q.; Wang, M.; Yin, B.; Sun, G. Nature-inspired semi-IPN hydrogels with tunable mechanical properties and multi-responsiveness. New J. Chem. 2021, 45, 861–871. [Google Scholar] [CrossRef]
- Zan, Z.; Zhang, B.; Nie, X.; Cheng, Y.; Hu, Z.; Liao, M.; Li, S. A sodium alginate-based sustained-release IPN hydrogel and its applications. RSC Adv. 2020, 10, 39722–39730. [Google Scholar]
- Li, Y.; Yang, H.Y.; Lee, D.S. Biodegradable and injectable hydrogels in biomedical applications. Biomacromolecules 2022, 23, 609–618. [Google Scholar] [CrossRef]
- Khan, S.A.; Azam, W.; Ashames, A.; Fehelelbom, K.M.; Ullah, K.; Mannan, A.; Murtaza, G. β-Cyclodextrin-based (IA-co-AMPS) Semi-IPNs as smart biomaterials for oral delivery of hydrophilic drugs: Synthesis, characterization, in-Vitro and in-Vivo evaluation. J. Drug. Deliv. Sci. Technol. 2020, 60, 101970. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Chen, J. Synthesis and characteristics of pH-sensitive semi-interpenetrating polymer network hydrogels based on konjac glucomannan and poly(aspartic acid) for in vitro drug delivery. Carbohydr. Polym. 2010, 79, 500–506. [Google Scholar] [CrossRef]
- Zenoozi, S.; Mohamad Sadeghi, G.M.; Rafiee, M. Synthesis and characterization of biocompatible semi-interpenetrating polymer networks based on polyurethan e and cross-linked poly (acrylic acid). Eur. Polym. J. 2020, 140, 109974. [Google Scholar] [CrossRef]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016, 23, 748–770. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.S.; Hudson, S.M. Stimuli-responsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Kashyap, N.; Kumar, N.; Kumar, M.R. Hydrogels for pharmaceutical and biomedical applications. Crit. Rev. Ther. Drug Carrier Syst. 2005, 22, 107–150. [Google Scholar] [CrossRef]
