Potato Starch-Based Film Incorporated with Tea Polyphenols and Its Application in Fruit Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Potato Starch-Based Film Incorporated with Tea Polyphenols
2.3. Determination of the Physical Properties (Thickness, Moisture Content, Color, Light Transmittance, Tensile Properties and Thermal Properties) of Potato Starch-Based Film Incorporated with TP
2.4. Determination of the Barrier Properties (Water Vapor Permeability and Oxygen Permeability) of Potato Starch-Based Film Incorporated with TP
2.5. Determination of the Antioxidant Activity of Potato Starch-Based Film Incorporated with TP
2.6. Determination of Wide-Angle X-ray Diffraction (WAXD) Pattern of Potato Starch-Based Film Incorporated with TP
2.7. Fourier Transform Infrared (FTIR) Spectrum Analysis of Potato Starch-Based Film Incorporated with TP
2.8. Scanning Electron Microscope (SEM) Observation of Potato Starch-Based Film Incorporated with TP
2.9. Fruit Packaging with Potato Starch-Based Film Incorporated with TP
2.9.1. Packaging of Blueberries and Fresh-Cut Bananas
2.9.2. Determination of the Weight Loss Ratio, Hardness and Chewiness of Blueberries
2.9.3. Determination of the Color of Fresh-Cut Bananas
2.10. Statistical Analysis
3. Results and Discussions
3.1. Effect of TP on the Physical Properties of Potato Starch-Based Film
3.1.1. Thickness, Moisture Content and Color
3.1.2. Light Transmittance and Tensile Properties
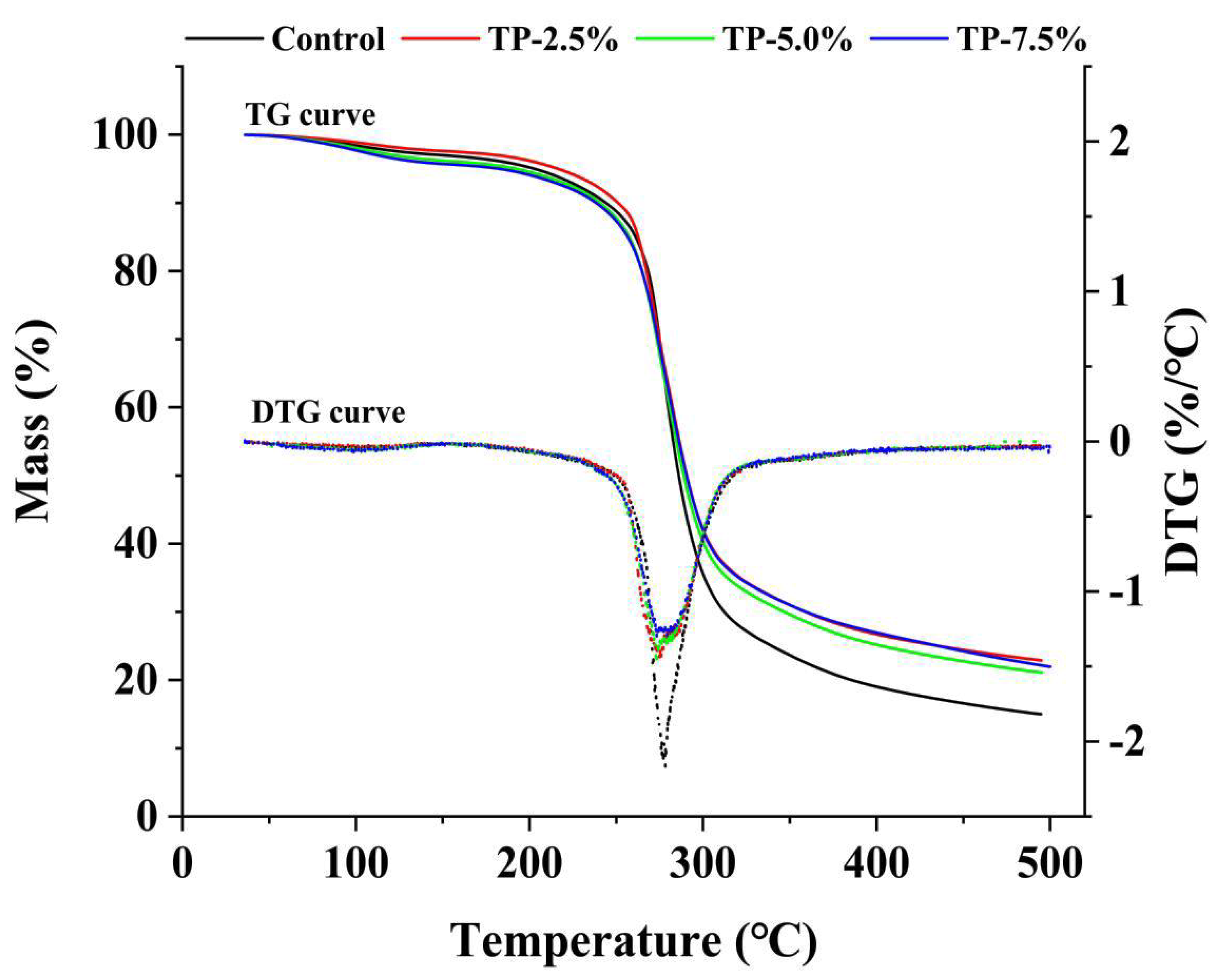
3.1.3. Thermal Properties
3.2. Effect of TP on the Barrier Properties and Functionality of Potato Starch-Based Film
3.2.1. Water Vapor Permeability (WVP) and Oxygen Permeability (OP)
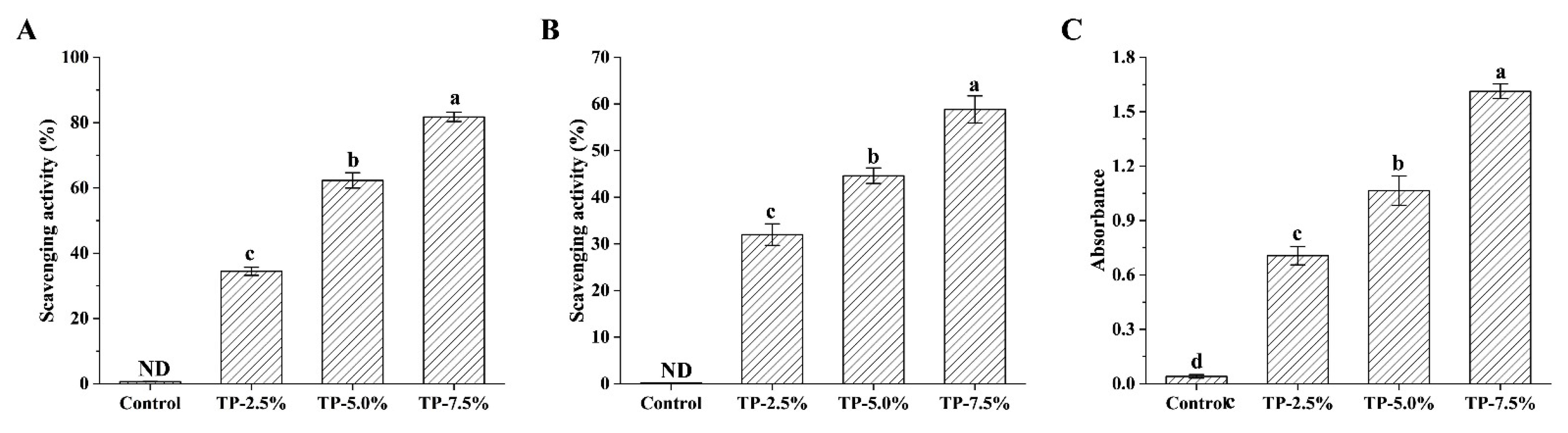
3.2.2. Antioxidant Activity
3.3. Interaction between Potato Starch and TP and the Structure of Film
3.3.1. WAXD and FTIR
3.3.2. SEM
3.4. Application of Potato Starch-Based Film Incorporated with TP in Fruit Packaging
3.4.1. Weight Loss Ratio, Hardness and Chewiness of Blueberries
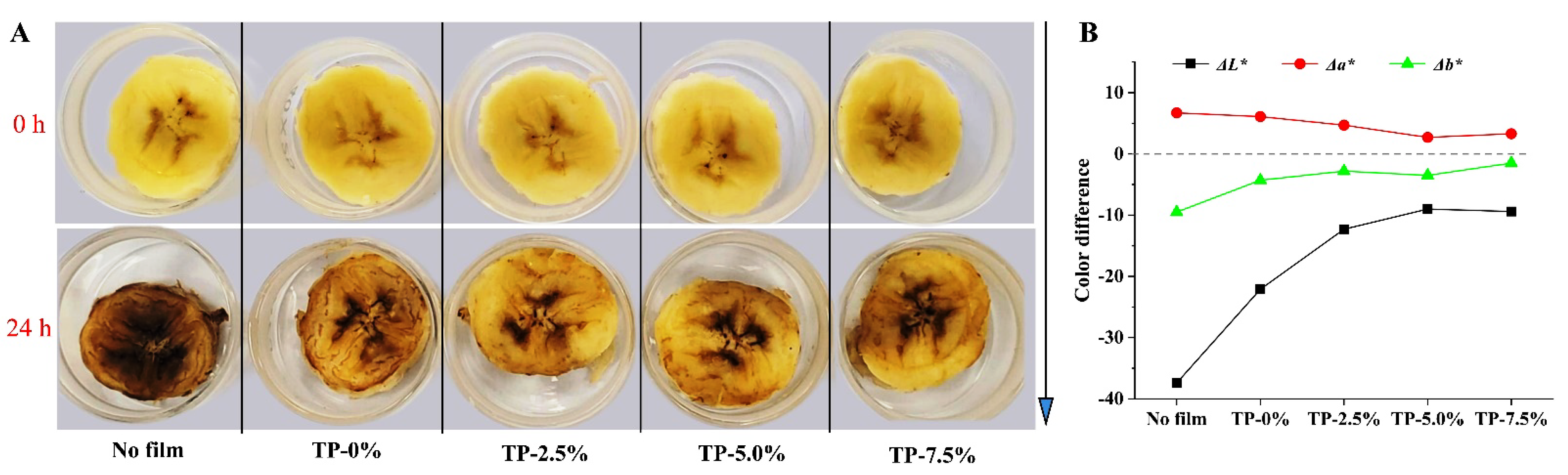
3.4.2. Color and Browning Degree of Fresh-Cut Bananas
