Novel Monomethoxy Poly(Ethylene Glycol) Modified Hydroxylated Tung Oil for Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of mPEG-HTO-mPEG
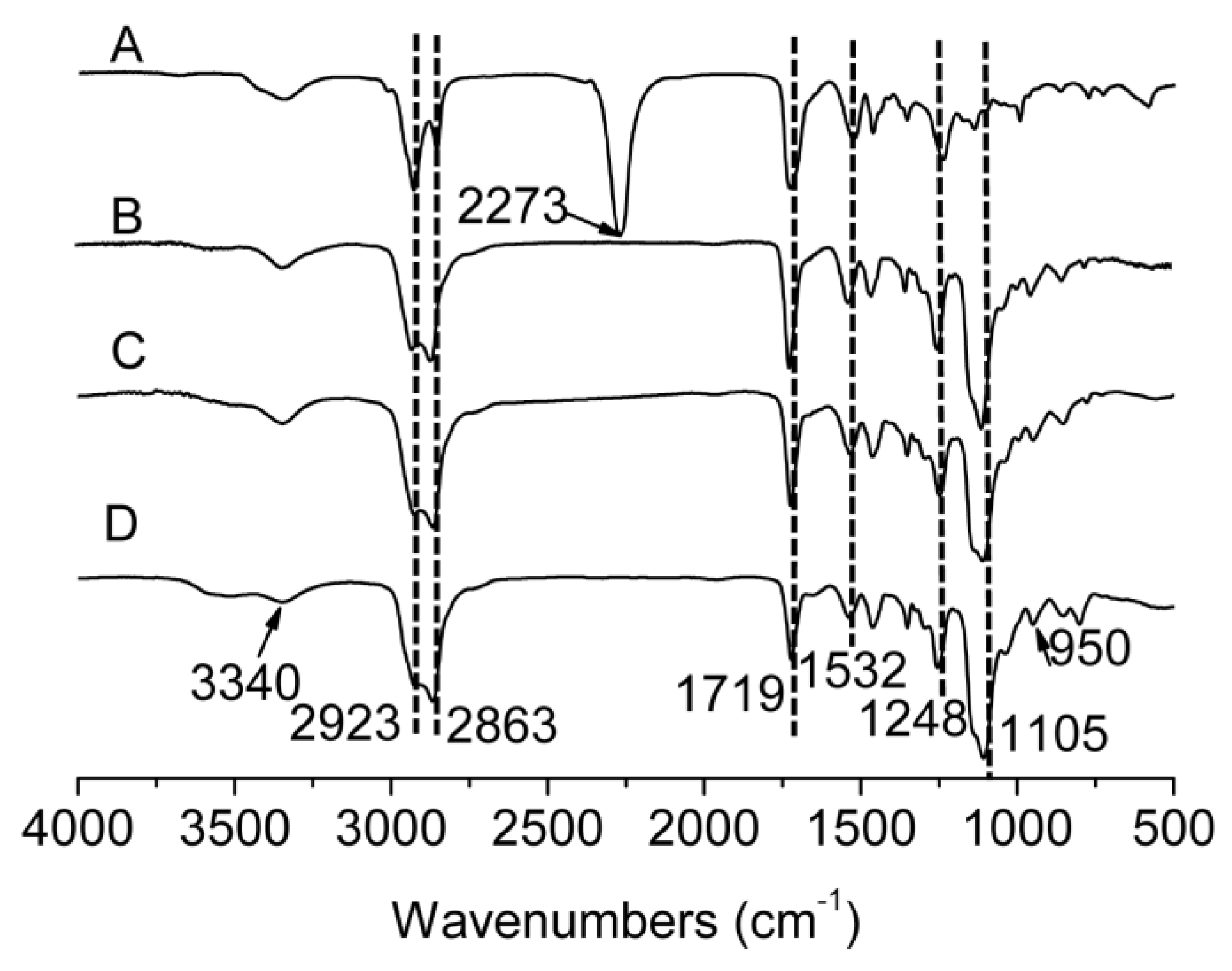
2.3. FT-IR Characterization
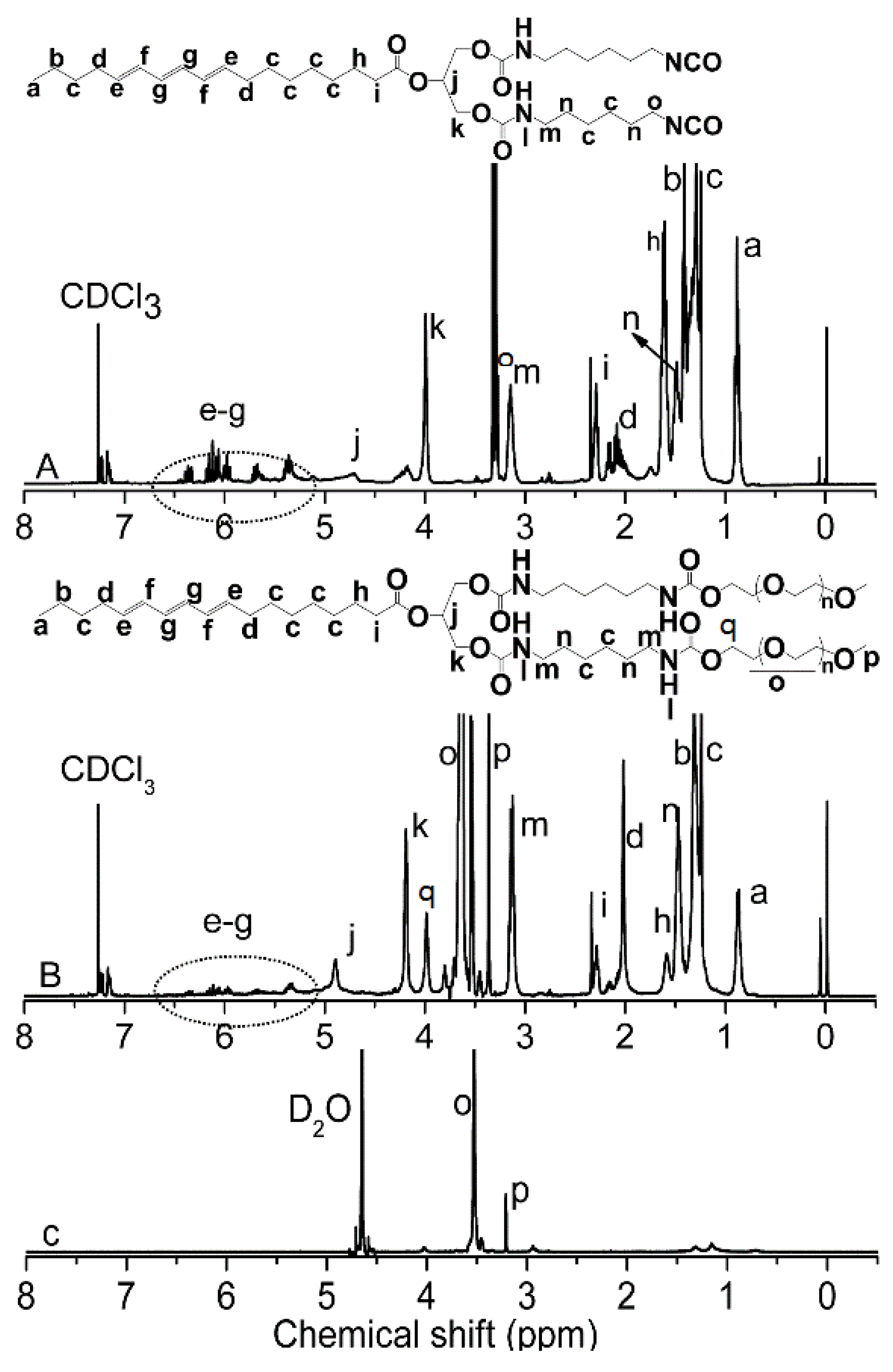
2.4. 1H NMR Characterization
2.5. GPC Measurement
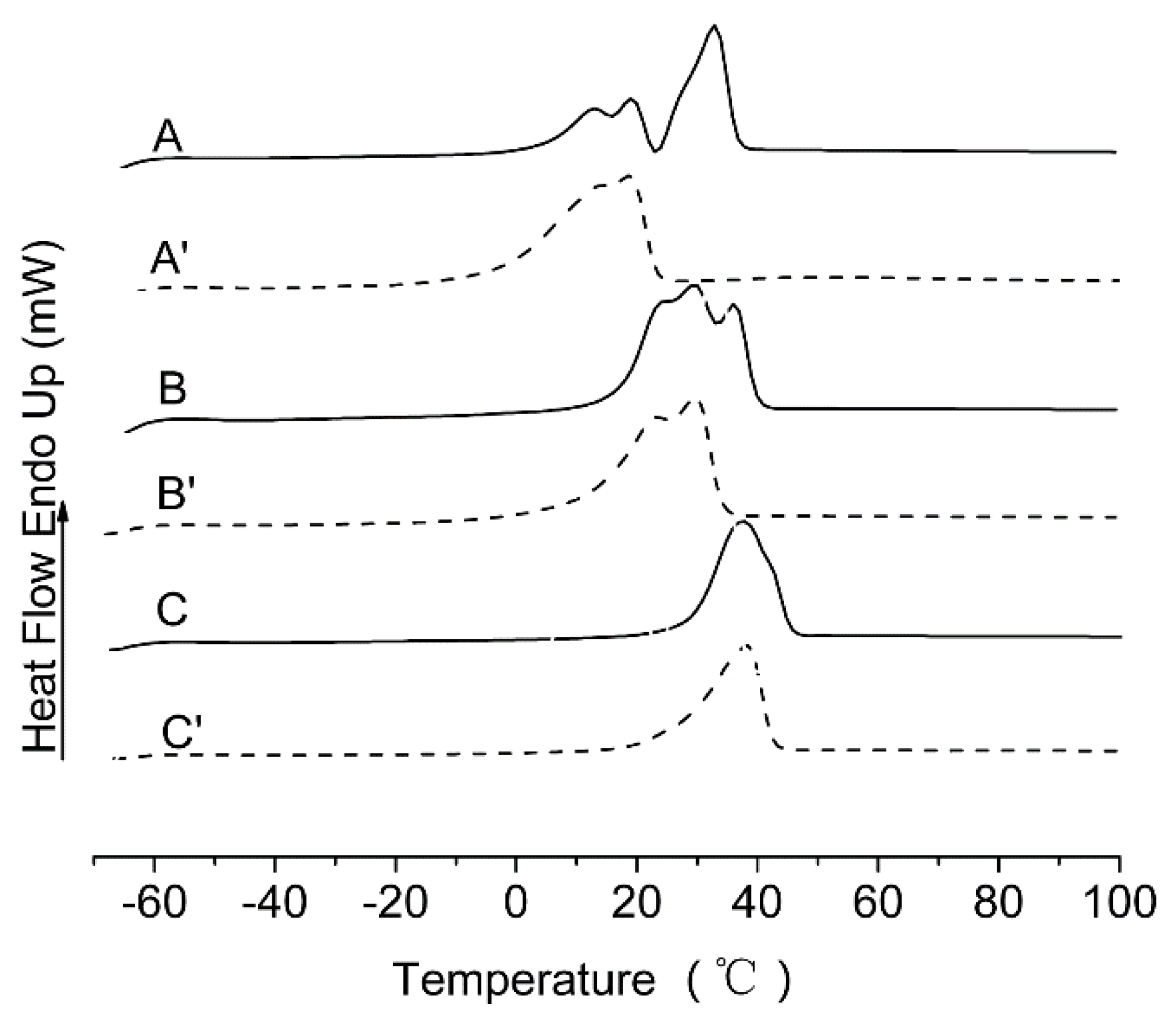
2.6. DSC Measurements
2.7. Preparation of mPEG-HTO-mPEG Micelles
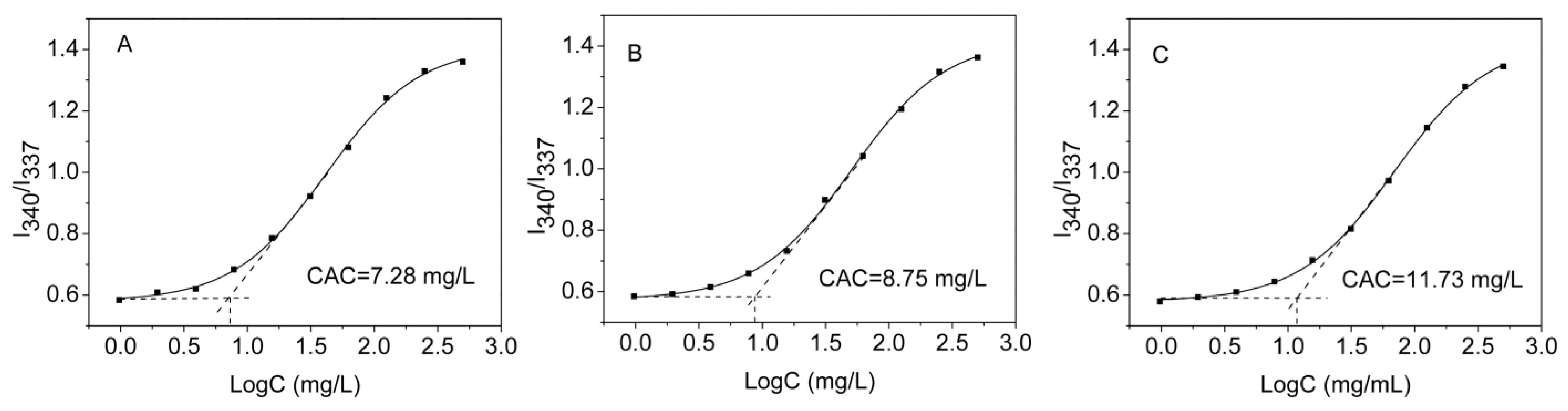
2.8. Determination of Critical Aggregation Concentration
2.9. Transmission Electron Microscopy (TEM) Observation
2.10. Size Distribution Measurement
2.11. Drug Loading
2.12. In Vitro Drug Release Study
2.13. In Vitro Cytotoxicity Test
3. Results and Discussion
3.1. Synthesis and Characterization of mPEG-HTO-mPEG
3.2. Thermal Properties of mPEG-HTO-mPEG
3.3. Self-Assembly of mPEG-HTO-mPEG Micelles
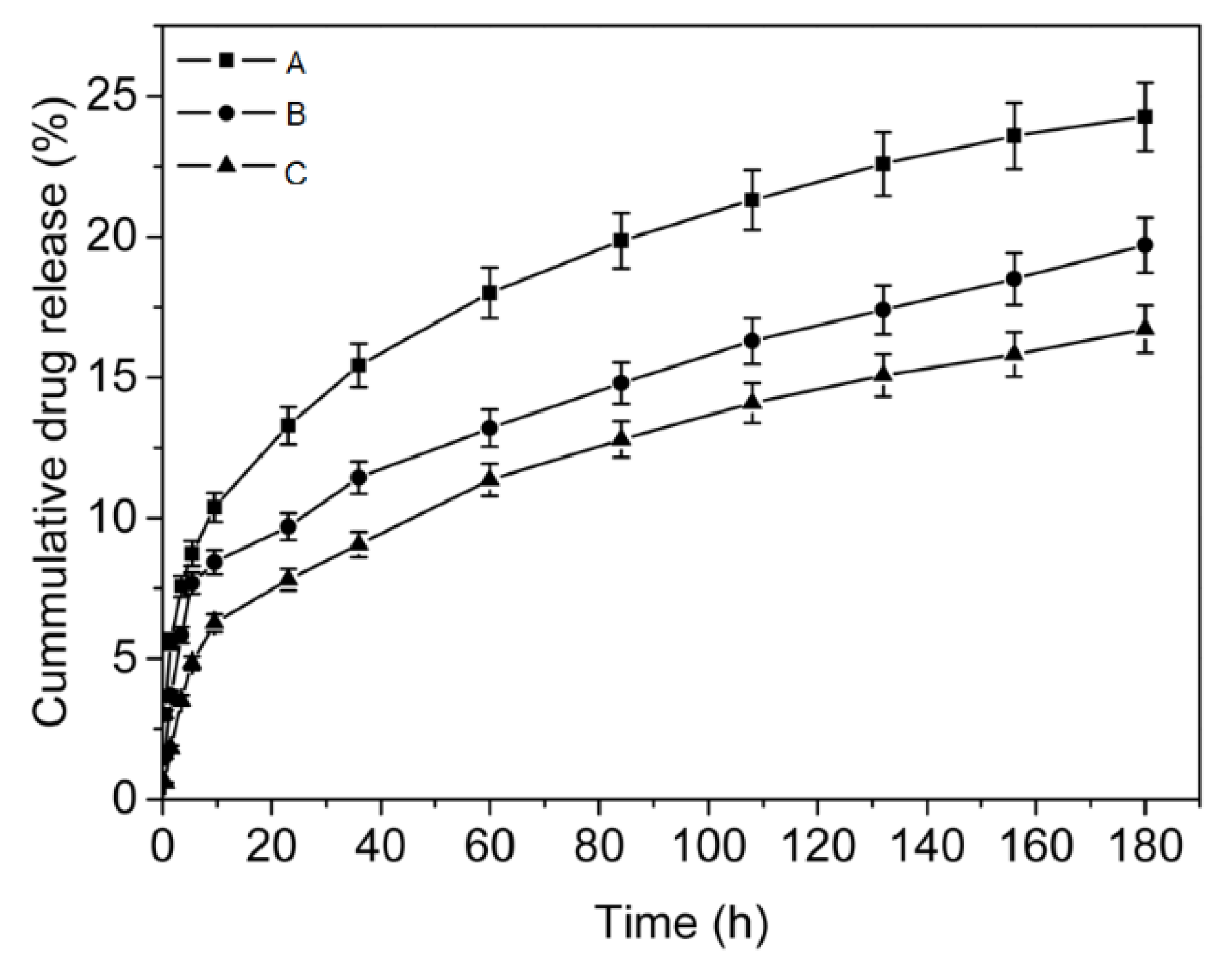
3.4. Drug Loading and In Vitro Drug Release
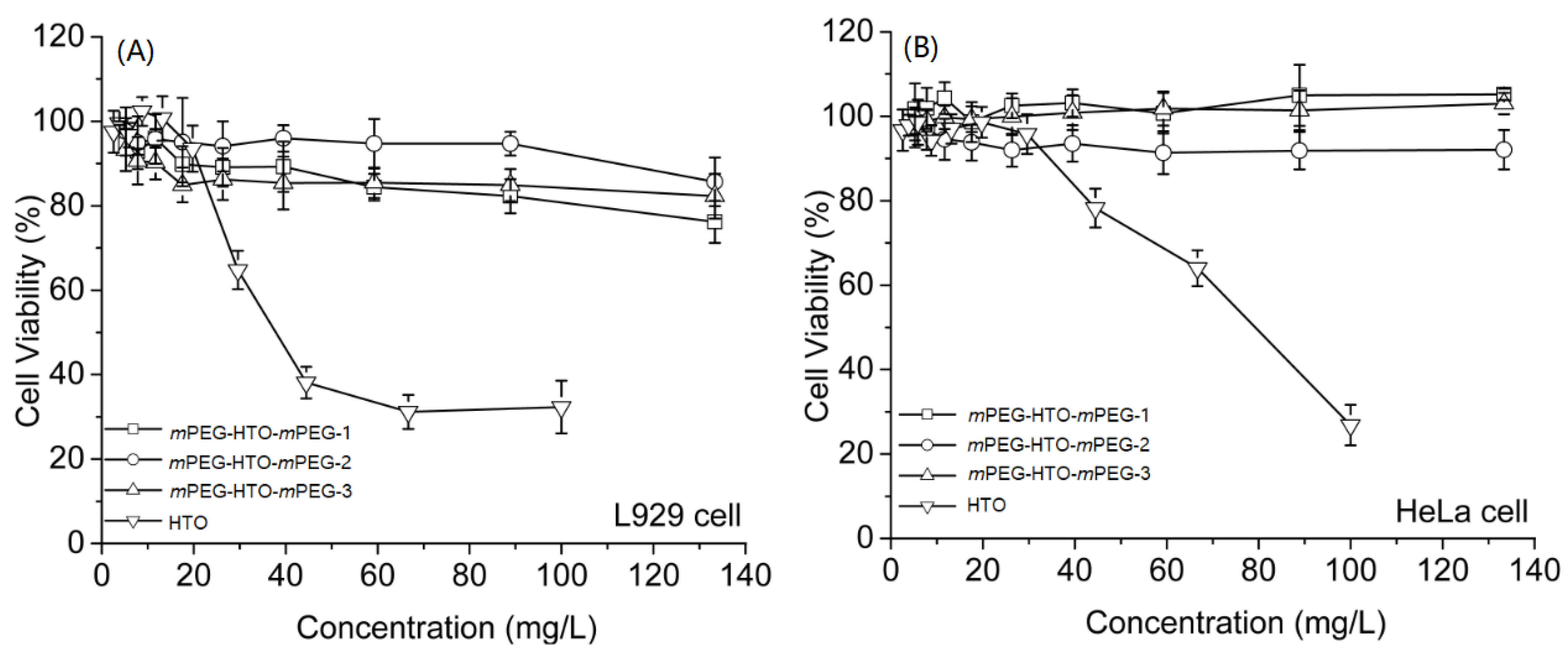
3.5. In Vitro Evaluation of Cell Viability
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Park, J.H.; Lee, D.S. Stimuli-Responsive Polymersomes for Cancer Therapy. Biomater. Sci. 2016, 4, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, L.; Wei, X.; Zhou, S. Polymer-Based Drug Delivery Systems for Cancer Treatment. J. Polym. Sci. Part A-Polym. Chem. 2016, 54, 3525–3550. [Google Scholar] [CrossRef]
