Green Composites Based on Animal Fiber and Their Applications for a Sustainable Future
Abstract
1. Introduction
2. Different Categories of Animal Fibers
2.1. Chicken Fibers
2.2. Silk
2.3. Wool
2.4. Human Hair
3. Applications of Animal Fibers
3.1. Biomedical Applications
3.2. Constructional
3.3. Automobile Applications
4. Conclusions
- A policy for extending the use of human hair while maintaining social and environmental norms can be framed by various governments.
- The enactment of rules and regulations, as well as the development of support structures for various uses of animal fibers based on their environmental impact, corporate requirements, and market reach can be framed.
- It is necessary to educate the community about the beneficial characteristics of animal fibers, as well as safe collecting and usage techniques.
- Complete human hair utilization systems may be established with the help of many stakeholders, reducing solid waste and environmental concerns, generating major social economic advantages for humans, and reducing pressure on other non-renewable resources and fossil fuels that can be saved for future needs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, A.; Vijay, R.; Singaravelu, D.L.; Sanjay, M.R.; Siengchin, S.; Verpoort, F.; Alamry, K.A.; Asiri, A.M. Characterization of Natural Fibers from Cortaderia Selloana Grass (Pampas) as Reinforcement Material for the Production of the Composites. J. Nat. Fibers 2020, 18, 1893–1901. [Google Scholar] [CrossRef]
- Khan, A.; Jawaid, M. Novel Spider Silk Fiber for High-Performance Textiles and Eco-friendly Armor Applications. J. Nat. Fibers 2020, 19, 1879–1884. [Google Scholar] [CrossRef]
- Ryszard, M.; Maria, M.; Malgorzata, M.; Jorge, B. Future of natural fibers, their coexistence and competition with man-made fibers in 21st century. Mol. Cryst. Liq. Cryst. 2012, 556, 200–222. [Google Scholar] [CrossRef]
- Harizi, T.; Msahli, S.; Sakli, F.; Khorchani, T. Evaluation of physical and mechanical properties of Tunisian camel hair. J. Text. Inst. 2007, 98, 15–21. [Google Scholar] [CrossRef]
- Joseph, P.; Joseph, V.; Thomas, S. Effect of processing variables on the mechanical properties of sisal-fiber-reinforced polypropylene composites. Compos. Sci. Technol. 1999, 59, 1625–1640. [Google Scholar] [CrossRef]
- Saheb, D.N.; Jog, J.P. Natural fiber polymer composites: A review. Advances in Polymer Technology. J. Polym. Process. Inst. 1999, 18, 351–363. [Google Scholar]
- Hill, C.A.S.; Khalil, A. Effect of fiber treatments on mechanical properties of coir or oil palm fiber reinforced polyester composites. J. Appl. Polym. Sci. 2000, 78, 1685–1697. [Google Scholar] [CrossRef]
- Sreekala, M.S.; Kumaran, M.G.; Thomas, S. Oil palm fibers: Morphology, chemical composition, surface modification, and mechanical properties. J. Appl. Polym. Sci. 1997, 66, 821–835. [Google Scholar] [CrossRef]
- Cunniff, P.M.; Fossey, S.A.; Auerbach, M.A.; Song, J.W.; Kaplan, D.L.; Adams, W.W.; Eby, R.K.; Mahoney, D.; Vezie, D.L. Mechanical and thermal properties of dragline silk from the spider Nephila clavipes. Polym. Adv. Technol. 1994, 5, 401–410. [Google Scholar] [CrossRef]
- Gosline, J.M.; Guerette, P.A.; Ortlepp, C.S.; Savage, K.N. The mechanical design of spider silks: From fibroin sequence to mechanical function. J. Exp. Biol. 1999, 202, 3295–3303. [Google Scholar] [CrossRef]
- Katori, S.; Kimura, T. Injection moulding of silk fiber reinforced biodegradable composites. WIT Trans. Built Environ. 2002, 59, 1–9. [Google Scholar]
- Baillie, C.; Jayasinghe, R. Green Composites: Polymer Composites and the Environment; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Cheung, H.-Y.; Lau, K.-T.; Ho, M.-P.; Mosallam, A. Study on the Mechanical Properties of Different Silkworm Silk Fibers. J. Compos. Mater. 2009, 43, 2521–2531. [Google Scholar] [CrossRef]
- Singh, G.; Dondapati, R.S.; Singh, L.P. Biomaterials Printing for Sustainability. In Sustainability for 3D Printing; Springer: Berlin/Heidelberg, Germany, 2021; pp. 15–28. [Google Scholar] [CrossRef]
- Czaplicki, Z. Properties and structure of polish alpaca wool. Fibres Text. East. Eur. 2012, 20, 8–12. [Google Scholar]
