The Current Status, Prospects, and Challenges of Shape Memory Polymers Application in Bone Tissue Engineering
Abstract
:1. Introduction
2. The Primary Materials of SMPs and Their Synthesis
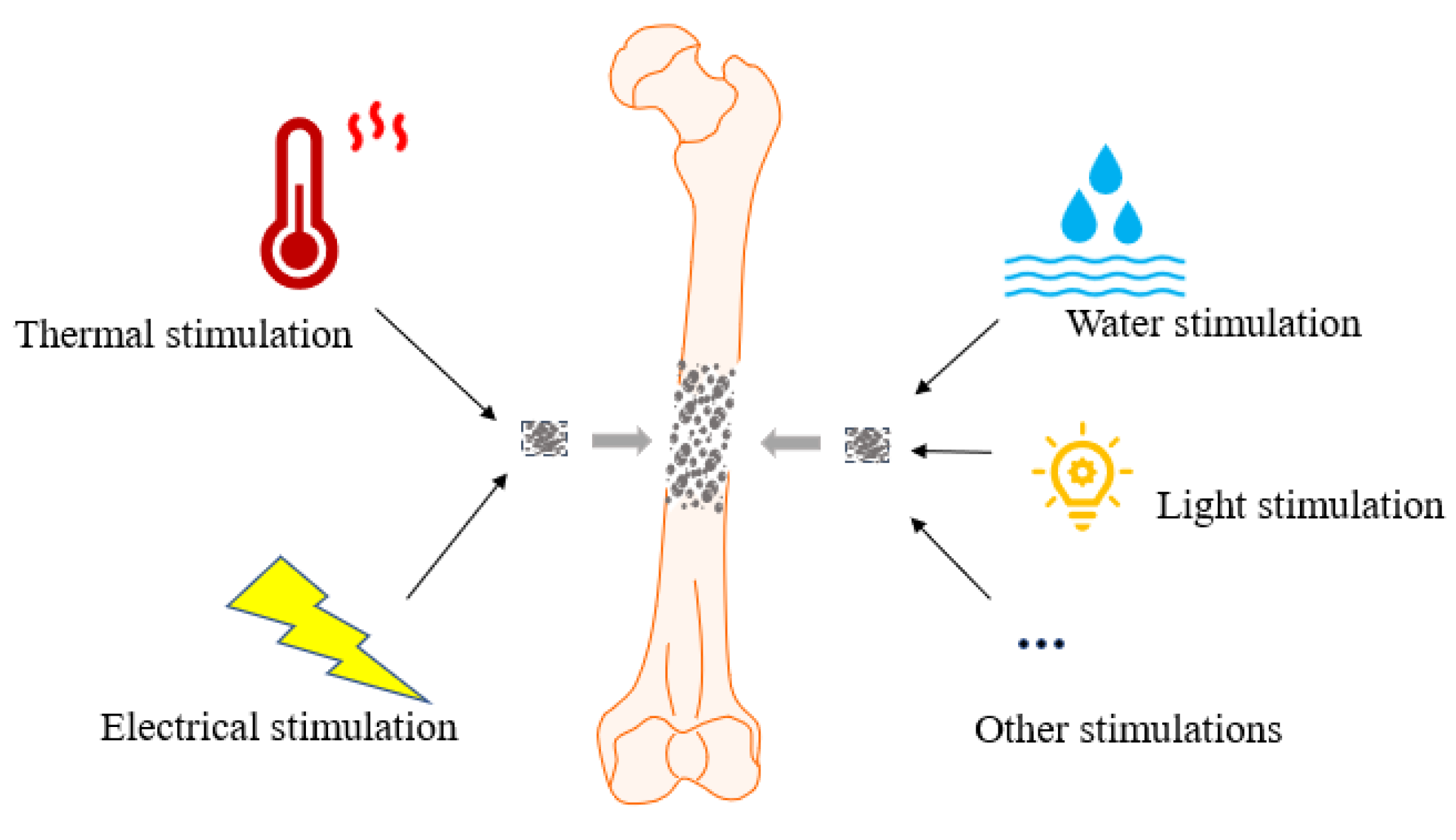
3. SMPs and Their Properties in Response to Different Stimulations
3.1. SMPs in Response to Different Stimulations
3.1.1. Thermology-Responsive SMPs
3.1.2. Water-Responsive SMPs
3.1.3. Electro-Responsive SMPs
3.1.4. Light-Responsive SMPs
3.2. Properties of SMPs
3.2.1. Biocompatibility and Biodegradability of SMPs
3.2.2. Mechanical Properties of SMPs
4. Application of SMP Scaffold in Bone Tissue Engineering
4.1. Osteogenic Properties of Bone Formation-Associated Cells in SMPs
4.1.1. Effect of SMPs on Osteoblasts and the Osteoblast Cell Line MC3T3-E1
4.1.2. Effect of SMPs on BMSCs
4.1.3. Effect of SMPs on h-MSCs and C2C12 in SMPs
4.2. Application of SMP in Craniomaxillofacial Defects
4.3. Application of SMP in Limb Bone Defects
5. Strategies and Challenges for Optimizing the Application of SMPs in Bone Tissue Engineering
5.1. Promote Bone Formation with Emphasis on Bone Angiogenesis
5.2. Increase the Gap between Bone and Periosteum through SMP to Promote Intramembranous Osteogenesis
6. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef] [PubMed]
- Mills, L.A.; Simpson, A.H. In vivo models of bone repair. J. Bone Jt. Surg. Br. 2012, 94, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Kashirina, A.; Yao, Y.; Liu, Y.; Leng, J. Biopolymers as bone substitutes: A review. Biomater. Sci. 2019, 7, 3961–3983. [Google Scholar] [CrossRef] [PubMed]
- Keeling, J.J.; Gwinn, D.E.; Tintle, S.M.; Andersen, R.C.; McGuigan, F.X. Short-term outcomes of severe open wartime tibial fractures treated with ring external fixation. J. Bone Jt. Surg. Am. 2008, 90, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Khan, A.M.; Azhar, M.H.; Javed, M.T.; Saleem, M.; Hafeez, Z. Outcome of minimally invasive plate osteosynthesis using locking compression plate in long bone fractures. J. Pak. Med. Assoc. 2021, 71 (Suppl. 5), S64–S69. [Google Scholar]
- Khalsa, A.S.; Toossi, N.; Tabb, L.P.; Amin, N.H.; Donohue, K.W.; Cerynik, D.L. Distal tibia fractures: Locked or non-locked plating? A systematic review of outcomes. Acta Orthop. 2014, 85, 299–304. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.B.; Li, S.; Deng, Y.; He, N.Y. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.U.A.; Razak, S.I.A.; Ansari, M.N.M.; Zulkifli, R.M.; Ahmad Zawawi, N.; Arshad, M. Development of Biodegradable Bio-Based Composite for Bone Tissue Engineering: Synthesis, Characterization and In Vitro Biocompatible Evaluation. Polymers 2021, 13, 3611. [Google Scholar] [CrossRef]
- Yakacki, C.M.; Shandas, R.; Lanning, C.; Rech, B.; Eckstein, A.; Gall, K. Unconstrained recovery characterization of shape-memory polymer networks for cardiovascular applications. Biomaterials 2007, 28, 2255–2263. [Google Scholar] [CrossRef] [Green Version]
- Vasita, R.; Shanmugam, I.K.; Katt, D.S. Improved biomaterials for tissue engineering applications: Surface modification of polymers. Curr. Top Med. Chem. 2008, 8, 341–353. [Google Scholar] [CrossRef]