- Kang, S.I.; Bae, Y.H. A sulfonamide based glucose-responsive hydrogel with covalently immobilized glucose oxidase and catalase. J. Control. Release 2003, 86, 115–121. [Google Scholar] [CrossRef]
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- La Gatta, A.; Schiraldi, C.; Esposito, A.; D´Agostino, A.; De Rosa, A. Novelpoly(HEMA-coMETAC)/alginate semi-interpenetrating hydrogels for biomedical applications: Synthesis and characterization. J. Biomed. Mater. Res. A 2009, 90, 292–302. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M.; Dong, W.; Zhang, J. Redox/pH dual stimuli-responsive degradable Salecan-g-SS-poly(IA-co-HEMA) hydrogel for release of doxorubicin. Carbohydr. Polym. 2017, 155, 242–251. [Google Scholar] [CrossRef]
- Patil, M.B.; Rajamani, S.B.; Mathad, S.N.; Patil, A.; Hussain, M.A.; Alorfii, H.S.; Khan, A.; Asiri, A.M.; Khan, I.; Puttegowda, M. Microwave-assisted synthesis of poly(acrylamide-co-2 hydroxyethyl methacrylate)/chitosan semi-IPN ZnO nanocomposite membranes for food packaging applications. J. Mater. Res. Technol. 2022, 20, 3537–3548. [Google Scholar] [CrossRef]
- Tanan, W.; Saengsuwan, S.M. Microwave-assisted synthesis of poly(acrylamide-co-2 hydroxyethyl methacrylate)/poly(vinyl alchol) semi-IPN hydrogel. Energy Procedia 2014, 56, 386–393. [Google Scholar] [CrossRef]
- Tamburini, G.; Canevali, C.; Ferrarlo, S.; Bianchi, A.; Sansonetti, A.; Simonutti, R. Optimized semi-interpenetrated p(HEMA)/PVP hydrogels for artistic surface cleaning. Materials 2022, 15, 6739. [Google Scholar] [CrossRef] [PubMed]
