Synthesis and Demulsification Properties of Poly (DMDAAC-co-DAMBAC) (9:1) Copolymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Poly (DMDAAC-co-DAMBAC) (9:1)
2.3. Single-Factor Exploratory Experiments
2.4. Process Optimization Experiments
2.5. Characterization
2.5.1. Determination of Intrinsic Viscosity
2.5.2. Determination of Double Bond Conversion
2.5.3. Refinement of the Product
2.5.4. Product Structure and Relative Molecular Mass Characterization
2.6. Demulsification Performance Experiment
3. Results and Discussion
3.1. Results Analysis of Response Surface Methodology
3.1.1. Surface Optimization of Feed Ratio Factors
3.1.2. Surface Optimization for Temperature Factors
3.2. Structural Analysis of Monomers and Copolymers
3.2.1. FTIR Characterization
3.2.2. NMR Characterization
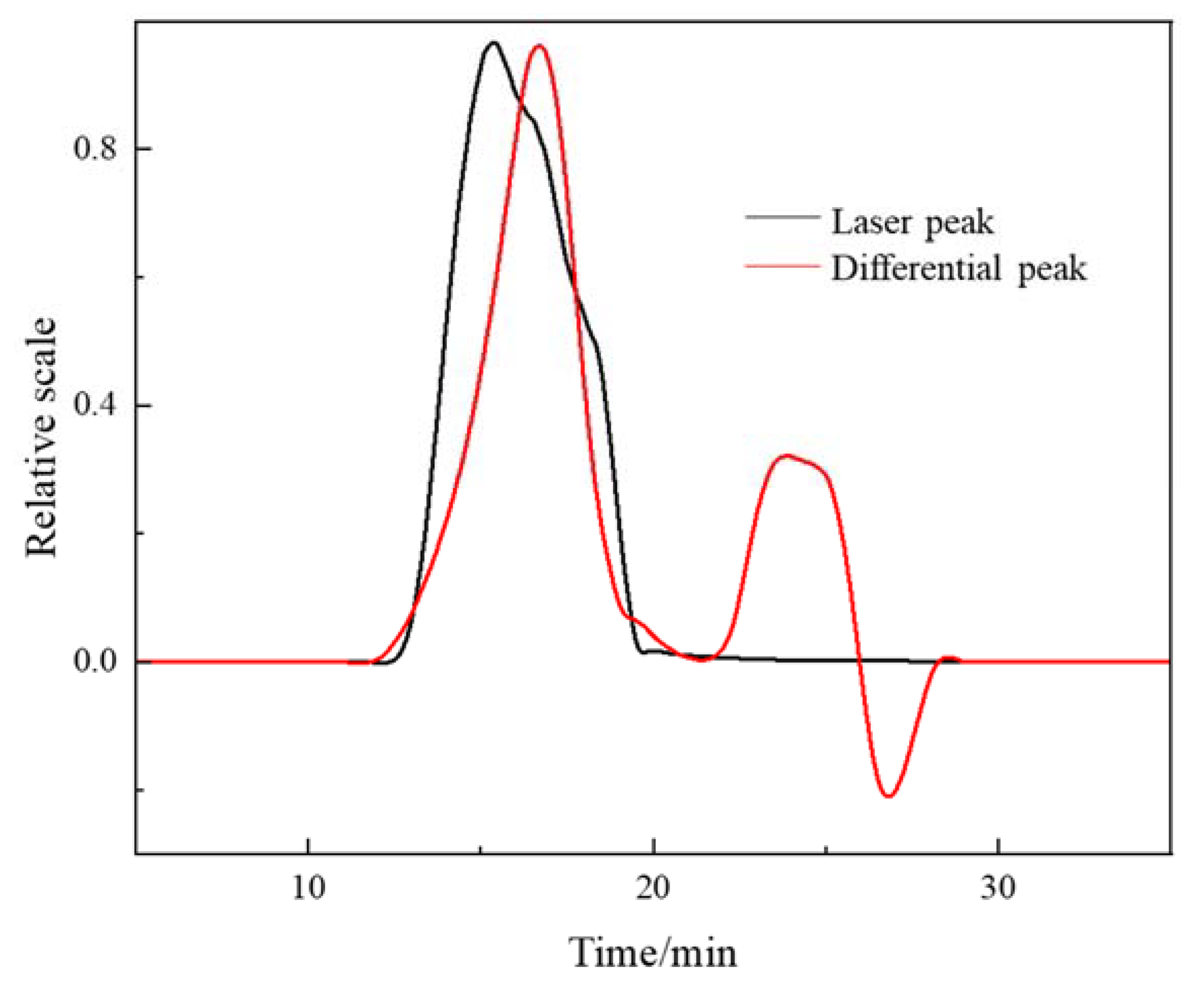
3.2.3. GPC-MALLS Characterization
3.3. Effect of Copolymerization Ratio on Polymerization Activity
3.4. Effect of Intrinsic Viscosity (Relative Molecular Weight) on Demulsification Properties of Polymers
3.5. Comparison of Demulsification Performance with Other Demulsifiers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, Z.; Guo, H.; Xu, J.; Li, Z.; Sun, G. Cationic poly(diallyldimethylammonium chloride) based hydrogel for effective anionic dyes adsorption from aqueous solution. React. Funct. Polym. 2022, 174, 105239. [Google Scholar] [CrossRef]