- Gong, T.; Li, W.; Chen, H.; Wang, L.; Shao, S.; Zhou, S. Remotely actuated shape memory effect of electrospun composite nanofibers. Acta Biomater. 2012, 8, 1248–1259. [Google Scholar] [CrossRef]
- Bao, M.; Zhou, Q.; Dong, W.; Lou, X.; Zhang, Y. Ultrasound-modulated shape memory and payload release effects in a biodegradable cylindrical rod made of chitosan-functionalized PLGA microspheres. Biomacromolecules 2013, 14, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Pfau, M.R.; Grunlan, M.A. Smart scaffolds: Shape memory polymers (SMPs) in tissue engineering. J. Mater. Chem. B 2021, 9, 4287–4297. [Google Scholar] [CrossRef] [PubMed]
- Lendlein, A.; Langer, R. Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science 2002, 296, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.M.; Small, W.t.; Wilson, T.S.; Benett, W.J.; Loge, J.M.; Maitland, D.J. A shape memory polymer dialysis needle adapter for the reduction of hemodynamic stress within arteriovenous grafts. IEEE Trans. Biomed. Eng. 2007, 54, 1722–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Small, W.t.; Wilson, T.S.; Buckley, P.R.; Benett, W.J.; Loge, J.M.; Hartman, J.; Maitland, D.J. Prototype fabrication and preliminary in vitro testing of a shape memory endovascular thrombectomy device. IEEE Trans. Biomed. Eng. 2007, 54, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; George, O.J.; Petersen, K.M.; Jimenez-Vergara, A.C.; Hahn, M.S.; Grunlan, M.A. A bioactive “self-fitting” shape memory polymer scaffold with potential to treat cranio-maxillo facial bone defects. Acta Biomater. 2014, 10, 4597–4605. [Google Scholar] [CrossRef]
- Bao, M.; Wang, X.; Yuan, H.; Lou, X.; Zhao, Q.; Zhang, Y. HAp incorporated ultrafine polymeric fibers with shape memory effect for potential use in bone screw hole healing. J. Mater. Chem. B 2016, 4, 5308–5320. [Google Scholar] [CrossRef]
- Vyas, C.; Zhang, J.; Ovrebo, O.; Huang, B.Y.; Roberts, I.; Setty, M.; Allardyce, B.; Haugen, H.; Rajkhowa, R.; Bartolo, P. 3D printing of silk microparticle reinforced polycaprolactone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2021, 118, 111433. [Google Scholar] [CrossRef]
- Balk, M.; Behl, M.; Wischke, C.; Zotzmann, J.; Lendlein, A. Recent advances in degradable lactide-based shape-memory polymers. Adv. Drug Deliver. Rev. 2016, 107, 136–152. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.H.; Shao, J.D.; Ma, Y.F.; Wang, J.H.; Huang, H.; Yang, N.; Wang, H.Y.; Ruan, C.S.; Luo, Y.F.; Wang, Q.Q.; et al. Biodegradable near-infrared-photoresponsive shape memory implants based on black phosphorus nanofillers. Biomaterials 2018, 164, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Musiol, M.; Jurczyk, S.; Kwiecien, M.; Smola-Dmochowska, A.; Domanski, M.; Janeczek, H.; Wlodarczyk, J.; Klim, M.; Rydz, J.; Kawalec, M.; et al. The impact of shape memory test on degradation profile of a bioresorbable polymer. J. Mech. Behav. Biomed. 2018, 81, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhou, T.; Liu, Y.; Leng, J. Microwave synthesis and actuation of shape memory polycaprolactone foams with high speed. Sci. Rep. 2015, 5, 11152. [Google Scholar] [CrossRef] [Green Version]