- Mann, G.S.; Khan, A.; Asiri, A.M. Glass and Natural Fiber Composite: Properties and Applications. Sustain. Nat. Fiber Compos. 2022, 122, 256–281. [Google Scholar]
- Sharma, A.; Pant, S. Studies on Camel Hair-Merino Wool Blended Knitted Fabrics; NISCAIR-CSIR: New Delhi, India, 2013. [Google Scholar]
- Alibeigloo, A. Effect of viscoelastic interface on three-dimensional static and vibration behavior of laminated composite plate. Compos. Part B Eng. 2015, 75, 17–28. [Google Scholar] [CrossRef]
- Stan, F.; Fetecau, C. Study of stress relaxation in polytetrafluoroethylene composites by cylindrical macroindentation. Compos. Part B Eng. 2013, 47, 298–307. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, J. Animal Hairs as Water-stimulated Shape Memory Materials: Mechanism and Structural Networks in Molecular Assemblies. Sci. Rep. 2016, 6, 26393. [Google Scholar] [CrossRef]
- Feughelman, M. Natural protein fibers. J. Appl. Polym. Sci. 2002, 83, 489–507. [Google Scholar] [CrossRef]
- Wortmann, F.-J.; Zahn, H. The Stress/Strain Curve of α-Keratin Fibers and the Structure of the Intermediate Filament. Text. Res. J. 1994, 64, 737–743. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, J.; Hui, D. Tensile-relaxation study of camel hair fiber at elastic stretching region: Analytical model and experiment. Compos. Part B Eng. 2016, 91, 559–568. [Google Scholar] [CrossRef]
- Viney, C.; Llorca, J.; Elices, M. Silkworm silk as an engineering material. J. Appl. Polym. Sci. 1998, 70, 2439–2447. [Google Scholar] [CrossRef]
- Pérez-Rigueiro, J.; Elices, M.; Llorca, J.; Viney, C. Tensile properties of Argiope trifasciata drag line silk obtained from the spider’s web. J. Appl. Polym. Sci. 2001, 82, 2245–2251. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, H.; Wang, C.; Wu, L.; Huang, J.; Guo, C. Tensile behavior and morphology of differently degummed silkworm (Bombyx mori) cocoon silk fibres. Mater. Lett. 2006, 60, 919–925. [Google Scholar] [CrossRef]
- Jawaid, M.; Salit, M.S.; Alothman, O.Y. (Eds.) Green Biocomposites: Design and Applications; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Uviesherhe, O.E.; Okonkwo, U.C.; Onwuamaeze, I.P. Optimization of chicken feather fibre reinforced composite with epoxy. Elixir Mater. Sci. 2015, 81, 31974–31977. [Google Scholar]
- Tran, C.D.; Prosenc, F.; Franko, M.; Benzi, G. Synthesis, structure and antimicrobial property of green composites from cellulose, wool, hair and chicken feather. Carbohydr. Polym. 2016, 151, 1269–1276. [Google Scholar] [CrossRef]
- Amieva, E.J.C.; Velasco-Santos, C.; Martínez-Hernández, A.L.; Rivera-Armenta, J.L.; Mendoza-Martínez, A.M.; Castaño, V.M. Composites from chicken feathers quill and recycled polypropylene. J. Compos. Mater. 2014, 49, 275–283. [Google Scholar] [CrossRef]
- Cui, L.; Gong, J.; Fan, X.; Wang, P.; Wang, Q.; Qiu, Y. Transglutaminase-modified wool keratin film and its potential application in tissue engineering. Eng. Life Sci. 2012, 13, 149–155. [Google Scholar] [CrossRef]
- Su, C.; Gong, J.-S.; Qin, J.; He, J.-M.; Zhou, Z.-C.; Jiang, M.; Xu, Z.-H.; Shi, J.-S. Glutathione enables full utilization of wool wastes for keratin production and wastewater decolorization. J. Clean. Prod. 2020, 270, 122092. [Google Scholar] [CrossRef]
- Shah, A.; Tyagi, S.; Bharagava, R.N.; Belhaj, D.; Kumar, A.; Saxena, G.; Saratale, G.D.; Mulla, S.I. Keratin Production and Its Applications: Current and Future Perspective. In Keratin as a Protein Biopolymer; Springer: Berlin/Heidelberg, Germany, 2018; pp. 19–34. [Google Scholar] [CrossRef]
- Martinez-Hernandez, A.L.; Velasco-Santos, C.; de Icaza, M.; Castano, V. Microstructural characterisation of keratin fibres from chicken feathers. Int. J. Environ. Pollut. 2005, 23, 162. [Google Scholar] [CrossRef]
- Gassner, G., III; Schmidt, W.; Line, M.J.; Thomas, C.; Waters, R.M. Fiber and Fiber Products Produced from Feather. U.S. Patent 5705030, 6 January 1998. [Google Scholar]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2012, 65, 457–470. [Google Scholar] [CrossRef]
- Rao, P.D.; Kiran, C.U.; Prasad, K.E. Tensile Studies on Random Oriented Human Hair Fiber Reinforced Polyester Composites. J. Mech. Eng. 2018, 47, 37–44. [Google Scholar] [CrossRef]