- Xu, H.; Yang, P.; Ma, H.; Yin, W.; Wu, X.; Wang, H.; Xu, D.; Zhang, X. Amphiphilic Block Copolymers-based Mixed Micelles for Noninvasive Drug Delivery. Drug Deliv. 2016, 23, 3063–3071. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block Copolymer Micelles for Drug Delivery: Design, Characterization and Biological Significance. Adv. Drug Deliv. Rev. 2012, 64, 37–48. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. Isocyanate Terminated Castor Oil-based Polyurethane Prepolymer: Synthesis and Characterization. Prog. Org. Coat. 2015, 80, 39–48. [Google Scholar] [CrossRef]
- Das, B.; Konwar, U.; Mandal, M.; Karak, N. Sunflower Oil Based Biodegradable Hyperbranched Polyurethane as a Thin Film Material. Ind. Crops Prod. 2013, 44, 396–404. [Google Scholar] [CrossRef]
- Meiorin, C.; Muraca, D.; Pirota, K.R.; Aranguren, M.I.; Mosiewicki, M.A. Nanocomposites with Superparamagnetic Behavior Based on a Vegetable Oil And Magnetite Nanoparticles. Eur. Polym. J. 2014, 53, 90–99. [Google Scholar] [CrossRef]
- Madbouly, S.A.; Liu, K.; Xia, Y.; Kessler, M.R. Semi-interpenetrating Polymer Networks Prepared from in Situ Cationic Polymerization of Bio-Based Tung Oil with Biodegradable Polycaprolactone. RSC Adv. 2014, 4, 6710. [Google Scholar] [CrossRef] [Green Version]
- Stemmelen, M.; Travelet, C.; Lapinte, V.; Borsali, R.; Robin, J.-J. Synthesis and self-assembly of amphiphilic polymers based on polyoxazoline and vegetable oil derivatives. Polym. Chem. 2013, 4, 1445–1458. [Google Scholar] [CrossRef]
- Zhuang, Y.; Ren, Z.; Jiang, L.; Zhang, J.; Wang, H.; Zhang, G. Raman and FTIR Spectroscopic Studies on Two Hydroxylated Tung Oils (HTO) Bearing Conjugated Double Bonds. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2018, 199, 146–152. [Google Scholar] [CrossRef]
- Uzaki, M.; Shibata, M. Preparation and Properties of Self-Healing Tung Oil-based Polymer Networks Driven by Thermo-Reversible Diels-Alder Reaction. J. Polym. Res. 2022, 29, 453. [Google Scholar] [CrossRef]
- He, Z.; Chapital, D.C.; Cheng, H.N.; Thomas Klasson, K.; Olanya, O.M.; Uknalis, J. Application of Tung Oil to Improve Adhesion Strength and Water Resistance Of Cottonseed Meal and Protein Adhesives On Maple Veneer. Ind. Crops Prod. 2014, 61, 398–402. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chen, J.H.; Chang, C.Y.; Chang, C.C. Biodiesel Production from Tung (Vernicia Montana) Oil and its Blending Properties in Different Fatty Acid Compositions. Bioresour. Technol. 2010, 101, 9521–9526. [Google Scholar] [CrossRef]
- Meiorin, C.; Aranguren, M.I.; Mosiewicki, M.A. Polymeric Networks Based On Tung Oil: Reaction and Modification With Green Oil Monomers. Eur. Polym. J. 2015, 67, 551–560. [Google Scholar] [CrossRef]
- Silva, J.A.C.; Grilo, L.M.; Gandini, A.; Lacerda, T.M. The Prospering of Macromolecular Materials Based on Plant Oils within the Blooming Field of Polymers from Renewable Resources. Polymers 2021, 13, 1722. [Google Scholar] [CrossRef]
- Shirke, A.G.; Dholakiya, B.Z.; Kuperkar, K. Modification of Tung Oil-Based Polyurethane Foam By Anhydrides and Inorganic Content Through Esterification Process. J. Appl. Polym. Sci. 2018, 135, 45786. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, L.; Wang, H.; Fu, Y.; Jiang, L.; Ren, B. Novel Amphoteric Polyurethane Dispersions with Postpolymerization Crosslinking Function Derived from Hydroxylated Tung Oil: Synthesis And Properties. Rsc Adv. 2015, 5, 27717–27721. [Google Scholar] [CrossRef]