- Nail, L.N.; Zhang, D.; Reinhard, J.L.; Grunlan, M.A. Fabrication of a Bioactive, PCL-based "Self-fitting" Shape Memory Polymer Scaffold. J. Vis. Exp. 2015, 104, e52981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Li, Y.; Hu, X.; Shen, J.; Guo, S. Biocompatible Shape Memory Blend for Self-Expandable Stents with Potential Biomedical Applications. ACS Appl. Mater. Interfaces 2017, 9, 13988–13998. [Google Scholar] [CrossRef]
- Defize, T.; Riva, R.; Thomassin, J.M.; Alexandre, M.; Herck, N.V.; Prez, F.D.; Jerome, C. Reversible TAD Chemistry as a Convenient Tool for the Design of (Re)processable PCL-Based Shape-Memory Materials. Macromol. Rapid Commun. 2017, 38, 1600517. [Google Scholar] [CrossRef]
- Arabiyat, A.S.; Pfau, M.R.; Grunlan, M.A.; Hahn, M.S. Intrinsic osteoinductivity of PCL-DA/PLLA semi-IPN shape memory polymer scaffolds. J. Biomed. Mater. Res. A 2021, 109, 2334–2345. [Google Scholar] [CrossRef]
- Morgan, R.A.; Loftus, I.; Ratnam, L.; Das, R.; Mailli, L.; Hamady, M.S.; Lobotesis, K. Clinical experience with a shape memory polymer peripheral vascular embolisation plug: A case series. CVIR Endovasc. 2021, 4, 29. [Google Scholar] [CrossRef]
- Lin, W.C.; Fan, F.Y.; Cheng, H.C.; Lin, Y.; Shen, Y.K.; Lai, J.S.; Wang, L.P.; Ruslin, M. Optimization Shape-Memory Situations of a Stimulus Responsive Composite Material. Polymers 2021, 13, 697. [Google Scholar] [CrossRef]
- Guo, Y.; Lv, Z.; Huo, Y.; Sun, L.; Chen, S.; Liu, Z.; He, C.; Bi, X.; Fan, X.; You, Z. A biodegradable functional water-responsive shape memory polymer for biomedical applications. J. Mater. Chem. B 2019, 7, 123–132. [Google Scholar] [CrossRef]
- Zhang, F.H.; Xia, Y.L.; Wang, L.L.; Liu, L.W.; Liu, Y.J.; Leng, J.S. Conductive Shape Memory Microfiber Membranes with Core-Shell Structures and Electroactive Performance. ACS Appl. Mater. Interfaces 2018, 10, 35526–35532. [Google Scholar] [CrossRef] [PubMed]
- Herath, M.; Epaarachchi, J.; Islam, M.; Fang, L.; Leng, J.S. Light activated shape memory polymers and composites: A review. Eur. Polym. J. 2020, 136, 109912. [Google Scholar] [CrossRef]
- Namhongsa, M.; Daranarong, D.; Sriyai, M.; Molloy, R.; Ross, S.; Ross, G.M.; Tuantranont, A.; Tocharus, J.; Sivasinprasasn, S.; Topham, P.D.; et al. Surface-Modified Polypyrrole-Coated PLCL and PLGA Nerve Guide Conduits Fabricated by 3D Printing and Electrospinning. Biomacromolecules 2022, 23, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Dayyoub, T.; Maksimkin, A.V.; Filippova, O.V.; Tcherdyntsev, V.V.; Telyshev, D.V. Shape Memory Polymers as Smart Materials: A Review. Polymers 2022, 14, 3511. [Google Scholar] [CrossRef]
- Wang, C.; Xie, J.; Xiao, X.; Chen, S.; Wang, Y. Development of Nontoxic Biodegradable Polyurethanes Based on Polyhydroxyalkanoate and L-lysine Diisocyanate with Improved Mechanical Properties as New Elastomers Scaffolds. Polymers 2019, 11, 1927. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.M.; Li, Y.; Tao, G.; Wang, L.; Zhou, S.B. Thermo- and water-induced shape memory poly(vinyl alcohol) supramolecular networks crosslinked by self-complementary quadruple hydrogen bonding. Polym. Chem. 2016, 7, 6637–6644. [Google Scholar] [CrossRef]