- Ushimaru, K.; Morita, T.; Fukuoka, T. A bio-based adhesive composed of polyelectrolyte complexes of lignosulfonate and cationic polyelectrolytes. J. Wood Chem. Technol. 2020, 40, 172–177. [Google Scholar] [CrossRef]
- Yang, S.; Wen, Y.; Duan, C.; Zhang, H.; Liu, X.; Ni, Y. Poly dimethyl diallyl ammonium chloride assisted cellulase pretreatment for pulp refining efficiency enhancement. Carbohydr. Polym. 2019, 203, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Gao, B.; Guo, K.; Yue, Q. Characterization and influence of floc under different coagulation systems on ultrafiltration membrane fouling. Chemosphere 2020, 238, 124659. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, W.; Zhai, C.; Liu, J. Effect of poly(diallyldimethylammonium chloride) adsorption on the dispersion features of SiC particles in aqueous media. New J. Chem. 2021, 45, 4638–4646. [Google Scholar] [CrossRef]
- Zhang, L.; Yi, D.; Hao, J. Poly(diallyldimethylammonium) and polyphosphate polyelectrolyte complex as flame retardant for char-forming epoxy resins. J. Fire Sci. 2020, 38, 333–347. [Google Scholar] [CrossRef]
- Ayatzhan, A.; Tashenov, A.; Nurgeldi, A.; Zhanar, O.; Zhexenbek, T.; Kaldibek, A.; Nuraje, N. P(DADMAAC-co-DMAA): Synthesis, thermal stability, and kinetics. Polym. Adv. Technol. 2021, 32, 2669–2675. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, X.; Peng, W.; Yang, K.; Xu, X.; Zhang, Y.; Wang, G.; Tao, X. Effect of Substituents on the Homopolymerization Activity of Methyl Alkyl Diallyl Ammonium Chloride. Molecules 2022, 27, 4677. [Google Scholar] [CrossRef]