- Lei, Y.; Lai, Y.; Li, Y.; Li, S.; Cheng, G.; Li, D.; Li, H.; He, B.; Gu, Z. Anticancer Drug Delivery of PEG Based Micelles With Small Lipophilic Moieties. Int. J. Pharm. 2013, 453, 579–586. [Google Scholar] [CrossRef]
- Warren, N.J.; Mykhaylyk, O.O.; Mahmood, D.; Ryan, A.J.; Armes, S.P. RAFT Aqueous Dispersion Polymerization Yields Poly(Ethylene Glycol)-Based Diblock Copolymer Nano-Objects With Predictable Single Phase Morphologies. J. Am. Chem. Soc. 2014, 136, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Tan, P.; Chen, X.; Kopytynski, M.; Pan, D.; Zheng, X.; Gu, L.; Gong, Q.; Tian, X.; Gu, Z.; et al. Stimuli-Sensitive Linear-Dendritic Block Copolymer-Drug Prodrug as a Nanoplatform for Tumor Combination Therapy. Adv. Mater. 2022, 34, 2108049. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.C.; Huang, S.Y.; Fan, W.L.; Lee, A.W.; Chiu, C.W.; Lee, D.J.; Lai, J.Y. Water-Soluble Single-Chain Polymeric Nanoparticles for Highly Selective Cancer Chemotherapy. Acs Appl. Polym. Mater. 2021, 3, 474–484. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Kumar, C.U.; Jewrajka, S.K. Effect of Polyethylene Glycol on Properties and Drug Encapsulation -Release Performance of Biodegradable/Cytocompatible Agarose-Polyethylene Glycol Polycaprolactone Amphiphilic Co-Network Gels. Acs Appl. Mater. Interfaces 2016, 8, 3182–3192. [Google Scholar] [CrossRef]