- Oliver, K.; Seddon, A.; Trask, R.S. Morphing in nature and beyond: A review of natural and synthetic shape-changing materials and mechanisms. J. Mater. Sci. 2016, 51, 10663–10689. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.T.; Jeong, H.J.; Park, H.C.; Jeong, H.M.; Bae, S.Y.; Kim, B.K. Electroactive shape memory performance of polyurethane/graphene nanocomposites. React. Funct. Polym. 2015, 88, 1–7. [Google Scholar] [CrossRef]
- Herath, H.M.C.M.; Epaarachchi, J.A.; Islam, M.M.; Al-Azzawi, W.; Leng, J.; Zhang, F. Structural performance and photothermal recovery of carbon fibre reinforced shape memory polymer. Compos. Sci. Technol. 2018, 167, 206–214. [Google Scholar] [CrossRef]
- Heo, M.S.; Kim, T.H.; Chang, Y.W.; Jang, K.S. Near-Infrared Light-Responsive Shape Memory Polymer Fabricated from Reactive Melt Blending of Semicrystalline Maleated Polyolefin Elastomer and Polyaniline. Polymers 2021, 13, 3984. [Google Scholar] [CrossRef]
- Dogan, S.K.; Boyacioglu, S.; Kodal, M.; Gokce, O.; Ozkoc, G. Thermally induced shape memory behavior, enzymatic degradation and biocompatibility of PLA/TPU blends: "Effects of compatibilization". J. Mech. Behav. Biomed. 2017, 71, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Rubio Hernandez-Sampelayo, A.; Navarro, R.; Gonzalez-Garcia, D.M.; Garcia-Fernandez, L.; Ramirez-Jimenez, R.A.; Aguilar, M.R.; Marcos-Fernandez, A. Biodegradable and Biocompatible Thermoplastic Poly(Ester-Urethane)s Based on Poly(epsilon-Caprolactone) and Novel 1,3-Propanediol Bis(4-Isocyanatobenzoate) Diisocyanate: Synthesis and Characterization. Polymers 2022, 14, 1288. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Kunkel, R.; Luo, J.S.; Li, Y.H.; Liu, H.; Bohnstedt, B.N.; Liu, Y.T.; Lee, C.H. Shape Memory Polyurethane with Porous Architectures for Potential Applications in Intracranial Aneurysm Treatment. Polymers 2019, 11, 631. [Google Scholar] [CrossRef] [Green Version]
- Peterson, G.I.; Dobrynin, A.V.; Becker, M.L. Biodegradable Shape Memory Polymers in Medicine. Adv. Healthc. Mater. 2017, 6, 1700694. [Google Scholar] [CrossRef] [Green Version]
- Paderni, K.; Fabbri, P.; Toselli, M.; Messori, M. Shape Memory Properties of PBS-Silica Hybrids. Materials 2014, 7, 751–768. [Google Scholar] [CrossRef]
- Zimkowski, M.M.; Rentschler, M.E.; Schoen, J.; Rech, B.A.; Mandava, N.; Shandas, R. Integrating a novel shape memory polymer into surgical meshes decreases placement time in laparoscopic surgery: An in vitro and acute in vivo study. J. Biomed. Mater. Res. Part A 2013, 101, 2613–2620. [Google Scholar] [CrossRef] [Green Version]
- Peng, K.; Zhao, Y.; Shahab, S.; Mirzaeifar, R. Ductile Shape-Memory Polymer Composite with Enhanced Shape Recovery Ability. ACS Appl. Mater. Interfaces 2020, 12, 58295–58300. [Google Scholar] [CrossRef]