- Novikova, A.A.; Vlasov, P.S.; Lin, S.-Y.; Sedláková, Z.; Noskov, B.A. Dynamic surface properties of poly(methylalkyldiallylammonium chloride) solutions. J. Taiwan Inst. Chem. Eng. 2017, 80, 122–127. [Google Scholar] [CrossRef]
- Timofeeva, L.M.; Kleshcheva, N.A.; Shleeva, M.O.; Filatova, M.P.; Simonova, Y.A.; Ermakov, Y.A.; Kaprelyants, A.S. Nonquaternary poly(diallylammonium) polymers with different amine structure and their biocidal effect on Mycobacterium tuberculosis and Mycobacterium smegmatis. Appl. Microbiol. Biotechnol. 2015, 99, 2557–2571. [Google Scholar] [CrossRef]
- Dauletov, Y.; Nuraje, N.; Abdiyev, K.; Toktarbay, Z.; Zhursumbaeva, M. Copolymers of Diallyldimethylammonium Chloride and Vinyl Ether of Monoethanolamine: Synthesis, Flocculating, and Antimicrobial Properties. J. Surfactants Deterg. 2019, 22, 1129–1137. [Google Scholar] [CrossRef]
- Santos, R.L.O.D.; Gallo, R.T.; Fernandes, L.G.; Coto, N.P.; Domaneschi, C.; Sugaya, N.N.; Paula, C.R. Inclusion and Antifungal Activity of Acrylic Resins Including Poly (Diallyldimethylammonium Chloride) Biocide. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, e285. [Google Scholar] [CrossRef]
- Lezov, A.A.; Lezova, A.A.; Vlasov, P.S.; Samokhvalova, S.A.; Rogozhin, V.B.; Polushina, G.E.; Tsvetkov, N.V. Cationic electrolyte copolymers of diallyldimethylammonium chloride with carboxybetaine 2-(diallyl(methyl) ammonio) acetate of various compositions in water solutions of different ionic strengths. J. Polym. Res. 2019, 26, 97. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Tao, X.; Jia, X.; Zhang, Y. Preparation and Decolorization of Polymethyl Benzyl Diallyl Ammonium Chloride. Polym. Mater. Sci. Eng. 2019, 35, 1–7. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, X.; Zhang, Y.; Tao, X. Synthesis and Characterization of Polymethylpropyldiallylammonium Chloride. Fine Chem. Ind. 2019, 36, 429–436. [Google Scholar] [CrossRef]
- Yang, J.Y.; Yan, L.; Li, S.P.; Xu, X.R. Treatment of aging oily wastewater by demulsification/flocculation. J. Environ. Sci. Health Part A 2016, 51, 798–804. [Google Scholar] [CrossRef]
- Wang, R.; Cai, Y.; Su, Z.; Ma, X.; Wu, W. High positively charged Fe3O4 nanocomposites for efficient and recyclable demulsification of hexadecane-water micro-emulsion. Chemosphere 2022, 291, 133050. [Google Scholar] [CrossRef]
- Nassiri, H.; Arabi, H.; Hakim, S.; Bolandi, S. Polymerization of propylene with Ziegler–Natta catalyst: Optimization of operating conditions by response surface methodology (RSM). Polym. Bull. 2011, 67, 1393–1411. [Google Scholar] [CrossRef]
- Shokri, A.-a.; Talebi, S.; Salami-Kalajahi, M. Polymerization of 1,3-butadiene using neodymium versatate: Optimization of NdV3/TEAL/EASC molar ratios via response surface methodology (RSM). Polym. Bull. 2019, 77, 5245–5260. [Google Scholar] [CrossRef]