- Gupta, A. Human hair “waste” and its utilization: Gaps and possibilities. J. Waste Manag. 2014, 2014, 498018. [Google Scholar] [CrossRef]
- Arunkumar, C.; Megwal, H.S.; Borkar, S.P.; Bhongade, A.L. Recycling of Chicken Feather and Wool Fibre Waste into Reinforced Multilayer Composite—A Review. Tech. Text. 2013, 73, 371–378. [Google Scholar]
- Müssig, D.-I.J.; Fischer, H.; Graupner, N.; Drieling, A. Testing Methods for Measuring Physical and Mechanical Fibre Properties (Plant and Animal Fibres). In Industrial Applications of Natural Fibres: Structure, Properties, and Technical Applications; Springer: Berlin/Heidelberg, Germany, 2010; pp. 267–309. [Google Scholar] [CrossRef]
- Ramamoorthy, S.K.; Skrifvars, M.; Persson, A. A Review of Natural Fibers Used in Biocomposites: Plant, Animal and Regenerated Cellulose Fibers. Polym. Rev. 2015, 55, 107–162. [Google Scholar] [CrossRef]
- Kock, J.W. Physical and Mechanical Properties of Chicken Feather Materials. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2006. [Google Scholar]
- Bansal, G.; Singh, V.K. Review of chicken feather fiber (CFF) a livestock waste in composite material development. Int. J. Waste Resour. 2016, 6, 1000254. [Google Scholar]
- Babalola, R.; Ayeni, A.O.; Joshua, P.S.; Ayoola, A.A.; Isaac, U.O.; Aniediong, U.; Efeovbokhan, V.E.; Omoleye, J.A. Synthesis of thermal insulator using chicken feather fibre in starch-clay nanocomposites. Heliyon 2020, 6, e05384. [Google Scholar] [CrossRef]
- Bartels, T. Variations in the morphology, distribution, and arrangement of feathers in domesticated birds. J. Exp. Zool. Part B Mol. Dev. Evol. 2003, 298, 91–108. [Google Scholar] [CrossRef]
- Winandy, J.E.; Muehl, J.H.; Micales, J.A.; Raina, A.; Schmidt, W. Potential of Chicken Feather Fibre in Wood MDF Composites. In Proceedings of the EcoComp 2003, Queen Mary, University of London, London, UK, 1–2 September 2003; Volume 6, p. 20. [Google Scholar]
- Zhan, M.; Wool, R.P. Mechanical properties of chicken feather fibers. Polym. Compos. 2011, 32, 937–944. [Google Scholar] [CrossRef]
- Huda, S.; Yang, Y. Composites from ground chicken quill and polypropylene. Compos. Sci. Technol. 2008, 68, 790–798. [Google Scholar] [CrossRef]
- Bullions, T.; Hoffman, D.; Gillespie, R.; Price-O’Brien, J.; Loos, A. Contributions of feather fibers and various cellulose fibers to the mechanical properties of polypropylene matrix composites. Compos. Sci. Technol. 2006, 66, 102–114. [Google Scholar] [CrossRef]
- Saucedo-Rivalcoba, V.; Martínez-Hernández, A.L.; Martínez-Barrera, G.; Velasco-Santos, C.; Castaño, V.M. (Chicken feathers keratin)/polyurethane membranes. Appl. Phys. A 2011, 104, 219–228. [Google Scholar] [CrossRef]
- Cheng, S.; Lau, K.-T.; Liu, T.; Zhao, Y.; Lam, P.-M.; Yin, Y. Mechanical and thermal properties of chicken feather fiber/PLA green composites. Compos. Part B Eng. 2009, 40, 650–654. [Google Scholar] [CrossRef]
- Oladele, I.O. Analysis of the influence of chemical treatments on the physico-chemical and tensile properties of avian fiber. Ann. Fac. Eng. Hunedoara 2016, 14, 87. [Google Scholar]
- Barone, J.R.; Schmidt, W.F. Polyethylene reinforced with keratin fibers obtained from chicken feathers. Compos. Sci. Technol. 2005, 65, 173–181. [Google Scholar] [CrossRef]
- Chang, K.C. The Archaeology of Ancient China, 4th ed.; Yale University Press: New Haven, CT, USA, 1986. [Google Scholar]
- Lucas, F.; Shaw, J.T.B.; Smith, S.G. The silk fibroins. In Advances in Protein Chemistry; Anfinsen, C.B., Ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Foelix, R.F. Biology of Spiders; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Engster, M.S. Studies on silk secretion in the trichoptera (F. Limnephilidae). Cell Tissue Res. 1976, 169, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Aldini, N.N.; Fini, M. Advances in Nanotechnologies for the Fabrication of Silk Fibroin-Based Scaffolds for Tissue Regeneration. In Extracellular Matrix for Tissue Engineering and Biomaterials; Springer: Berlin/Heidelberg, Germany, 2018; pp. 151–160. [Google Scholar] [CrossRef]