- Iqbal, D.; Samiullah, M.H. Photo-Responsive Shape-Memory and Shape-Changing Liquid-Crystal Polymer Networks. Materials 2013, 6, 116–142. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.S.; Cha, J.R.; Gong, M.S. Biodegradable shape-memory polymers using polycaprolactone and isosorbide based polyurethane blends. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 426–435. [Google Scholar] [CrossRef]
- Park, B.; Jung, Y.; Ko, J.S.; Park, J.; Cho, H. Self-Restoring Capacitive Pressure Sensor Based on Three-Dimensional Porous Structure and Shape Memory Polymer. Polymers 2021, 13, 824. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Al-Arjan, W.S.; Ashammakhi, N.; Haider, S.; Amin, R.; Hasan, A. Multifunctional Bioactive Scaffolds from ARX-g-(Zn@rGO)-HAp for Bone Tissue Engineering: In Vitro Antibacterial, Antitumor, and Biocompatibility Evaluations. ACS Appl. Bio. Mater. 2022, 5, 5445–5456. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Rizwan, M.; Razak, S.I.A.; Hassan, A.; Rasheed, T.; Bilal, M. Electroactive polymeric nanocomposite BC-g-(Fe(3)O(4)/GO) materials for bone tissue engineering: In vitro evaluations. J. Biomater. Sci. Polym. Ed. 2022, 33, 1349–1368. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Teraoka, F. Application of shape memory polymer to dental materials (part 1). Physical properties. J. Osaka Univ. Dent. Sch. 1986, 26, 59–65. [Google Scholar] [PubMed]
- Xia, Y.; He, Y.; Zhang, F.; Liu, Y.; Leng, J. A Review of Shape Memory Polymers and Composites: Mechanisms, Materials, and Applications. Adv. Mater. 2021, 33, e2000713. [Google Scholar] [CrossRef]
- Bao, M.; Lou, X.; Zhou, Q.; Dong, W.; Yuan, H.; Zhang, Y. Electrospun biomimetic fibrous scaffold from shape memory polymer of PDLLA-co-TMC for bone tissue engineering. ACS Appl. Mater. Interfaces 2014, 6, 2611–2621. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Hu, J.; Hoffmann, O.; Zhang, Y.; Ng, F.; Qin, T.; Guo, X. Self-fitting shape memory polymer foam inducing bone regeneration: A rabbit femoral defect study. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Erndt-Marino, J.D.; Munoz-Pinto, D.J.; Samavedi, S.; Jimenez-Vergara, A.C.; Diaz-Rodriguez, P.; Woodard, L.; Zhang, D.; Grunlan, M.A.; Hahn, M.S. Evaluation of the Osteoinductive Capacity of Polydopamine-Coated Poly(epsilon-caprolactone) Diacrylate Shape Memory Foams. ACS Biomater. Sci. Eng. 2015, 1, 1220–1230. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Xie, R.; Yang, Y.; Cao, J.; Tu, Y.; Zhang, Y.; Qin, T.; Zhao, X. A programmable, fast-fixing, osteo-regenerative, biomechanically robust bone screw. Acta Biomater. 2020, 103, 293–305. [Google Scholar] [CrossRef]
- Huang, K.; Yang, M.S.; Tang, Y.J.; Ling, S.Y.; Pan, F.; Liu, X.D.; Chen, J. Porous shape memory scaffold of dextran and hydroxyapatite for minimum invasive implantation for bone tissue engineering applications. J. Biomater. Appl. 2021, 35, 823–837. [Google Scholar] [CrossRef]
- Wang, X.; Yan, H.; Shen, Y.; Tang, H.; Yi, B.; Qin, C.; Zhang, Y. Shape Memory and Osteogenesis Capabilities of the Electrospun Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Modified Poly(l-Lactide) Fibrous Mats. Tissue Eng. Part A 2021, 27, 142–152. [Google Scholar] [CrossRef]