- Huval, C.C.; Holmes-Farley, S.R.; Mandeville, W.H.; Sacchiero, R.; Dhal, P.K. Syntheses of hydrophobically modified cationic hydrogels by copolymerization of alkyl substituted diallylamine monomers and their use as bile acid sequestrants. Eur. Polym. J. 2004, 40, 693–701. [Google Scholar] [CrossRef]
- Hao, H.; Wang, G.; Xu, M.; Chang, Q. Optimization of the Preparation of Thioglycol Acetylated Polyacrylamide as a Chelating Flocculant by Response Surface Methodology. Environ. Chem. 2016, 35, 1269–1279. [Google Scholar]
- Hong, M.; Liu, W.; Liu, Y.; Dai, X.; Kang, Y.; Li, R.; Bao, F.; Qiu, X.; Pan, Y.; Ji, X. Improved characterization on molecular weight of polyamic acids using gel permeation chromatography coupled with differential refractive index and multi-angle laser light scattering detectors. Polymer 2022, 260, 125370. [Google Scholar] [CrossRef]
- Hu, B.; Han, L.; Kong, H.; Nishinari, K.; Phillips, G.O.; Yang, J.; Fang, Y. Preparation and emulsifying properties of trace elements fortified gum arabic. Food Hydrocoll. 2019, 88, 43–49. [Google Scholar] [CrossRef]
- Rozada, R.; Paredes, J.I.; López, M.J.; Villar-Rodil, S.; Cabria, I.; Alonso, J.A.; Martínez-Alonso, A.; Tascón, J.M.D. From graphene oxide to pristine graphene: Revealing the inner workings of the full structural restoration. Nanoscale 2015, 7, 2374–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Guo, R.; Xie, C.; Wang, M.; Wang, E. Synthesis and Performance of Oil in Water Demulsifier for Crude Oil Emulsion. Petrochem. Ind. 2014, 43, 1053–1057. [Google Scholar]