- Meinel, L.; Hofmann, S.; Karageorgiou, V.; Kirker-Head, C.; McCool, J.; Gronowicz, G.; Zichner, L.; Langer, R.; Vunjak-Novakovic, G.; Kaplan, D.L. The inflammatory responses to silk films in vitro and in vivo. Biomaterials 2005, 26, 147–155. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, H.-J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef] [PubMed]
- Vehoff, T.; Glišović, A.; Schollmeyer, H.; Zippelius, A.; Salditt, T. Mechanical Properties of Spider Dragline Silk: Humidity, Hysteresis, and Relaxation. Biophys. J. 2007, 93, 4425–4432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Q. Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 2002, 20, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Kikuchi, Y.; Takagi, T.; Kikuchi, A.; Oyama, K.; Shimura, K.; Mizuno, K. The primary structure of the silk fibroin light chain is determined by cDNA sequencing and peptide analysis. J. Mol. Biol. 1989, 210, 127–139. [Google Scholar] [CrossRef]
- Putthanarat, S.; Stribeck, N.; Fossey, S.; Eby, R.; Adams, W. Investigation of the nanofibrils of silk fibers. Polymer 2000, 41, 7735–7747. [Google Scholar] [CrossRef]
- Bhat, N.V.; Nadiger, G.S. Crystallinity in silk fibers: Partial acid hydrolysis and related studies. J. Appl. Polym. Sci. 1980, 25, 921–932. [Google Scholar] [CrossRef]
- Glišovic, A.; Vollrath, F. Mulberry Silk, Spider Dragline and Recombinant Silks. In Industrial Applications of Natural Fibres: Structure, Properties and Technical Applications; Wiley: Hoboken, NJ, USA, 2010; p. 237. [Google Scholar]
- Xu, M.; Lewis, R.V. Structure of a protein superfiber: Spider dragline silk. Proc. Natl. Acad. Sci. USA 1990, 87, 7120–7124. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, X.; Shao, Z.; Zhou, P.; Porter, D.; Knight, D.P.; Vollrath, F. Toughness of Spider Silk at High and Low Temperatures. Adv. Mater. 2005, 17, 84–88. [Google Scholar] [CrossRef]
- Kaplan, D.; Adams, W.W.; Farmer, B.; Viney, C. Silk polymers: Materials science and biotechnology. Am. Chem. Soc. 1993, 544, 2–16. [Google Scholar]
- Bell, F.I.; McEwen, I.J.; Viney, C. Supercontraction stress in wet spider dragline. Nature 2002, 416, 37. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Baldassarre, H.; Tao, T.; Gauthier, M.; Neveu, N.; Zhou, J.; Leduc, M.; Duguay, F.; Bilodeau, A.; Lazaris, A.; et al. Transgenic goats produced by DNA pronuclear microinjection of in vitro derived zygotes. Mol. Reprod. Dev. 2002, 63, 437–443. [Google Scholar] [CrossRef]
- Scheibel, T. Protein fibers as performance proteins: New technologies and applications. Curr. Opin. Biotechnol. 2005, 16, 427–433. [Google Scholar] [CrossRef]
- Scheller, J.; Gührs, K.-H.; Grosse, F.; Conrad, U. Production of spider silk proteins in tobacco and potato. Nat. Biotechnol. 2001, 19, 573–577. [Google Scholar] [CrossRef]
- Prudhomme, J.C.; Couble, P. Perspectives in silkworm (Bombyx mori) transgenesis. Curr. Sci. 2002, 83, 432–438. [Google Scholar]
- Zhou, B.; Zhou, Q.; Wang, P.; Yuan, J.; Yu, Y.; Deng, C.; Wang, Q.; Fan, X. HRP-mediated graft polymerization of acrylic acid onto silk fibroins and in situ biomimetic mineralization. J. Mater. Sci. Mater. Med. 2018, 29, 1–14. [Google Scholar] [CrossRef]
- Wang, P.; Yu, M.; Cui, L.; Yuan, J.; Wang, Q.; Fan, X. Modification of B ombyx mori silk fabrics by tyrosinase-catalyzed grafting of chitosan. Eng. Life Sci. 2014, 14, 211–217. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, B.; Li, M.; Zuo, B.; Kaplan, D.L.; Huang, Y.; Zhu, H. Degradation Mechanism and Control of Silk Fibroin. Biomacromolecules 2011, 12, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhu, X.; Yuan, J.; Yu, Y.; Cui, L.; Duan, Y.; Wang, Q.; Fan, X. Grafting of tyrosine-containing peptide onto silk fibroin membrane for improving enzymatic reactivity. Fibers Polym. 2016, 17, 1323–1329. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, C.; Ma, X.; Hu, L.; Chen, L.; Wang, C. In vitro and in vivo degradation behavior of aqueous-derived electrospun silk fibroin scaffolds. Polym. Degrad. Stab. 2010, 95, 1679–1685. [Google Scholar] [CrossRef]
- Shao, W.; He, J.; Han, Q.; Sang, F.; Wang, Q.; Chen, L.; Ding, B. A biomimetic multilayer nanofiber fabric fabricated by electrospinning and textile technology from polylactic acid and Tussah silk fibroin as a scaffold for bone tissue engineering. Mater. Sci. Eng. C 2016, 67, 599–610. [Google Scholar] [CrossRef]