- Domingues, J.A.L.; Bonelli, N.; Giorgi, R.; Fratini, E.; Gorel, F.; Baglioni, P. Innovative hydrogels based on semi-interpenetrating p(HEMA)/PVP networks for the cleaning of water-sensitive cultural heritage artifacts. Langmuir 2013, 29, 2746–2755. [Google Scholar] [CrossRef]
- Racovita, S.; Trofin, M.A.; Loghin, D.F.; Zaharia, M.M.; Bucatariu, F.; Mihai, M.; Vasiliu, S. Polybetaines in biomedical applications. Int. J. Mol. Sci. 2021, 22, 9321. [Google Scholar] [CrossRef]
- Luca, C.; Neagu, V.; Vasiliu, S.; Barboiu, V. Synthetic polybetaines: Synthesis and properties. In Focus in Ionic Polymers; Dragan, S., Ed.; Research Singpost: Kerala, India, 2005; pp. 117–153. [Google Scholar]
- Barboiu, V.; Streba, E.; Luca, C.; Simionescu, C.I. Reactions on polymers with amine groups. I. Reactions of vinylpiridine polymers and of their model compound with unsaturated carboxylic acids. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 389–398. [Google Scholar] [CrossRef]
- Michalowski, T.; Asuero, A.G.; Wybraniec, S. The titration in the Kjeldahl method of nitrogen determination: Base or acid as titrant? J. Chem. Educ. 2013, 90, 191–197. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turk, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clim. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Mostafa, K.; Samarkandy, A.R.; El-Sanabary, A. Grafting onto carbohydrate polymer using novel potassium persulfate/tetrametlene diamine redox system for initiating grafting. Adv. Polym. Technol. 2011, 30, 138–149. [Google Scholar] [CrossRef]
- Chen, L.; Yu, Q.; Wang, Y.; Li, H. Bis GMA/TEGDMA dental composite containing high aspect-ratio hydroxyapatite nanofibers. Dental. Mater. 2011, 27, 1187–1195. [Google Scholar] [CrossRef]