- Xu, J.; Ji, P.; Yu, H.; Chen, G. Progress in demulsification of complex crude oil. Chem. Technol. Dev. 2018, 47, 25–28. [Google Scholar]
- Zhang, S.; Ma, Z.; Yi, X.; Zhao, M. Research progress of reverse demulsifier at home and abroad. Fine Spec. Chem. 2009, 17, 18–20. [Google Scholar]
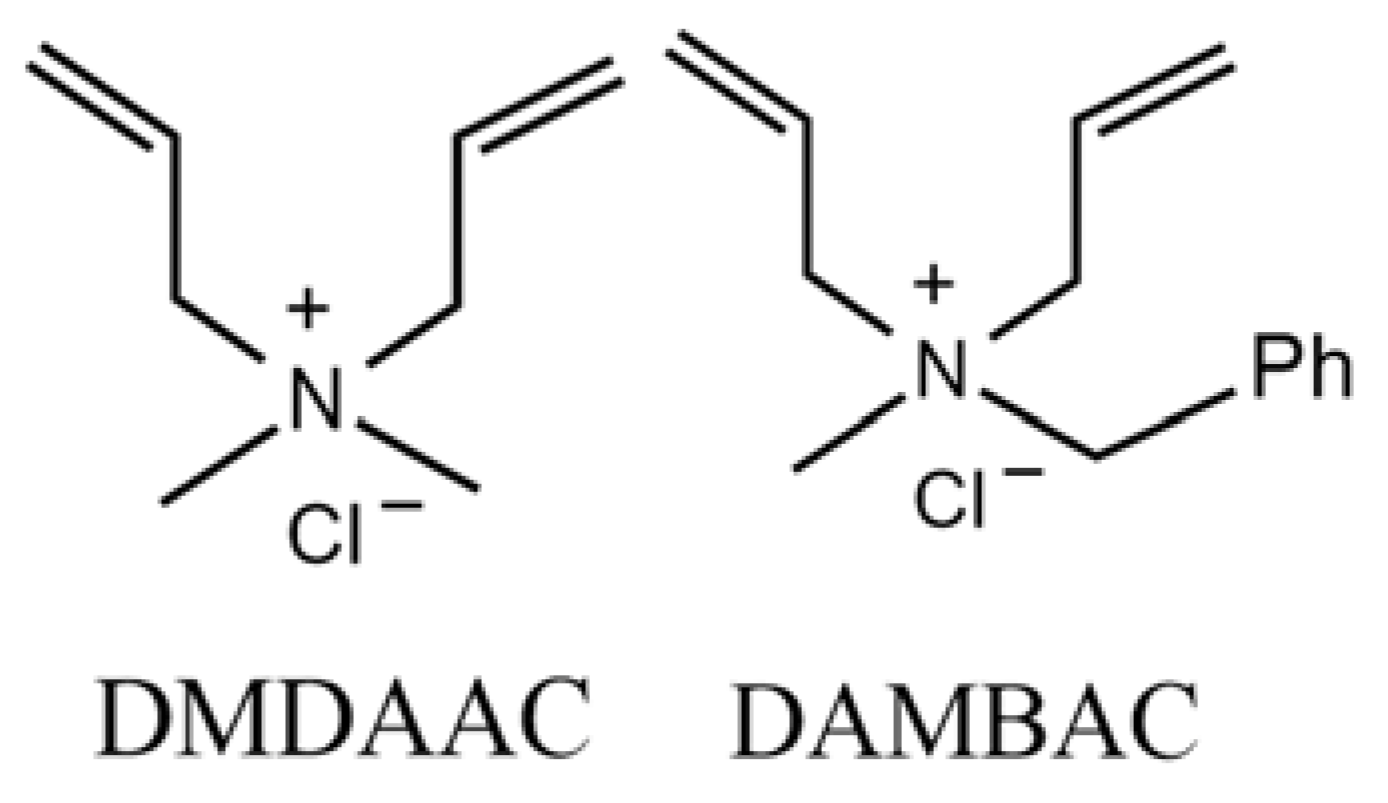
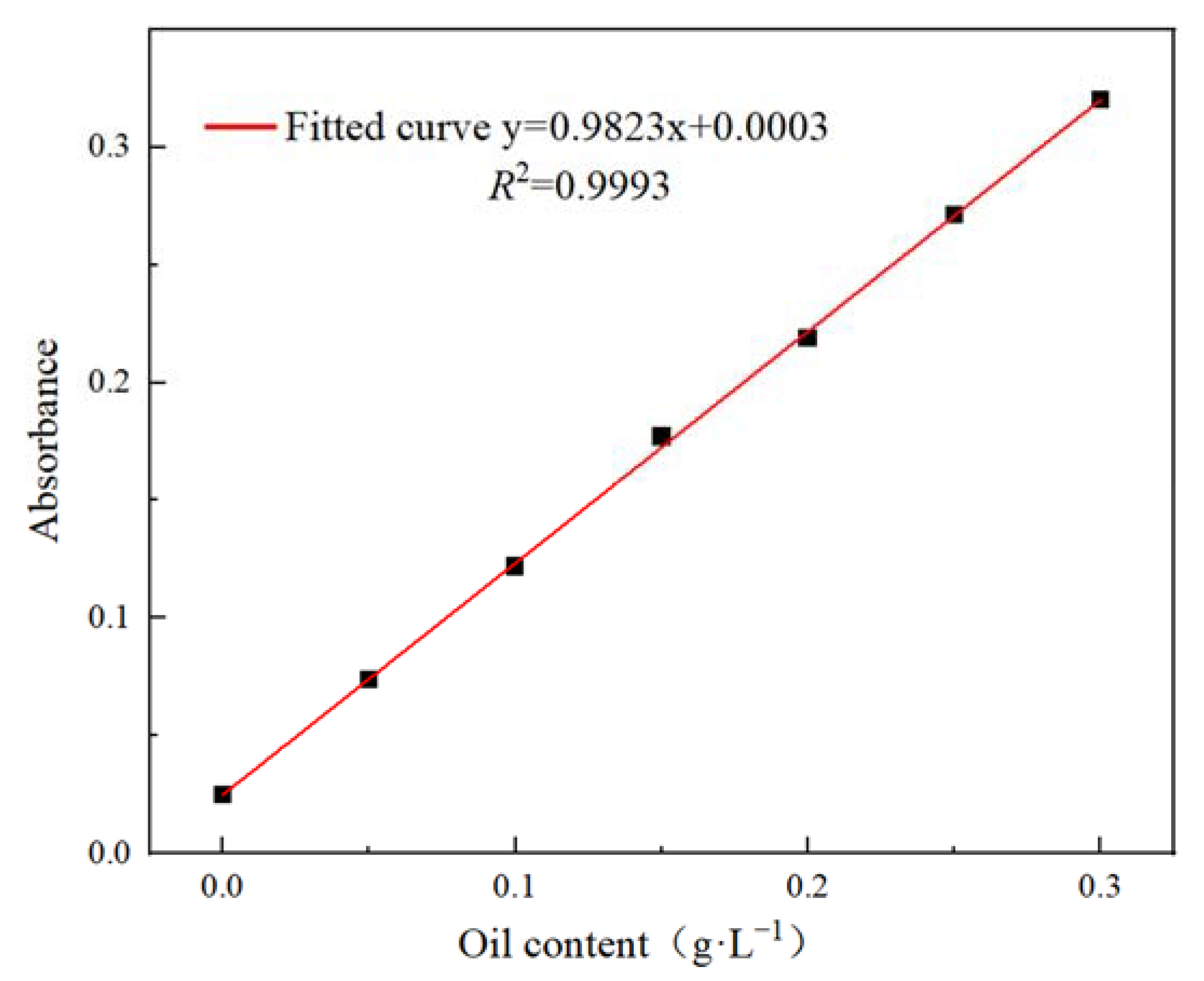
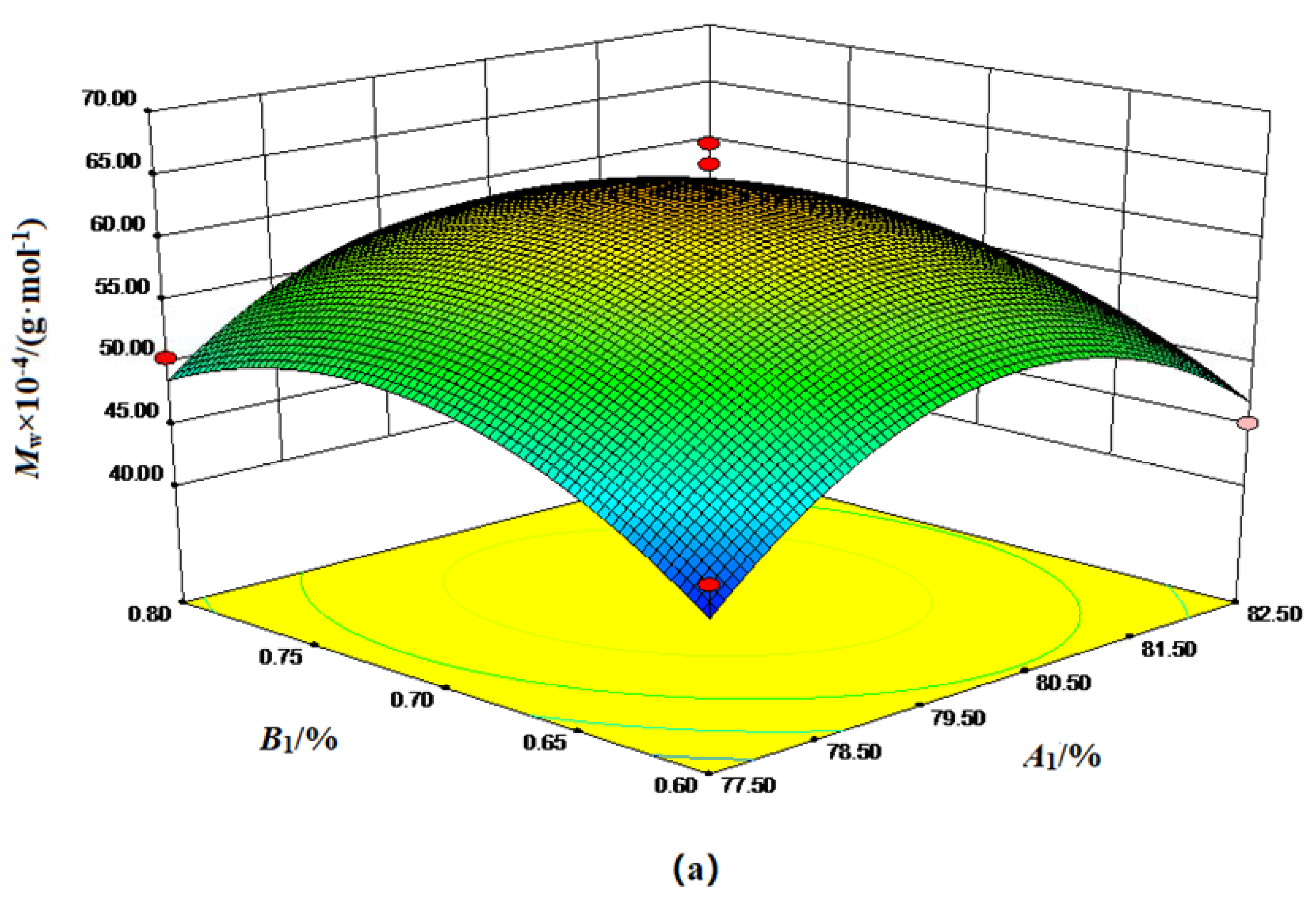





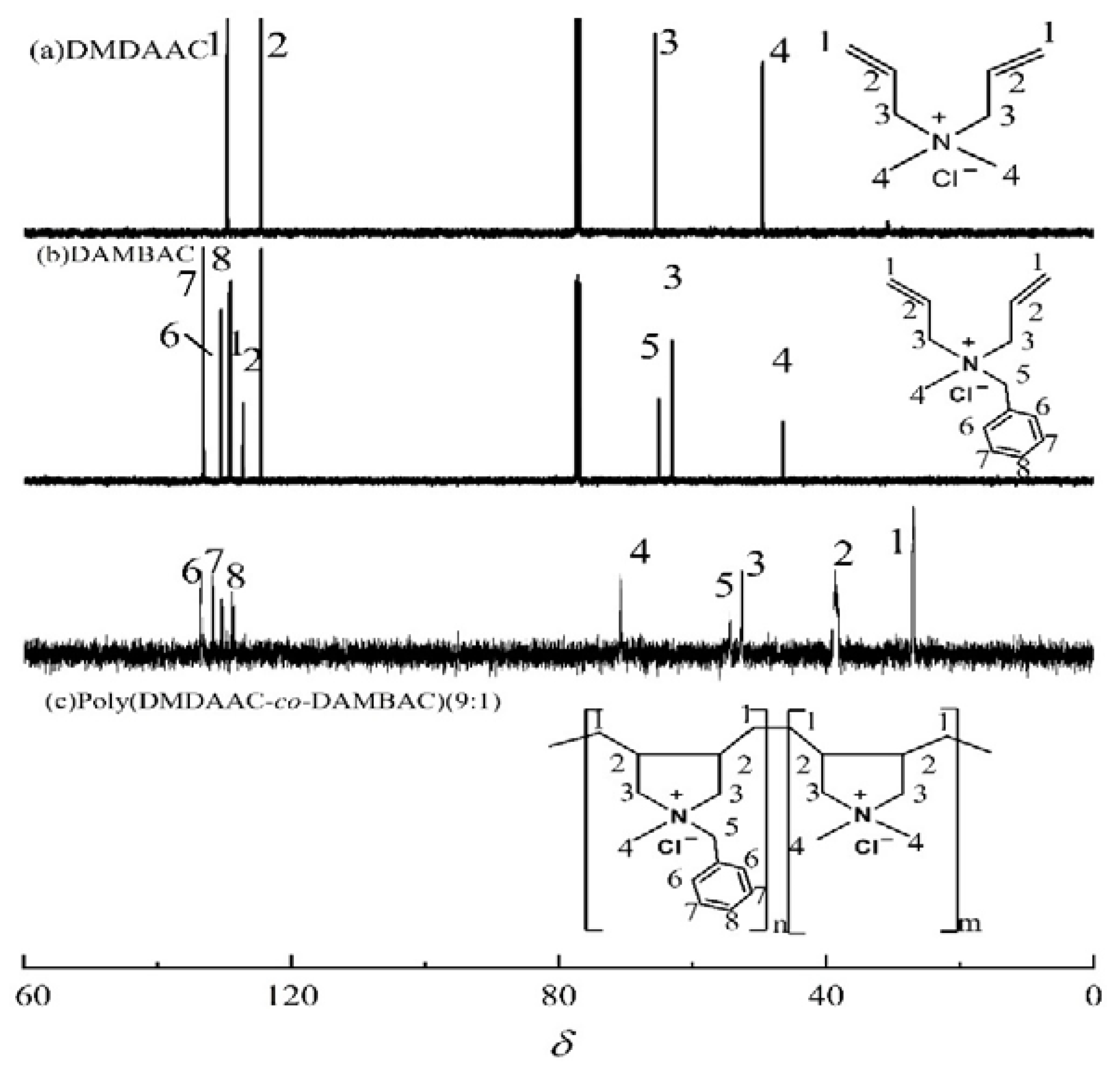


| Factor | Code | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| w (M)/% | A1 | 77.50 | 80.00 | 82.50 |
| m (V50):m (M)/% | B2 | 0.60 | 0.70 | 0.80 |
| m (Na4EDTA):m (M)/% | C3 | 0.002 | 0.004 | 0.006 |
| Factor | Code | Level | ||
|---|---|---|---|---|
| −1 | 0 | −1 | ||
| T1/°C | A2 | 45.00 | 50.00 | 55.00 |
| T2/°C | B2 | 55.00 | 60.00 | 65.00 |
| T3/°C | C2 | 67.50 | 72.50 | 77.50 |
| Source | SS | DF | MS | F | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 1045.42 | 9 | 116.16 | 8.18 | 0.0056 | Significant |
| A1 | 9.10 | 1 | 9.10 | 0.64 | 0.4499 | |
| B1 | 30.65 | 1 | 30.65 | 2.16 | 0.1852 | |
| C1 | 0.03 | 1 | 0.03 | 1.94 × 10−3 | 0.9661 | |
| A1B1 | 8.56 | 1 | 8.56 | 0.60 | 0.4631 | |
| A1C1 | 0.96 | 1 | 0.96 | 0.07 | 0.8023 | |
| B1C1 | 8.85 | 1 | 8.85 | 0.62 | 0.4558 | |
| A12 | 404.26 | 1 | 404.26 | 28.47 | 0.0011 | |
| B12 | 203.14 | 1 | 203.14 | 14.30 | 0.0069 | |
| C12 | 278.54 | 1 | 278.54 | 19.61 | 0.0030 | |
| Residual | 99.41 | 7 | 14.20 | |||