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Polysaccharide based films and coatings for food packaging: Effect of added polyphenols. Food Chem. 2021, 359, 129871. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.X.; He, Z.; Sun, Q.; He, Q.; Zeng, W.C. A functional polysaccharide film forming by pectin, chitosan, and tea polyphenols. Carbohydr. Polym. 2019, 215, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chorfa, N.; Nlandu, H.; Belkacemi, K.; Hamoudi, S. Physical and Enzymatic Hydrolysis Modifications of Potato Starch Granules. Polymers 2022, 14, 2027. [Google Scholar] [CrossRef]
- Versino, F.; Lopez, O.V.; Garcia, M.A.; Zaritzky, N.E. Starch-based films and food coatings: An overview. Starch-Starke 2016, 68, 1026–1037. [Google Scholar] [CrossRef]
- Mohammed, A.A.B.A.; Hasan, Z.; Omran, A.A.B.; Elfaghi, A.M.; Khattak, M.A.; Ilyas, R.A.; Sapuan, S.M. Effect of Various Plasticizers in Different Concentrations on Physical, Thermal, Mechanical, and Structural Properties of Wheat Starch-Based Films. Polymers 2023, 15, 63. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, R.; Wang, B.; Chen, K. Development and characterization of bilayer films based on pea starch/polylactic acid and use in the cherry tomatoes packaging. Carbohydr. Polym. 2019, 222, 114912. [Google Scholar] [CrossRef]
- Pinto, T.; Aires, A.; Cosme, F.; Bacelar, E.; Morais, M.C.; Oliveira, I.; Vilela, A. Bioactive (poly)phenols, volatile compounds from vegetables, medicinal and aromatic plants. Foods 2021, 10, 106. [Google Scholar] [CrossRef]
- Chen, N.; Feng, Z.J.; Gao, H.X.; He, Q.; Zeng, W.C. Effects of phenols with different structure characteristics on properties of potato starch: Action rule and molecular mechanism. J. Food Process. Preserv. 2022, 46, 16679. [Google Scholar] [CrossRef]
- Chen, N.; Gao, H.X.; He, Q.; Yu, Z.L.; Zeng, W.C. Interaction and action mechanism of starch with different phenolic compounds. Int. J. Food Sci. Nutr. 2020, 71, 726–737. [Google Scholar] [CrossRef]
- Chen, N.; Chen, L.; He, Q.; Sun, Q.; Zeng, W.C. Effects of tea polyphenols on physicochemical properties of wheat starch and bread quality and their action mechanism. Food Sci. 2021, 42, 8–16. [Google Scholar] [CrossRef]