- Wang, H.F.; Luo, X.H.; Liu, C.W.; Feng, J.; Zhang, X.Z.; Zhuo, R.X. A Smart Micellar System with an Amine-Containing Polycarbonate Shell. Acta Biomater. 2012, 8, 589–598. [Google Scholar] [CrossRef]
- Liu, J.; Ma, X.; Tong, Y.; Lang, M. Self-Healing Polyurethane Based on Ditelluride Bonds. Appl. Surf. Sci. 2018, 455, 318–325. [Google Scholar] [CrossRef]
- Ye, H.M.; Song, Y.Y.; Xu, J.; Guo, B.H.; Zhou, Q. Melting Behavior of Inclusion Complex Formed Between Polyethylene Glycol Oligomer And Urea. Polymer 2013, 54, 3385–3391. [Google Scholar] [CrossRef]
- Song, N.; Ding, M.; Pan, Z.; Li, J.; Zhou, L.; Tan, H.; Fu, Q. Construction of Targeting-Clickable And Tumor-Cleavable Polyurethane Nanomicelles for Multifunctional Intracellular Drug Delivery. Biomacromolecules 2013, 14, 4407–4419. [Google Scholar] [CrossRef]
- Nutan, B.; Chandel, A.K.S.; Jewrajka, S.K. Synthesis and Multi-Responsive Self-Assembly of Cationic Poly(caprolactone)-Poly(ethylene glycol) Multiblock Copolymers. Chem. -A Eur. J. 2017, 23, 8166–8170. [Google Scholar] [CrossRef]
- Hu, G.; Cui, L.; Zhang, S.; Xie, B. Solubility of Prednisone Acetate in Different Mixed Solvents at Different Temperatures. J. Mol. Liq. 2012, 173, 13–17. [Google Scholar] [CrossRef]