| Lack of Fit | 48.00 | 3 | 16.00 | 1.24 | 0.4043 | Insignificant |
| Pure Error | 51.41 | 4 | 12.85 | |||
| Total | 1144.83 | 16 |
| Number | Mw × 10−5/(g·mol−1) | Conv./% | Remark |
|---|---|---|---|
| 1 | 6.03 | 80.11 | The first group |
| 2 | 6.22 | 75.21 | The second group |
| 3 | 6.45 | 85.73 | The third group |
| 4 | 6.24 | 80.35 | Average value |
| Source | SS | DF | MS | F | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 6927.64 | 9 | 769.74 | 10.79 | 0.0024 | Significant |
| A2 | 167.26 | 1 | 167.26 | 2.35 | 0.1695 | |
| B2 | 625.52 | 1 | 625.52 | 8.77 | 0.0211 | |
| C2 | 0.05 | 1 | 0.05 | 7.18 × 10−4 | 0.9794 | |
| A2B2 | 5.55 | 1 | 5.55 | 0.08 | 0.7884 | |
| A2C2 | 0.17 | 1 | 0.17 | 2.42 × 10−3 | 0.9622 | |
| B2C2 | 17.94 | 1 | 17.94 | 0.25 | 0.6314 | |
| A22 | 668.07 | 1 | 668.07 | 9.37 | 0.0183 | |
| B22 | 4078.03 | 1 | 4078.03 | 57.18 | 0.0001 | |
| C22 | 854.55 | 1 | 854.55 | 11.98 | 0.0105 | |
| Residual | 499.19 | 7 | 71.31 | |||
| Lack of Fit | 400.69 | 3 | 133.56 | 5.42 | 0.0680 | Insignificant |
| Pure Error | 98.51 | 4 | 24.63 | |||
| Total | 7426.83 | 16 |
| Number | Mw × 10−5/(g·mol−1) | Conv./% | Remark |
|---|---|---|---|
| 1 | 6.32 | 86.22 | The first group |
| 2 | 6.59 | 88.65 | The second group |
| 3 | 6.26 | 87.29 | The third group |
| 4 | 6.39 | 85.05 | Average value |
| Samples | w (M) /% | m (V50):m (M) /% | m (Na4EDTA): m (M)/% | T1 /°C | T2 /°C | T3 /°C | [η] /(dL·g−1) | Mw × 105 /(g·mol−1) | Conv. /% |