- Feng, M.; Yu, L.; Zhu, P.; Zhou, X.; Liu, H.; Yang, Y.; Zhou, J.; Gao, C.; Bao, X.; Chen, P. Development and preparation of active starch films carrying tea polyphenol. Carbohydr. Polym. 2018, 196, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Gao, W.; Zhang, X.; Wang, B.; Zou, F.; Yu, B.; Cui, B. Effects of ultrasonication on the properties of maize starch/stearic acid/sodium carboxymethyl cellulose composite film. Ultrason. Sonochemistry 2021, 72, 105447. [Google Scholar] [CrossRef]
- Sun, S.; Liu, P.; Ji, N.; Hou, H.; Dong, H. Effects of low polyhydroxyalkanoate content on the properties of films based on modified starch acquired by extrusion blowing. Food Hydrocoll. 2017, 72, 81–89. [Google Scholar] [CrossRef]
- Li, Q.; Li, J.; Li, H.; Xu, R.; Yuan, Y.; Cao, J. Physicochemical properties and functional bioactivities of different bonding state polysaccharides extracted from tomato fruit. Carbohydr. Polym. 2019, 219, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Rosa, F.V.P.; Aguilar, P.E.; de Jesús, Z.M.J.; Odin, V.G.M.; Enrique, V.M.J.; Martinez, B.F.; Jacobo, V.N. Physicochemical and microstructural characterization of corn starch edible films obtained by a combination of extrusion technology and casting technique. J. Food Sci. 2016, 81, E2224–E2232. [Google Scholar] [CrossRef]
- Rymal, K.S. Portable micro method for quantitative determination of Vitamin C in fruit and vegetable juices. AOAC 1983, 66, 810–813. [Google Scholar] [CrossRef]
- Yu, Z.L.; Zeng, W.C. Antioxidant, antibrowning, and cytoprotective activities of Ligustrum robustum (Rxob.) Blume extract. J. Food Sci. 2013, 78, C1354–C1362. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Gao, H.X.; He, Q.; Zeng, W.C. Wheat Starch Modified with Ligustrum robustum (Rxob.) Blume Extract and Its Action Mechanism. Foods 2022, 11, 3187. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Waldron, K.W. Crosslinking in polysaccharide and protein films and coatings for food contact—A review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Ju, A.; Song, K.B. Active biodegradable films based on water soluble polysaccharides from white jelly mushroom (Tremella fuciformis) containing roasted peanut skin extract. LWT-Food Sci. Technol. 2020, 126, 109293. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Langroodi, A.M.; Molaei, R.; Mahmoudian, A. Chitosan-starch film containing pomegranate peel extract and Thymus kotschyanus essential oil can prolong the shelf life of beef. Meat Sci. 2022, 163, 108073. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Li, C.; Qin, Y.; Xiao, L.; Liu, J. Comparison of the structural, physical and functional properties of κ-carrageenan films incorporated with pomegranate flesh and peel extracts. Int. J. Biol. Macromol. 2020, 147, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- Menzel, C. Improvement of starch films for food packaging through a threeprinciple approach: Antioxidants, cross-linking and reinforcement. Carbohydr. Polym. 2020, 250, 116828. [Google Scholar] [CrossRef] [PubMed]
- Pineros, H.D.; Medina, J.C.; Lopez, C.A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Yu, Z.L.; Sun, L.; Wang, W.; Zeng, W.C.; Mustapha, A.; Lin, M.S. Soy protein-based films incorporated with cellulose nanocrystals and pine needle extract for active packaging. Industrial Crops Prod. 2018, 112, 412–419. [Google Scholar] [CrossRef]
- Shahbazi, Y. The properties of chitosan and gelatin films incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil as biodegradable materials for active food packaging. Int. J. Biol. Macromol. 2017, 99, 746–753. [Google Scholar] [CrossRef]
- Cui, C.; Ji, N.; Wang, Y.; Xiong, L.; Sun, Q. Bioactive and intelligent starch-based films: A review. Trends Food Sci. Technol. 2021, 116, 854–869. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Li, B. Nature-inspired one-step green procedure for enhancing the antibacterial and antioxidant behavior of a chitin film: Controlled interfacial assembly of tannic acid onto a chitin film. J. Agric. Food Chem. 2016, 64, 5736–5741. [Google Scholar] [CrossRef]
- Wei, X.; Li, J.; Li, B. Multiple steps and critical behaviors of the binding of tannic acid to wheat starch: Effect of the concentration of wheat starch and the mass ratio of tannic acid to wheat starch. Food Hydrocoll. 2019, 94, 174–182. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, Y.; Li, Q. Preparation and characterization of corn starch-based composite films containing corncob cellulose and Cassia oil. Starch-Starke 2020, 72, 1900209. [Google Scholar] [CrossRef]