- Popescu, C.; Höcker, H. Hair—The most sophisticated biological composite material. Chem. Soc. Rev. 2007, 36, 1282–1291. [Google Scholar] [CrossRef]
- Feughelman, M. A note on the water-impenetrable component of α-keratin fibers. Text. Res. J. 1989, 59, 739–742. [Google Scholar] [CrossRef]
- Chen, D.; Tan, L.; Liu, H.; Hu, J.; Li, Y.; Tang, F. Fabricating Superhydrophilic Wool Fabrics. Langmuir 2009, 26, 4675–4679. [Google Scholar] [CrossRef]
- Idris, A.; Vijayaraghavan, R.; Rana, U.A.; Patti, A.F.; Macfarlane, D.R. Dissolution and regeneration of wool keratin in ionic liquids. Green Chem. 2014, 16, 2857–2864. [Google Scholar] [CrossRef]
- Parsons, Y.M.; Cooper, D.W.; Piper, L.R. Evidence of linkage between high-glycine-tyrosine keratin gene loci and wool fibre diameter in a Merino half-sib family. Anim. Genet. 1994, 25, 105–108. [Google Scholar] [CrossRef]
- Sukumar, A.; Subramanian, R. Elements in the Hair of Non-Mining Workers of a Lignite Open Mine in Neyveli. Ind. Health 2003, 41, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Hoecker, H.; Wortmann, G. Unconventional uses of wool. In Proceedings of the IWTO Meeting, Buenos Aires, Argentina, 10–13 December 2003. [Google Scholar]
- Berger, W.; Faulstich, H.; Fischer, P.; Heger, A.; Jacobasch, H.J.; Mally, A.; Mikut, I. Textile Faserstoffe: Beschaffenheit und Eigenschaften; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Zach, J.; Korjenic, A.; Petránek, V.; Hroudová, J.; Bednar, T. Performance evaluation and research of alternative thermal insulations based on sheep wool. Energy Build. 2012, 49, 246–253. [Google Scholar] [CrossRef]
- Jeon, S.-H.; Hwang, K.-H.; Lee, J.S.; Boo, J.-H.; Yun, S.H. Plasma treatments of wool fiber surface for microfluidic applications. Mater. Res. Bull. 2015, 69, 65–70. [Google Scholar] [CrossRef]
- Goudarzi, G.; Sepehrizadeh, Z.; Yazdi, M.T.; Jamshidiha, M. Comparison of surface modification of wool fibres using Pronase, Trypsin, Papain and Pepsin. Lipids 2015, 2, 6. [Google Scholar]
- Salih, R.M.; Al-Zubaydi, A.S.J. Study of the insulation and mechanical properties of hair-reinforced epoxy. Prog. Rubber Plast. Recycl. Technol. 2021, 37, 247–263. [Google Scholar] [CrossRef]
- Senthilnathan, D.; Babu, A.G.; Bhaskar, G.B.; Gopinath, K.G.S. Characterization of glass fibre–coconut coir–human hair hybrid composites. Int. J. Eng. Technol. 2014, 6, 75–82. [Google Scholar]
- Vineis, C.; Aluigi, A.; Tonin, C. Outstanding traits and thermal behaviour for the identification of speciality animal fibres. Text. Res. J. 2010, 81, 264–272. [Google Scholar] [CrossRef]
- Robbins, C.R. Dyeing human hair. In Chemical and Physical Behavior of Human Hair; Springer: Berlin/Heidelberg, Germany, 2012; pp. 445–488. [Google Scholar]
- Wang, F.; Zieman, A.; Coulombe, P.A. Skin keratins. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2016; Volume 568, pp. 303–350. [Google Scholar]
- Yu, J.; Yu, D.W.; Checkla, D.M.; Freedberg, I.M.; Bertolino, A.P. Human hair keratins. J. Investig. Dermatol. 1993, 101, S56–S59. [Google Scholar] [CrossRef]
- Bongu, C.S.; Karuppiah, S.; Nallathamby, K. Validation of green composite containing nanocrystalline Mn2O3 and biocarbon derived from human hair as a potential anode for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 23981–23989. [Google Scholar] [CrossRef]
- Deepmala, K.; Jain, N.; Singh, V.K.; Chauhan, S. Fabrication and characterization of chitosan coated human hair reinforced phytagel modified soy protein-based green composite. J. Mech. Behav. Mater. 2018, 27. [Google Scholar] [CrossRef]
- Shao, Z.; Vollrath, F. Surprising strength of silkworm silk. Nature 2002, 418, 741. [Google Scholar] [CrossRef] [PubMed]
- Wray, L.S.; Hu, X.; Gallego, J.; Georgakoudi, I.; Omenetto, F.G.; Schmidt, D.; Kaplan, D.L. Effect of processing on silk-based biomaterials: Reproducibility and biocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 99, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Q.; Zhao, H.P.; Feng, X.Q.; Cui, W.; Lin, Z.; Xu, M.Q. Mechanical properties of cocoons constructed consecutively by a single silkworm caterpillar, Bombyx mori. Acta Mech. Sin. 2008, 24, 151–160. [Google Scholar] [CrossRef]