- Tseng, L.F.; Wang, J.; Baker, R.M.; Wang, G.; Mather, P.T.; Henderson, J.H. Osteogenic Capacity of Human Adipose-Derived Stem Cells is Preserved Following Triggering of Shape Memory Scaffolds. Tissue Eng. Part A 2016, 22, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.S.; Sohn, J.S.; Cha, S.W. Shape-Memory-Recovery Characteristics of Microcellular Foamed Thermoplastic Polyurethane. Polymers 2020, 12, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, M.; Wang, L.; Ge, J.; Guo, B.; Ma, P.X. Strong electroactive biodegradable shape memory polymer networks based on star-shaped polylactide and aniline trimer for bone tissue engineering. ACS Appl. Mater. Interfaces 2015, 7, 6772–6781. [Google Scholar] [CrossRef]
- Deng, Z.; Guo, Y.; Zhao, X.; Li, L.; Dong, R.; Guo, B.; Ma, P.X. Stretchable degradable and electroactive shape memory copolymers with tunable recovery temperature enhance myogenic differentiation. Acta Biomater. 2016, 46, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, K.; Gong, T.; Song, J.; Bao, C.; Luo, E.; Weng, J.; Zhou, S. Delivery of growth factors using a smart porous nanocomposite scaffold to repair a mandibular bone defect. Biomacromolecules 2014, 15, 1019–1030. [Google Scholar] [CrossRef]
- Wang, S.; Lu, L.; Gruetzmacher, J.A.; Currier, B.L.; Yaszemski, M.J. Synthesis and characterizations of biodegradable and crosslinkable poly(epsilon-caprolactone fumarate), poly(ethylene glycol fumarate), and their amphiphilic copolymer. Biomaterials 2006, 27, 832–841. [Google Scholar] [CrossRef]
- Woodard, L.N.; Page, V.M.; Kmetz, K.T.; Grunlan, M.A. PCL-PLLA Semi-IPN Shape Memory Polymers (SMPs): Degradation and Mechanical Properties. Macromol. Rapid Commun. 2016, 37, 1972–1977. [Google Scholar] [CrossRef]
- Woodard, L.N.; Kmetz, K.T.; Roth, A.A.; Page, V.M.; Grunlan, M.A. Porous Poly(epsilon-caprolactone)-Poly(l-lactic acid) Semi-Interpenetrating Networks as Superior, Defect-Specific Scaffolds with Potential for Cranial Bone Defect Repair. Biomacromolecules 2017, 18, 4075–4083. [Google Scholar] [CrossRef]
- Pfau, M.R.; McKinzey, K.G.; Roth, A.A.; Graul, L.M.; Maitland, D.J.; Grunlan, M.A. Shape memory polymer (SMP) scaffolds with improved self-fitting properties. J. Mater. Chem. B 2021, 9, 3826–3837. [Google Scholar] [CrossRef]
- Lawson, Z.T.; Han, J.; Saunders, W.B.; Grunlan, M.A.; Moreno, M.R.; Robbins, A.B. Methodology for performing biomechanical push-out tests for evaluating the osseointegration of calvarial defect repair in small animal models. MethodsX 2021, 8, 101541. [Google Scholar] [CrossRef]