- Huang, F.; Cheng, R.; Meng, F.; Deng, C.; Zhong, Z. Micelles Based on Acid Degradable Poly(acetal urethane): Preparation, pH-Sensitivity, and Triggered Intracellular Drug Release. Biomacromolecules 2015, 16, 2228–2236. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, J.; Wang, Q.; Zhong, Z.; Zhuo, R. Miktoarm Copolymers Bearing One Poly(ethylene glycol) Chain and Several Poly(epsilon-caprolactone) Chains on a Hyperbranched Polyglycerol Core. Macromolecules 2010, 43, 6671–6677. [Google Scholar] [CrossRef]
- Luo, Y.L.; Chen, L.L.; Miao, Y.; Xu, F. Novel AB4-Type CTBN-b-mPEG PU Micelle-like Amphiphilic Block Copolymer Micelles for Prednisone Drug Release. Ind. Eng. Chem. Res. 2013, 52, 1571–1580. [Google Scholar] [CrossRef]
- Madbouly, S.A.; Kessler, M.R. Preparation of Nanoscale Semi-IPNs with an Interconnected Microporous Structure via Cationic Polymerization of Bio-Based Tung Oil in a Homogeneous Solution of Poly(epsilon-caprolactone). Acs Omega 2020, 5, 9977–9984. [Google Scholar] [CrossRef] [Green Version]
- Cherng, J.Y.; Hou, T.Y.; Shih, M.F.; Talsma, H.; Hennink, W.E. Polyurethane-based Drug Delivery Systems. Int. J. Pharm. 2013, 450, 145–162. [Google Scholar] [CrossRef]