- Reddy, N.; Jiang, Q.; Jin, E.; Shi, Z.; Hou, X.; Yang, Y. Bio-thermoplastics from grafted chicken feathers for potential biomedical applications. Colloids Surf. B Biointerfaces 2013, 110, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Martelli, S.M.; Laurindo, J.B. Chicken feather keratin films plasticized with polyethylene glycol. Int. J. Polym. Mater. 2012, 61, 17–29. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Chen, G.; He, J.; Han, Y.; Wang, X.; Kaplan, D.L. Silk-Based Biomaterials in Biomedical Textiles and Fiber-Based Implants. Adv. Health Mater. 2015, 4, 1134–1151. [Google Scholar] [CrossRef]
- Seves, A.; Romanò, M.; Maifreni, T.; Sora, S.; Ciferri, O. The microbial degradation of silk: A laboratory investigation. Int. Biodeterior. Biodegradation 1998, 42, 203–211. [Google Scholar] [CrossRef]
- Koyanagi, R.; Zhu, Z.; Asakura, T. Regenerated Bombyx mori silk fiber with enhanced biodegradability. J. Insect Biotechnol. Sericol. 2010, 79, 27–30. [Google Scholar]
- Altman, G.H.; Horan, R.L.; Lu, H.H.; Moreau, J.; Martin, I.; Richmond, J.C.; Kaplan, D.L. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials 2002, 23, 4131–4141. [Google Scholar] [CrossRef]
- Gow, R.; Thomson, S.; Rieder, M.; van Uum, S.; Koren, G. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci. Int. 2010, 196, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Forrest, R.D. Early history of wound treatment. J. R. Soc. Med. 1982, 75, 198. [Google Scholar] [CrossRef] [PubMed]
- Sood, G.C.; Sen, D.K.; Sota, L.D. Human hair sutures in ophthalmic surgery. Br. J. Ophthalmol. 1970, 54, 335–337. [Google Scholar] [CrossRef]
- Luangjarmekorn, P.; Kitidumrongsook, P.; Honsawek, S. Do-It-Yourself Microsuture from Human Hair for Basic Microsurgical Training. J. Reconstr. Microsurg. 2018, 35, 315–321. [Google Scholar] [CrossRef]
- Vitus, V.; Ibrahim, F.; Wan Kamarul Zaman, W.S. Valorisation of Human Hair and Its Derivatives in Tissue Engineering: A Review. Tissue Eng. 2022, 28, 529–544. [Google Scholar] [CrossRef]
- Eroğlu, L.; Güneren, E.; Akbaş, H.; Demir, A.; Uysal, A.A. Using human hair as suture material in microsurgical practice. J. Reconstr. Microsurg. 2003, 19, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Allafi, F.; Hossain, S.; Lalung, J.; Shaah, M.; Salehabadi, A.; Ahmad, M.I.; Shadi, A. Advancements in Applications of Natural Wool Fiber: Review. J. Nat. Fibers 2020, 19, 497–512. [Google Scholar] [CrossRef]
- Reddy, R.M. Innovative and multidirectional applications of natural fibre, silk-a review. Acad. J. Entomol. 2009, 2, 71–75. [Google Scholar]
- Dweib, M.; Hu, B.; O’Donnell, A.; Shenton, H.; Wool, R. All natural composite sandwich beams for structural applications. Compos. Struct. 2004, 63, 147–157. [Google Scholar] [CrossRef]
- Savio, L.; Bosia, D.; Patrucco, A.; Pennacchio, R.; Piccablotto, G.; Thiebat, F. Applications of Building Insulation Products Based on Natural Wool and Hemp Fibers. In Advances in Natural Fibre Composites; Springer: Cham, Switzerland, 2017; pp. 237–247. [Google Scholar] [CrossRef]
- Acada, M.N. Waste chicken feather as reinforcement in cement-bonded composites. Philipp. J. Sci. 2010, 139, 161–166. [Google Scholar]
- Koronis, G.; Silva, A.; Fontul, M. Green composites: A review of adequate materials for automotive applications. Compos. Part B Eng. 2013, 44, 120–127. [Google Scholar] [CrossRef]
- Mann, G.S.; Singh, L.P.; Kumar, P.; Singh, S. Green composites: A review of processing technologies and recent applications. J. Thermoplast. Compos. Mater. 2018, 33, 1145–1171. [Google Scholar] [CrossRef]
- Mann, G.S.; Singh, L.P.; Kumar, P.; Singh, S.; Prakash, C. On briefing the surface modifications of polylactic acid: A scope for betterment of biomedical structures. J. Thermoplast. Compos. Mater. 2019, 34, 977–1005. [Google Scholar] [CrossRef]
- Akampumuza, O.; Wambua, P.M.; Ahmed, A.; Li, W.; Qin, X.-H. Review of the applications of biocomposites in the automotive industry. Polym. Compos. 2016, 38, 2553–2569. [Google Scholar] [CrossRef]