- Li, S.; Ma, Y.; Ji, T.; Sameen, D.E.; Liu, Y. Cassava starch/carboxymethylcellulose edible films embedded with lactic acid bacteria to extend the shelf life of banana. Carbohydr. Polym. 2020, 248, 116805. [Google Scholar] [CrossRef] [PubMed]




| Sample | Thickness (mm) | Moisture Content (%) | L* | a* | b* | ΔE |
|---|---|---|---|---|---|---|
| control | 0.089 ± 0.002 a | 10.27 ± 0.70 a | 33.18 ± 0.66 a | −0.23 ± 0.01 d | −1.20 ± 0.14 d | - |
| TP-2.5% | 0.087 ± 0.004 a | 9.68 ± 0.54 b | 28.59 ± 0.22 b | 0.77 ± 0.04 c | 1.83 ± 0.04 c | 5.41 ± 0.18 c |
| TP-5.0% | 0.083 ± 0.001 a | 9.45 ± 0.77 bc | 25.76 ± 0.65 c | 1.53 ± 0.13 b | 3.30 ± 0.17 b | 8.68 ± 0.60 b |
| TP-7.5% | 0.086 ± 0.002 a | 9.27 ± 0.63 c | 24.05 ± 0.75 d | 1.56 ± 0.03 a | 4.18 ± 0.14 a | 10.55 ± 0.52 a |
| Sample | Weight Loss Ratio (%) | Hardness (g) | Chewiness | |
|---|---|---|---|---|
| 1 d | - | - | 995 ± 33 | 226 ± 29 |
| 4 d | No film | 13.97 ± 3.13 a | 671 ± 47 e | 103 ± 37 d |
| Control | 9.67 ± 1.42 b | 715 ± 61 d | 138 ± 23 c | |
| TP-2.5% | 9.59 ± 1.65 b | 803 ± 55 c | 147 ± 29 c | |
| TP-5.0% | 9.22 ± 2.11 c | 851 ± 73 b | 168 ± 36 b | |
| TP-7.5% | 7.89 ± 2.04 d | 906 ± 52 a | 174 ± 41 a | |
| 7 d | No film | 24.69 ± 3.81 a | 346 ± 29 d | 66 ± 26 d |
| Control | 18.70 ± 1.98 b | 543 ± 33 c | 85 ± 11 c | |
| TP-2.5% | 17.53 ± 2.45 c | 590 ± 59 b | 89 ± 20 b | |
| TP-5.0% | 16.12 ± 1.98 d | 590 ± 46 b | 84 ± 18 c | |
| TP-7.5% | 15.93 ± 2.56 e | 631 ± 31 a | 101 ± 17 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, N.; Gao, H.-X.; He, Q.; Zeng, W.-C. Potato Starch-Based Film Incorporated with Tea Polyphenols and Its Application in Fruit Packaging. Polymers 2023, 15, 588. https://doi.org/10.3390/polym15030588
Chen N, Gao H-X, He Q, Zeng W-C. Potato Starch-Based Film Incorporated with Tea Polyphenols and Its Application in Fruit Packaging. Polymers. 2023; 15(3):588. https://doi.org/10.3390/polym15030588
Chicago/Turabian StyleChen, Nan, Hao-Xiang Gao, Qiang He, and Wei-Cai Zeng. 2023. "Potato Starch-Based Film Incorporated with Tea Polyphenols and Its Application in Fruit Packaging" Polymers 15, no. 3: 588. https://doi.org/10.3390/polym15030588