| Sample | mPEG | Mn a | Mn (GPC) b | Mw (GPC) b | PDI (GPC) b |
|---|---|---|---|---|---|
| mPEG-HTO-mPEG-1 | 550 | 1834 | 2111 | 4001 | 1.89 |
| mPEG-HTO-mPEG-2 | 750 | 2234 | 2435 | 4699 | 1.93 |
| mPEG-HTO-mPEG-3 | 1000 | 2734 | 2200 | 3128 | 1.42 |
| Samples | EE(%) | DLE(%) |
|---|---|---|
| mPEG-HTO-mPEG-1 | 66.0 | 25.1 |
| mPEG-HTO-mPEG-2 | 46.9 | 20.3 |
| mPEG-HTO-mPEG-3 | 45.6 | 17.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; He, H.; Zhang, J.; Liu, J.; Zhuang, Y.; Yin, Y.; Ren, Z.; Fu, Y.; He, S. Novel Monomethoxy Poly(Ethylene Glycol) Modified Hydroxylated Tung Oil for Drug Delivery. Polymers 2023, 15, 564. https://doi.org/10.3390/polym15030564
Wang H, He H, Zhang J, Liu J, Zhuang Y, Yin Y, Ren Z, Fu Y, He S. Novel Monomethoxy Poly(Ethylene Glycol) Modified Hydroxylated Tung Oil for Drug Delivery. Polymers. 2023; 15(3):564. https://doi.org/10.3390/polym15030564
Chicago/Turabian StyleWang, Huafen, Huanhuan He, Jiaxiang Zhang, Juntao Liu, Yuwei Zhuang, Yuanyuan Yin, Zhiyong Ren, Yang Fu, and Suqin He. 2023. "Novel Monomethoxy Poly(Ethylene Glycol) Modified Hydroxylated Tung Oil for Drug Delivery" Polymers 15, no. 3: 564. https://doi.org/10.3390/polym15030564