- Pfau, M.R.; Beltran, F.O.; Woodard, L.N.; Dobson, L.K.; Gasson, S.B.; Robbins, A.B.; Lawson, Z.T.; Brian Saunders, W.; Moreno, M.R.; Grunlan, M.A. Evaluation of a self-fitting, shape memory polymer scaffold in a rabbit calvarial defect model. Acta Biomater. 2021, 136, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.M.; Tseng, L.F.; Iannolo, M.T.; Oest, M.E.; Henderson, J.H. Self-deploying shape memory polymer scaffolds for grafting and stabilizing complex bone defects: A mouse femoral segmental defect study. Biomaterials 2016, 76, 388–398. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, Y.; Liu, X.; Sun, Y.; Zhao, Q.; Liu, L.; Zhu, Z.; Luo, E. Smart Porous Scaffold Promotes Peri-Implant Osteogenesis under the Periosteum. ACS Biomater. Sci. Eng. 2020, 6, 6321–6330. [Google Scholar] [CrossRef]
- Guiducci, S.; Distler, O.; Distler, J.H.; Matucci-Cerinic, M. Mechanisms of vascular damage in SSc--implications for vascular treatment strategies. Rheumatology 2008, 47 (Suppl. 5), v18–v20. [Google Scholar] [CrossRef] [Green Version]
- Richardson, T.P.; Peters, M.C.; Ennett, A.B.; Mooney, D.J. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001, 19, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Saik, J.E.; Gould, D.J.; Keswani, A.H.; Dickinson, M.E.; West, J.L. Biomimetic hydrogels with immobilized ephrinA1 for therapeutic angiogenesis. Biomacromolecules 2011, 12, 2715–2722. [Google Scholar] [CrossRef] [Green Version]
- Levenberg, S.; Rouwkema, J.; Macdonald, M.; Garfein, E.S.; Kohane, D.S.; Darland, D.C.; Marini, R.; van Blitterswijk, C.A.; Mulligan, R.C.; D’Amore, P.A.; et al. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 2005, 23, 879–884. [Google Scholar] [CrossRef]
- Van Tomme, S.R.; Hennink, W.E. Biodegradable dextran hydrogels for protein delivery applications. Expert Rev. Med. Devices 2007, 4, 147–164. [Google Scholar] [CrossRef]
- Sun, G.; Mao, J.J. Engineering dextran-based scaffolds for drug delivery and tissue repair. Nanomedicine 2012, 7, 1771–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pokharel, M.; Park, K. Light mediated drug delivery systems: A review. J. Drug Target. 2022, 30, 368–380. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Friess, F.; Wischke, C.; Lendlein, A. A multifunctional multimaterial system for on-demand protein release. J. Control. Release 2018, 284, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, H.; Esworthy, T.; Mei, D.; Wang, Y.; Zhang, L.G. Emerging 4D Printing Strategies for Next-Generation Tissue Regeneration and Medical Devices. Adv Mater. 2022, 34, e2109198. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Kinoshita, K.; Ueda, M. Effects of cortical bone perforation on periosteal distraction: An experimental study in the rabbit mandible. J. Oral Maxillofac. Surg. 2009, 67, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Duchamp de Lageneste, O.; Colnot, C. Periostin in Bone Regeneration. Adv. Exp. Med. Biol. 2019, 1132, 49–61. [Google Scholar] [CrossRef]
- Kojimoto, H.; Yasui, N.; Goto, T.; Matsuda, S.; Shimomura, Y. Bone lengthening in rabbits by callus distraction. The role of periosteum and endosteum. J. Bone Jt. Surg. Br. 1988, 70, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Verdugo, F.; D’Addona, A.; Ponton, J. Clinical, tomographic, and histological assessment of periosteal guided bone regeneration with cortical perforations in advanced human critical size defects. Clin. Implant. Dent. Relat. Res. 2012, 14, 112–120. [Google Scholar] [CrossRef]