|---|---|---|---|---|---|---|---|---|---|
| DMDAAC | 65.00 | 0.70 | 0.0035 | 50.0 | 60.0 | 70.0 | 3.44 | 11.44 | 100.00 |
| Poly (DMDAAC-co-DAMBAC) (9:1) | 80.11 | 0.71 | 0.0040 | 51.2 | 59.2 | 72.4 | 2.27 | 6.59 | 88.65 |
| DAMBAC | 82.50 | 3.03 | 0.0147 | 61.8 | 70.6 | 85.0 | 0.38 | 0.79 | 75.12 |
| Number | [ƞ]/(dL·g−1) | D/(g·L−1) | T/°C | t/h | R/% |
|---|---|---|---|---|---|
| 1 | 0.31 | 1.0 | 55.0 | 7.0 | 97.73 |
| 2 | 0.98 | 0.5 | 50.0 | 5.0 | 97.20 |
| 3 | 2.75 | 0.1 | 45.0 | 5.0 | 96.81 |
| Number | Demulsifier Sample | [ƞ]/(dL·g−1) | D/(g·L−1) | t/h | T/°C | R/% |
|---|---|---|---|---|---|---|
| 1 | BQ-05 | - | 5.0 | 4.0 | 60.0 | 84.12 |
| 2 | PDMDAAC | 0.32 | 2.0 | 4.0 | 55.0 | 37.34 |
| 3 | PDAMBAC | 0.33 | 2.0 | 5.0 | 55.0 | 91.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, X.; Qian, M.; Peng, W.; Xu, X.; Zhang, Y.; Zhao, X. Synthesis and Demulsification Properties of Poly (DMDAAC-co-DAMBAC) (9:1) Copolymer. Polymers 2023, 15, 562. https://doi.org/10.3390/polym15030562
Jia X, Qian M, Peng W, Xu X, Zhang Y, Zhao X. Synthesis and Demulsification Properties of Poly (DMDAAC-co-DAMBAC) (9:1) Copolymer. Polymers. 2023; 15(3):562. https://doi.org/10.3390/polym15030562
Chicago/Turabian StyleJia, Xu, Minghuan Qian, Wenhui Peng, Xiao Xu, Yuejun Zhang, and Xiaolei Zhao. 2023. "Synthesis and Demulsification Properties of Poly (DMDAAC-co-DAMBAC) (9:1) Copolymer" Polymers 15, no. 3: 562. https://doi.org/10.3390/polym15030562