- Huda, M.S.; Drzal, L.T.; Ray, D.; Mohanty, A.K.; Mishra, M. Natural-fiber composites in the automotive sector. In Properties and Performance of Natural-Fibre Composites; Woodhead Publishing: Sawston, UK, 2008; pp. 221–268. [Google Scholar]
- Kurien, R.A.; Biju, A.; Raj, K.A.; Chacko, A.; Joseph, B.; Koshy, C.P. Chicken feather fiber reinforced composites for sustainable applications. Mater. Today Proc. 2021, 58, 862–866. [Google Scholar] [CrossRef]
- Mann, G.S.; Singh, L.P.; Kumar, P.; Khan, A.; Khan, A.A.P.; Asiri, A.M. Effect of Process Parameters on the Fabrication of Hybrid Natural Fiber Composites Fabricated via Compression Moulding Process. J. Nat. Fibers 2022, 19, 14803–14812. [Google Scholar] [CrossRef]




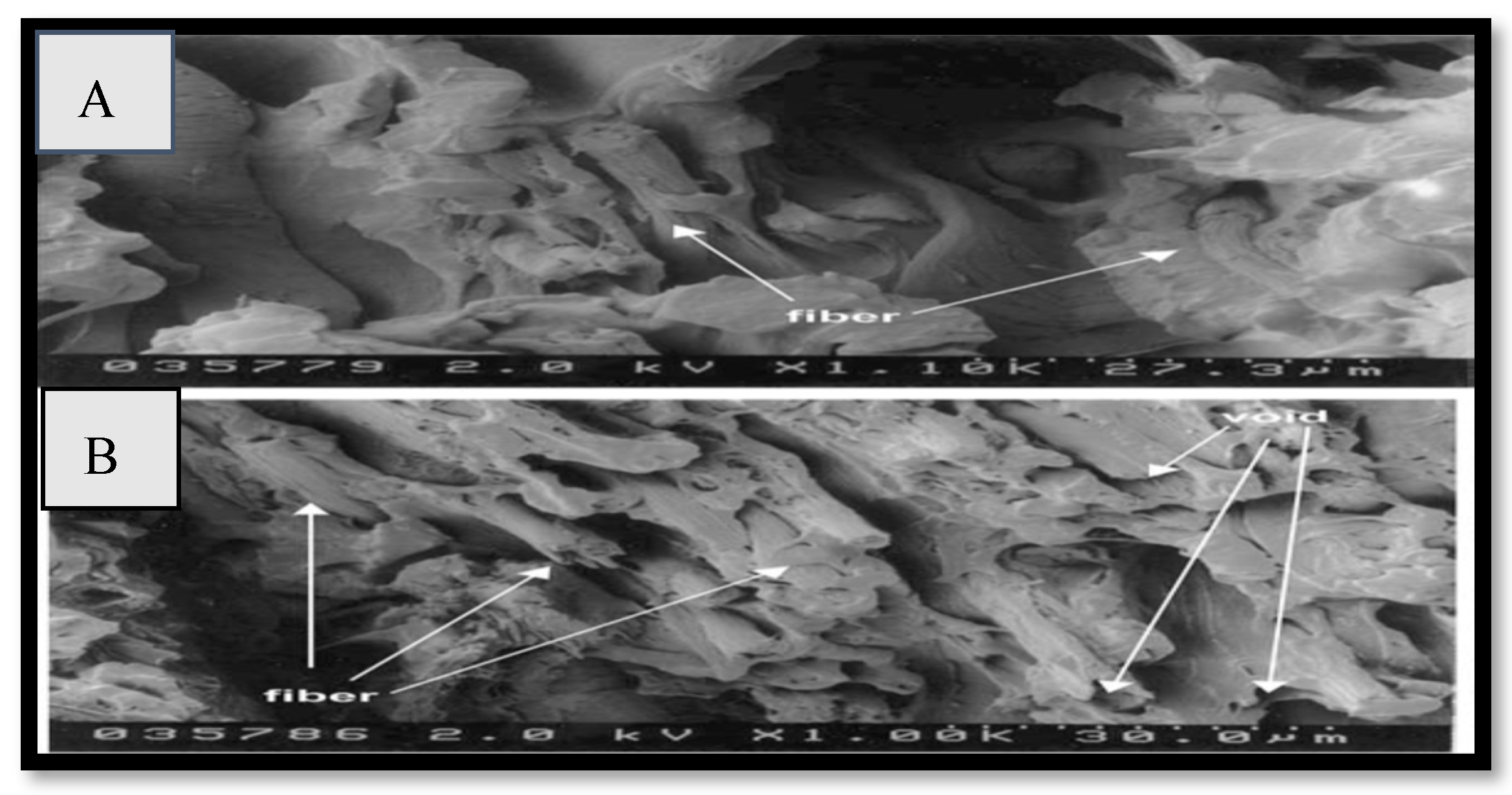

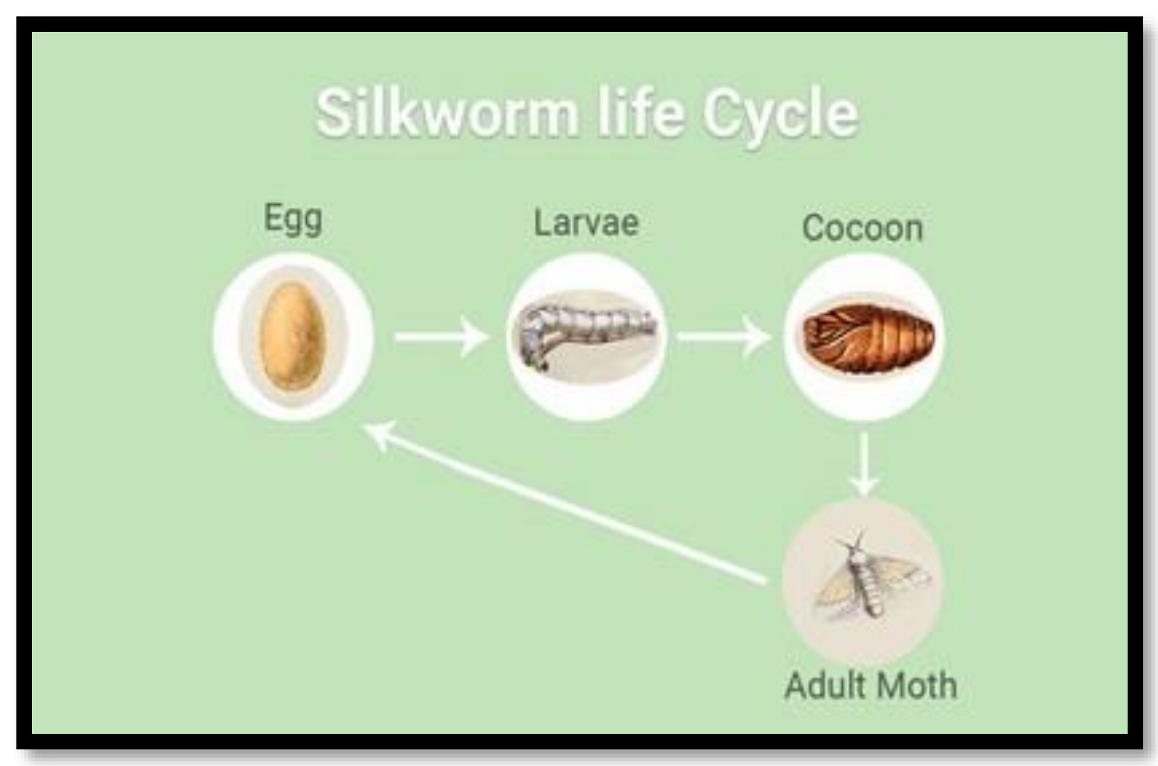
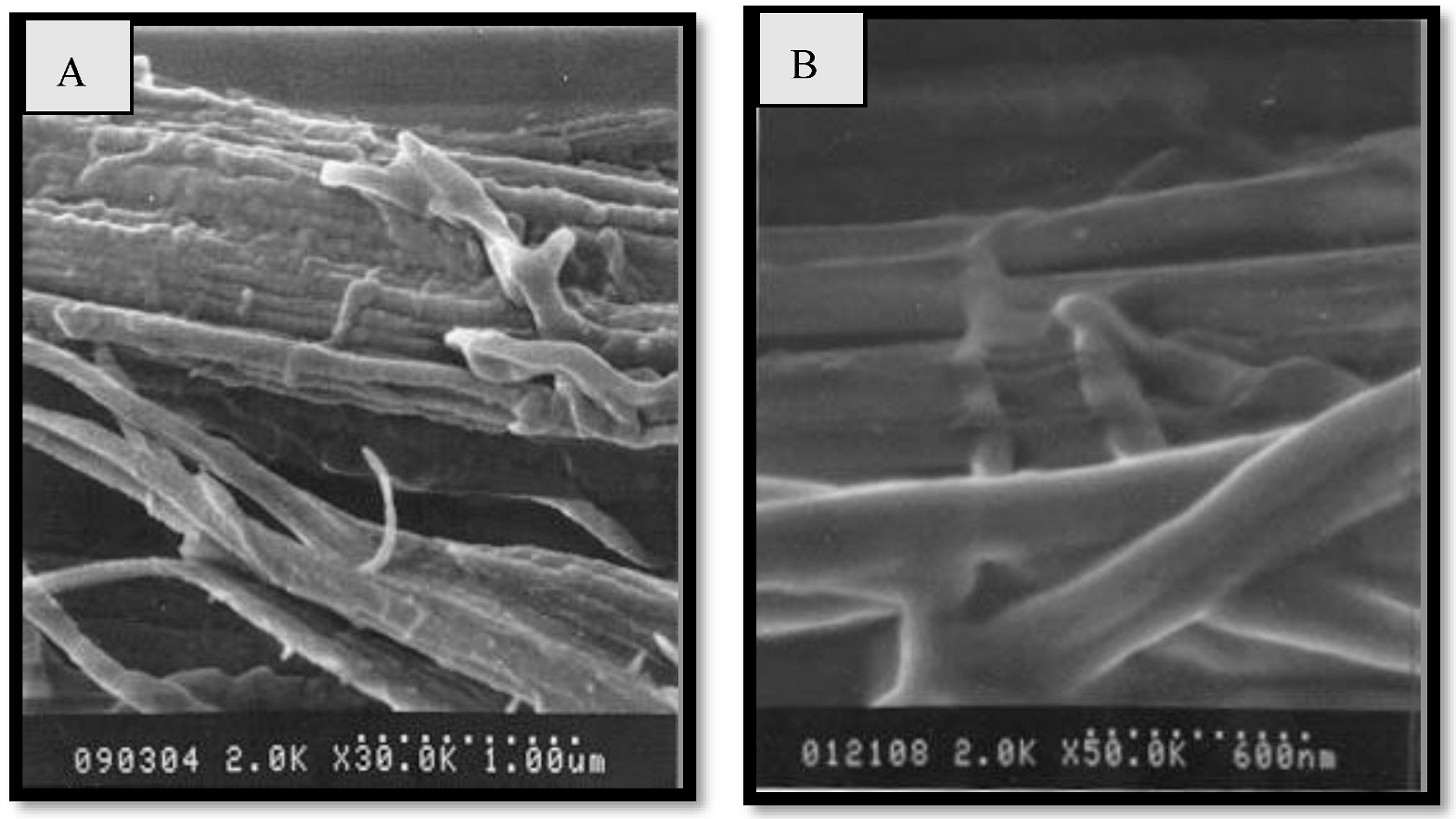


| Fibers | Properties | Diameter (µm) | UTS (MPa) | Elongation at Break (in µm) | E (GPa) | Density (g/cm3) |
|---|---|---|---|---|---|---|
| Flax | Lightweight, absorbent | 12–16 | 300–1500 | 1.3–10 | 24.80 | 1.4–1.5 |
| Jute | Strength, durability | 17–20 | 200–800 | 1.16–8 | 10–55 | 1.3–1.5 |
| Sisal | Strength, durability | 200–400 | 80–840 | 2–25 | 9–38 | 1.5 |
| Kenaf | Rough | 25–35 | 296–1191 | 3.5 | 2.86 | - |
| Abaca | Thin, lightweight | 40 | 980 | - | 7.31 × 10−4 | - |
| Pineapple | Soft, lightweight | 10–28 | 170–1627 | 2.4 | 60–82 | 0.8–1.6 |
| Banana | Warm, thick, durable | 200 | 529–914 | 3 | 27–32 | - |
| Coir | Strength, durability | 10–20 | 106–175 | 14.21–49 | 4–6 | 1.2 |
| Ramie | Heavy, tough | 20 | 348–938 | 1.2–8 | 44–128 | - |
| Hemp | Strength, durability | 16–50 | 310–900 | 1.6–6 | 30–70 | 1.48 |
| Wool | Warmth | 16–40 | 120–174 | 25–35 | 2.3–3.4 | - |
| Spider silk | Smooth fabric finish with high shine | 10–13 | 875–972 | 17–18 | 11–13 | - |
| Cotton | Lightweight, absorbent | 11–22 | 264–800 | 3–8 | 5–12.6 | - |
| Mulberry silkworm fiber | White-toned and more reproducible | 10 | 208.45 | 19.55 | 6.10 | 1.33 |
| Wild (Tussah) silkworm fiber | Beige to brownish-toned | 25 | 165.27 | 20.57 | 3.82 | 1.32 |
| Twisted B. mori silk | 10 | 248.77 | 33.48 | 5.79 | - | |
| Camel hair | Softness, warmth | 20.04 | 212.15 | 37.05 | 3.87 | - |
| Catgut fiber | 790 | 100 | - | |||
| Angora wool | Softness, thin fibers | 12–16 | ||||
| Yak fiber | Warmth, softness, breathability, odor-resistant | 15–19 | 270.05 | 14.53 | 45.0943 | 3.41 |
| Alpaca | Lightweight, soft, fine, glossy, and luxurious | 12–29 | 53.5 | 42.3 | 1.38 | |
| Bison | Red brown, soft | 59 | ||||
| Llama | Fine, soft | 30–40 | ||||
| Qiviut | Long, smooth, 8 times warmer than sheep | 15–20 |
| Species | Silk | Diet | Domestication |
|---|---|---|---|
| Bombyx mori (silkworm) | Mulberry (cocoon) | Morus spp. (mulberry) exclusively | Yes |
| Antheraea pernyi (Chinese oak silk moth) | Tussah (cocoon) | Quercus spp. (oak) exclusively | Semi |
| Antheraea assamensis | Muga (cocoon) | Machilus bombycina Latsaea polyantha | Semi |
| Philosamia cynthia ricini | Eri (cocoon) | Ricinus communis (castor) exclusively | Yes |
| Nephila sp. (Golden orbweaver) | Dragline (among others) | Insects | No |
| Araneus sp. (European garden spider) | Dragline (among others) | Insects | No |
| Trichoptera sp. (Caddiesfly) | Part of cases | Herbivorous Carnivorous | No |
| Material | Tensile Strength in GPa | Extensibility (% of Initial Length) | Young’s Modulus in Gpa | Toughness in MJ/m3 |
|---|---|---|---|---|
| High-tensile strength steel | 1.5 | 0.8 | 200 | 6 |
| Aramide (Kevlar) | 47 | 3.6 | 2.7 | 130 |
| Polyamide 6.6 (Nylon, DuPont) | 0.95 | 18 | 4 | 80 |
| Mulberry silk (Bombyx mori) | 0.6 | 18 | 6 | 70 |
| Dragline (Nephila) | 1.1 | 30 | 20 | 170 |
| Property | Bombyx Mori | Nephila Dragline |
|---|---|---|
| Degree of crystallinity in % | 38–66 | 20–45 |
| Density in g/cm3 | 1.35–1.42 | |
| Crystallite size in nm | 1.0–2.5 | 4.7 × 5.3 × 6.0 |
| Index of refraction | 1.591 parallel to fiber | 1.538 perpendicular to fiber |
| Maximum use temperature in °C | 170 | 150 |
| Thermal degradation in °C | 250 | 234 |
| Heat capacity in J/g K | 1.38 | |
| Glass transition temperature | 178 °C at 0% RH | 39 °C at 75% RH |
| Super contraction in water | No | ∼50% |