- Bravo, S.A.; Lamas, M.C.; Salomon, C.J. In-vitro studies of diclofenac sodium controlled release from biopolymeric hydrophilic matrices. J. Pharm. Pharm. Sci. 2002, 5, 213–219. [Google Scholar] [PubMed]
- Higuchi, W.I. Diffusional models useful in biopharmaceutics. Drug release rate processes. J. Pharm. Sci. 1967, 56, 315–324. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]

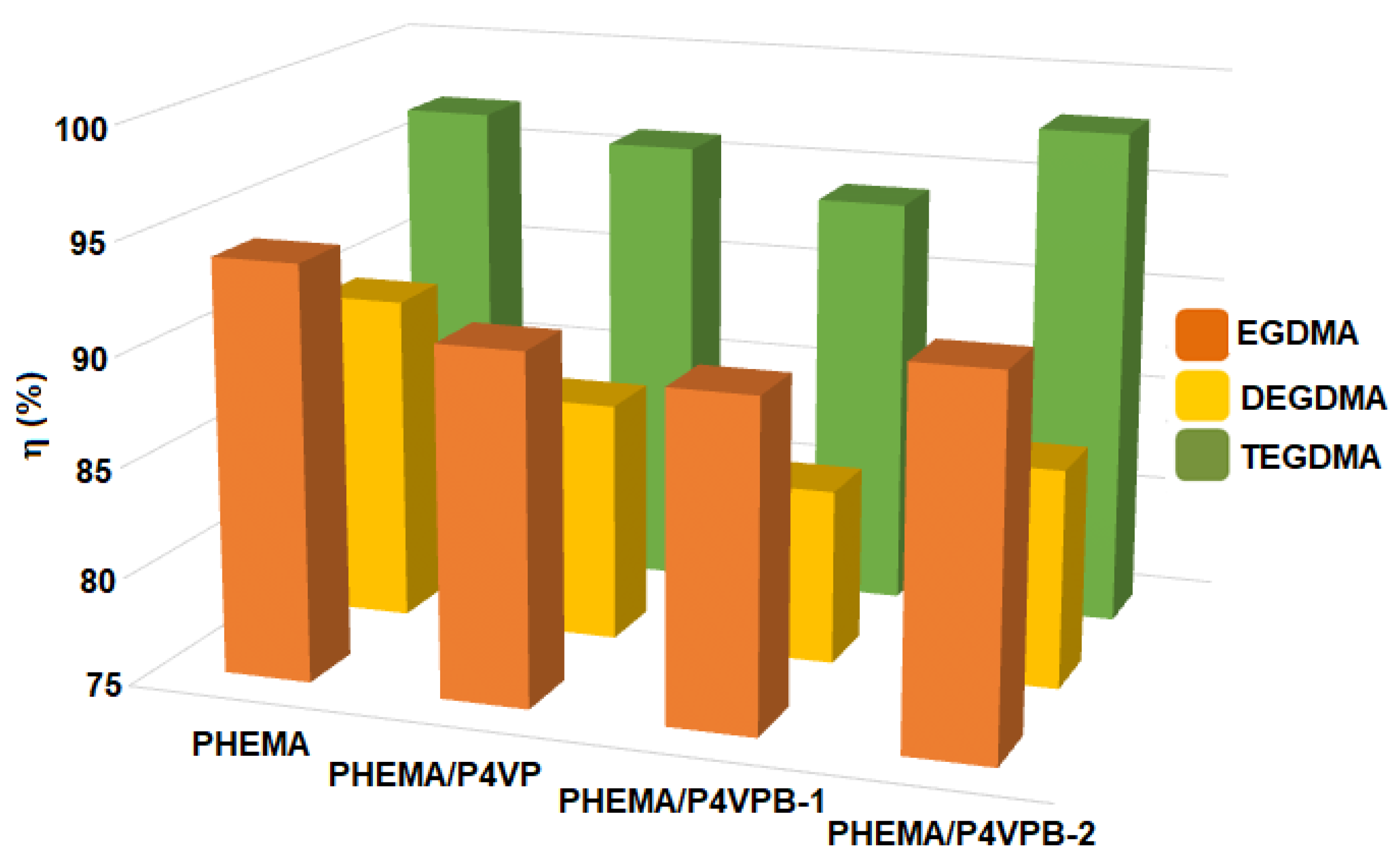
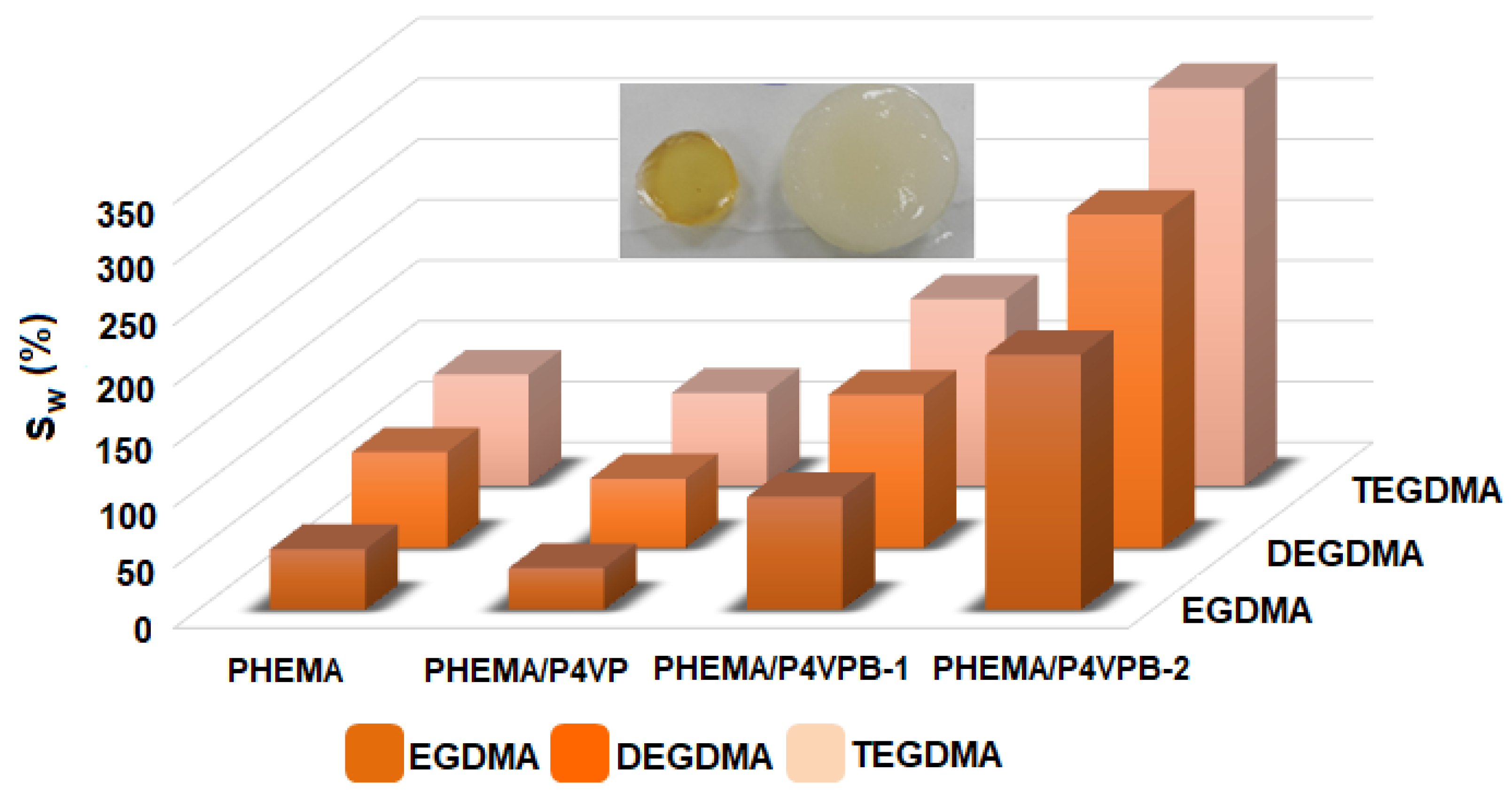

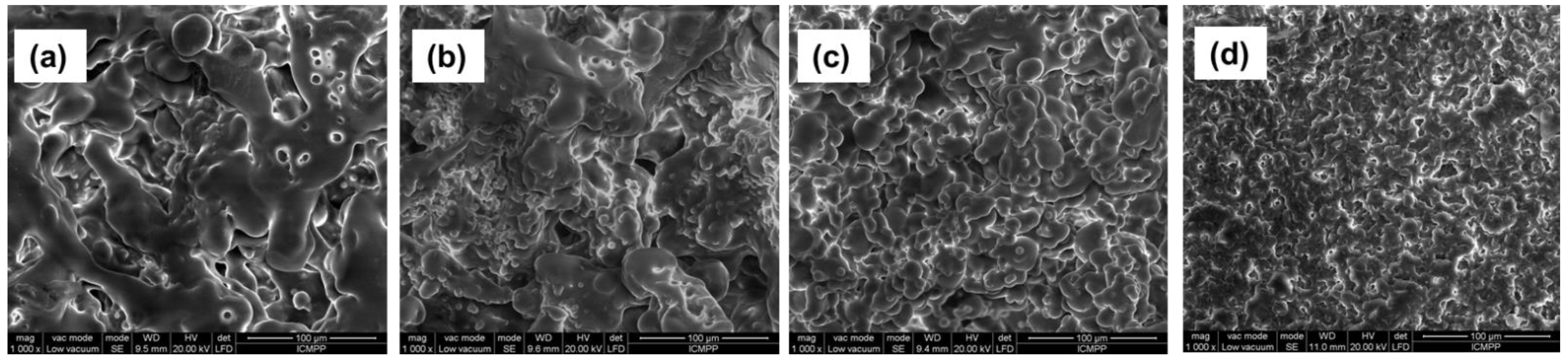




| Sample Codes | TEGDMA:HEMA (mol/mol) | η (%) | SW (%) | Images of Semi-IPN |
|---|---|---|---|---|
| 1:80 | 90 | 285.7 |  | |
| 1:67 | 92 | 270.9 | - | |
| PHEMA | 1:50 | 93 | 222.5 |  |
| 1:40 | 98 | 200.8 | - | |
| 1:20 | 98 | 70.70 |  | |
| 1:80 | 85 | 242.7 |  | |
| 1:67 | 87 | 226.3 | - | |
| PHEMA/P4VP | 1:50 | 90 | 200.7 |  |
| 1:40 | 96 | 138.6 | - | |
| 1:20 | 96 | 60.10 |  | |
| 1:80 | 88 | 386.91 |  | |
| 1:67 | 89 | 330.16 | - | |
| PHEMA/P4VPB-1 | 1:50 | 91 | 246.26 |  |
| 1:40 | 94 | 153.70 | - | |
| 1:20 | 97 | 136.31 |  | |
| 1:80 | 91 | 550.72 |  | |
| 1:67 | 93 | 472.54 | - | |
| PHEMA/P4VPB-2 | 1:50 | 94 | 391.60 |  |
| 1:40 | 97 | 327.61 | - | |
| 1:20 | 98 | 298.26 |  |
| Sample Codes | TEGDMA/HEMA (mol/mol) | HEMA/P4VPB-2 (g/g) | N (%) | P4VPB-2 (%) | |
|---|---|---|---|---|---|
| Calc. | Exp. | Found in Semi-IPN | |||
| 1:0.2 | 1.292 | 1.204 | 93.19 | ||
| P4VPB-2 | 1:50 | 1:0.3 | 1.795 | 1.710 | 95.26 |
| 1:0.4 | 2.227 | 2.140 | 96.09 | ||
| 1:0.5 | 2.603 | 2.534 | 97.35 | ||
| PHEMA/P4VP | PHEMA/P4VPB-1 | PHEMA/P4VPB-2 | ||
|---|---|---|---|---|
| First-order kinetic model [42] * | k1 (h−1) | 0.087 | 0.085 | 0.079 |
| R2 | 0.989 | 0.986 | 0.984 | |