| SMP Material | Cell/Tissue | Species | Effects on Bone Engineering | Disadvantage | References |
|---|---|---|---|---|---|
| PDLLA-co-TMC | Calvarial osteoblasts | Rat | Support adhesion and proliferation of osteoblasts and functionally promote the expression of alkaline phosphatases and mineral deposition | - | [55] |
| PCL, polydopamine-coated scaffolds | Osteoblasts | Human | Support osteoblasts adhesion, proliferation, and osteogenic gene expression/extracellular matrix deposition | - | [17] |
| PCL-HA | BMSCs | Rat | Promote adhesion, proliferation, and osteogenic differentiation of BMSCs, and promote angiogenesis | [59] | |
| PCL-DA | - | - | - | - | [24] |
| PCL-DA | Skull | Rabbit | Promote osseointegration at the scaffold/defect interface | - | [71] |
| PD-PCLDA | h-MSCs | Human | Promote the osteogenic differentiation of h-MSCs | - | [57] |
| tBA-BA and TPU | ASCs | Human | Both foam scaffolds and fiber scaffolds promote osteogenic differentiation of h-ASCs | - | [61] |
| PCL-PLLA | - | - | To a certain extent, the thermal properties, shape memory behavior, degradation rate, and mechanical properties meet the requirements for repairing irregular cranial bone defects | - | [68] |
| PCL-HA | MC3T3-E1/Femur | Rabbit | Support MCET3-E1 cell infiltration and growth, promote angiogenesis and bone formation in the femoral defects | Slow degradation rate | [56] |
| BMP-loaded cPCL-HA | BMSCs/mandibular | Rabbit | Support the growth of BMSCs in vitro and promote the healing of mandibular defects in rabbits in vivo | Slow degradation rate | [65] |
| PBF | Calvarial osteoblasts | Rat | Promote osteoblast adhesion, viability, and alkaline phosphatase activity | - | [30] |
| SMPU-HA-RGD | BMSCs | Rabbit | Support survival, proliferation, and osteogenic differentiation of BMSCs in vitro; promote bone formation in the femoral defect in vivo | The modulus is not sufficient to replace metal screws for cortical bone repair | [58] |
| PCL-HA | BMSCs/mandibular | Rabbit | Increase the stability of titanium implants and promote bone formation | Lack of examination of degradation rate in vivo | [73] |
| PLLA-PHBV | BMSCs | Mouse | Promote osteogenic differentiation of BMSCs | - | [60] |
| PCL-DA/PLLA semi-IPN | H-MSCs | Human | Promote osteogenic differentiation of h-MSCs | - | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Chen, L.; Yuan, Y.; Shi, R. The Current Status, Prospects, and Challenges of Shape Memory Polymers Application in Bone Tissue Engineering. Polymers 2023, 15, 556. https://doi.org/10.3390/polym15030556
Li T, Chen L, Yuan Y, Shi R. The Current Status, Prospects, and Challenges of Shape Memory Polymers Application in Bone Tissue Engineering. Polymers. 2023; 15(3):556. https://doi.org/10.3390/polym15030556
Chicago/Turabian StyleLi, Tingting, Liang Chen, Yu Yuan, and Rengfei Shi. 2023. "The Current Status, Prospects, and Challenges of Shape Memory Polymers Application in Bone Tissue Engineering" Polymers 15, no. 3: 556. https://doi.org/10.3390/polym15030556