| Species | Year-Round Production | Advantage | Disadvantage |
|---|---|---|---|
| Capra aegagrus hircus (domestic goat) | Yes | Easy to keep and can be constrained in stables | High space consumption |
| Escherichia coli | Yes | Can be kept constrained in high densities | Only 30 kDa proteins |
| Nicotiana tabacum (tobacco) | No, seasonal | 100 kDa proteins | Poor acceptance in Europe (genetic engineering for agriculture) |
| Solanum tuberosum (potato) | No, seasonal | 100 kDa proteins | Poor acceptance in Europe (genetic engineering for agriculture) |
| Bombyx mori (silkworm) | No, seasonal | Produces fibers and raw protein, easy to keep and to keep constrained | Fibers are not pure spider silk protein, exclusive diet of mulberry leaves = mulberry plant age required |
| Amino Acid in mol % | Wool | Cashmere | Yak |
|---|---|---|---|
| Glycine | 8.1 | 9.9 | 9.8 |
| Alanine | 5 | 5.8 | 5.6 |
| Serine | 10.2 | 12.2 | 10 |
| Glutamine + glutamic acid | 12.1 | 12.4 | 12.5 |
| Cystine | 11.2 | 6 | 6.4 |
| Proline | 7.5 | 6.7 | 6.6 |
| Arginine | 7.2 | 7 | 7.1 |
| Leucine | 6.9 | 7.5 | 8.3 |
| Threonine | 6.5 | 6.6 | 6.6 |
| Asparagine + aspartic acid | 6 | 6.2 | 6.7 |
| Valine | 5.1 | 5.5 | 5.9 |
| Tyrosine | 4.2 | 3.5 | 3.4 |
| Isoleucine | 2.8 | 3.2 | 3.5 |
| Phenylalanine | 2.5 | 2.8 | 3 |
| Lysine | 2.3 | 2.8 | 3 |
| Tryptophan | 1.2 | - | - |
| Histidine | 0.7 | 1.2 | 1 |
| Methionine | 0.5 | 0.5 | 0.5 |
| Breaking Stress | |
|---|---|
| Dry | 250–350 MPa |
| Wet | 100–200 MPa |
| Strength loss when wet | 20% |
| Breaking strain | |
| Dry | 28–48% |
| Wet | 40–61% |
| Elasticity modulus | |
| Dry | 4.0–5.0 GPa |
| Wet | 2.0–3.0 GPa |
| Recovery at strain | |
| 2% | 95–99% |
| 5% | 60–70% |
| 10% | 40–50% |
| Bending modulus | 4.0–5.5 GPa |
| Stretching modulus | 5.0–6.0 GPa |
| Torsion modulus parallel | 1.1–1.3 GPa |
| Stretching modulus/torsion modulus | 3.0–4.0 GPa |
| Shear modulus in torsion | |
| Dry | 1.2 GPa |
| Wet | 0.1GPa |
| Wool Type | Grease and Suint | Sand and Dirt | Vegetable Matter | Fiber |
|---|---|---|---|---|
| Merino (<25 μm) | 15–30 | 5–40 | 0.5–10 | 30–60 |
| Cross-bred (25–33 μm) | 15–30 | 5–20 | 1–5 | 40–65 |
| Long wool (>33 μm) | 5–15 | 5–10 | 0–2 | 60–75 |
| Amino Acid | Amount in Residues Extracted |
|---|---|
| Cysteine | 17.5 |
| Serine | 11.7 |
| Glutamic acid | 11.1 |
| Threonine | 6.9 |
| Glycine | 6.5 |
| Valine | 5.9 |
| Arginine | 5.6 |
| Aspartic acid | 5 |
| Alanine | 4.8 |
| Proline | 3.6 |
| Isoleucine | 2.7 |
| Tyrosine | 1.9 |
| Amino Acid | Micromoles per Gram of Hair | Significant Difference for | |
|---|---|---|---|
| Non-Frosted Fibers | Frosted Fibers | Frequencies at Alpha = 0.01 Level | |
| Aspartic acid | 437 | 432 | _ |
| Threonine | 616 | 588 | _ |
| Serine | 1085 | 973 | _ |
| Glutamic acid | 1030 | 999 | _ |
| Proline | 639 | 582 | _ |
| Glycine | 450 | 415 | _ |
| Alanine | 370 | 357 | _ |
| Half cystine | 1509 | 731 | Yes |
| Valine | 487 | 464 | _ |
| Methionine | 50 | 38 | Yes |
| Isoleucine | 227 | 220 | _ |
| Leucine | 509 | 485 | _ |
| Tyrosine | 183 | 146 | Yes |
| Phenylalanine | 139 | 129 | _ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mann, G.S.; Azum, N.; Khan, A.; Rub, M.A.; Hassan, M.I.; Fatima, K.; Asiri, A.M. Green Composites Based on Animal Fiber and Their Applications for a Sustainable Future. Polymers 2023, 15, 601. https://doi.org/10.3390/polym15030601
Mann GS, Azum N, Khan A, Rub MA, Hassan MI, Fatima K, Asiri AM. Green Composites Based on Animal Fiber and Their Applications for a Sustainable Future. Polymers. 2023; 15(3):601. https://doi.org/10.3390/polym15030601
Chicago/Turabian StyleMann, Guravtar Singh, Naved Azum, Anish Khan, Malik Abdul Rub, Md Imtaiyaz Hassan, Kisa Fatima, and Abdullah M. Asiri. 2023. "Green Composites Based on Animal Fiber and Their Applications for a Sustainable Future" Polymers 15, no. 3: 601. https://doi.org/10.3390/polym15030601
APA StyleMann, G. S., Azum, N., Khan, A., Rub, M. A., Hassan, M. I., Fatima, K., & Asiri, A. M. (2023). Green Composites Based on Animal Fiber and Their Applications for a Sustainable Future. Polymers, 15(3), 601. https://doi.org/10.3390/polym15030601