| Higuchi model [43] * | kH (min−0.5) | 4.813 | 4.426 | 3.831 |
| R2 | 0.996 | 0.996 | 0.997 | |
| Korsmeyer–Peppas model [44] * | kr (min−n) | 0.025 | 0.017 | 0.012 |
| n | 0.659 | 0.725 | 0.761 | |
| R2 | 0.998 | 0.999 | 0.999 |
| Sample Codes | E. coli ATCC 25922 | S. aureus ATCC 25923 |
|---|---|---|
| PHEMA/P4VP-tetracycline | 12 mm | 20 mm |
| PHEMA/P4VPB-1-tetracycline | 15 mm | 23 mm |
| PHEMA/P4VPB-2-tetracycline | 17 mm | 31 mm |
| Tetracycline | 20 mm | 28 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gugoasa, A.I.; Racovita, S.; Vasiliu, S.; Popa, M. Semi-Interpenetrating Polymer Networks Based on Hydroxy-Ethyl Methacrylate and Poly(4-vinylpyridine)/Polybetaines, as Supports for Sorption and Release of Tetracycline. Polymers 2023, 15, 490. https://doi.org/10.3390/polym15030490
Gugoasa AI, Racovita S, Vasiliu S, Popa M. Semi-Interpenetrating Polymer Networks Based on Hydroxy-Ethyl Methacrylate and Poly(4-vinylpyridine)/Polybetaines, as Supports for Sorption and Release of Tetracycline. Polymers. 2023; 15(3):490. https://doi.org/10.3390/polym15030490
Chicago/Turabian StyleGugoasa, Aurica Ionela, Stefania Racovita, Silvia Vasiliu, and Marcel Popa. 2023. "Semi-Interpenetrating Polymer Networks Based on Hydroxy-Ethyl Methacrylate and Poly(4-vinylpyridine)/Polybetaines, as Supports for Sorption and Release of Tetracycline" Polymers 15, no. 3: 490. https://doi.org/10.3390/polym15030490
APA StyleGugoasa, A. I., Racovita, S., Vasiliu, S., & Popa, M. (2023). Semi-Interpenetrating Polymer Networks Based on Hydroxy-Ethyl Methacrylate and Poly(4-vinylpyridine)/Polybetaines, as Supports for Sorption and Release of Tetracycline. Polymers, 15(3), 490. https://doi.org/10.3390/polym15030490









