Development and Characterization of a Sustainable Bio-Polymer Concrete with a Low Carbon Footprint
Abstract
1. Introduction
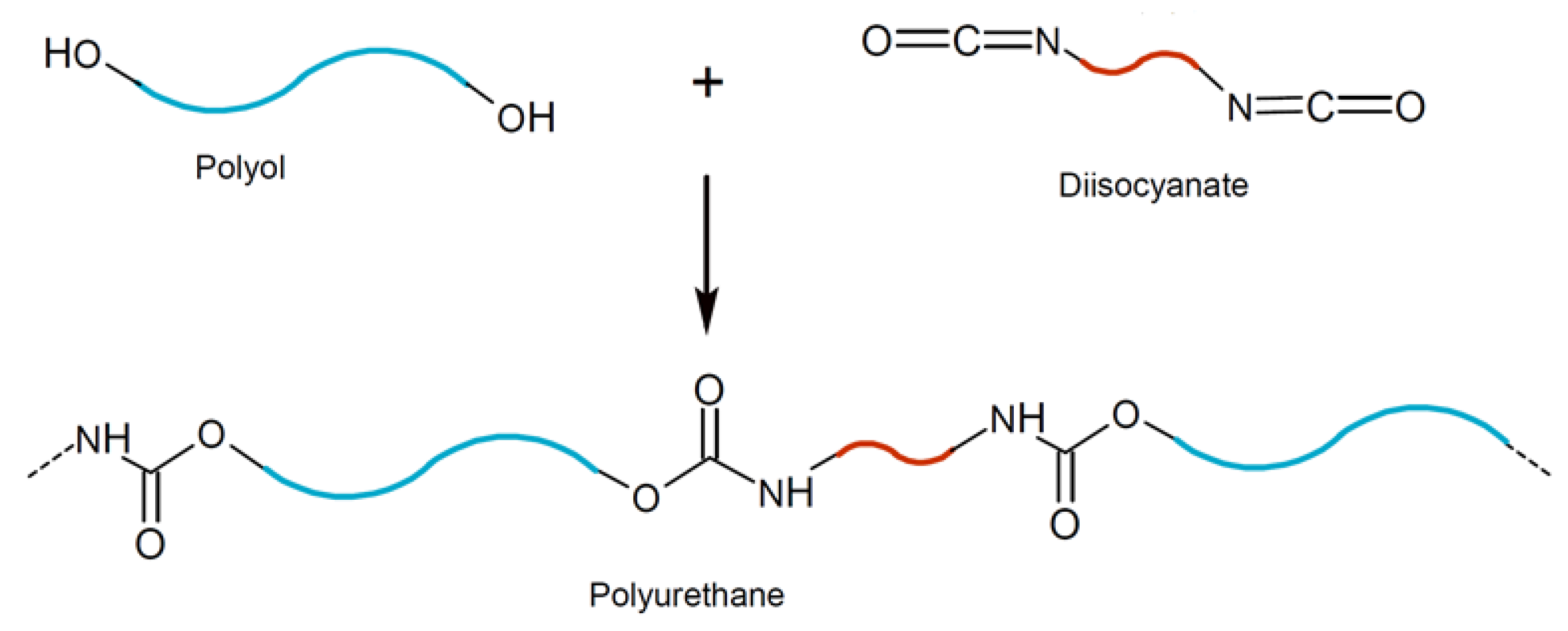
2. Materials and Mix Design
3. Experimental Methods
4. Results and Discussion
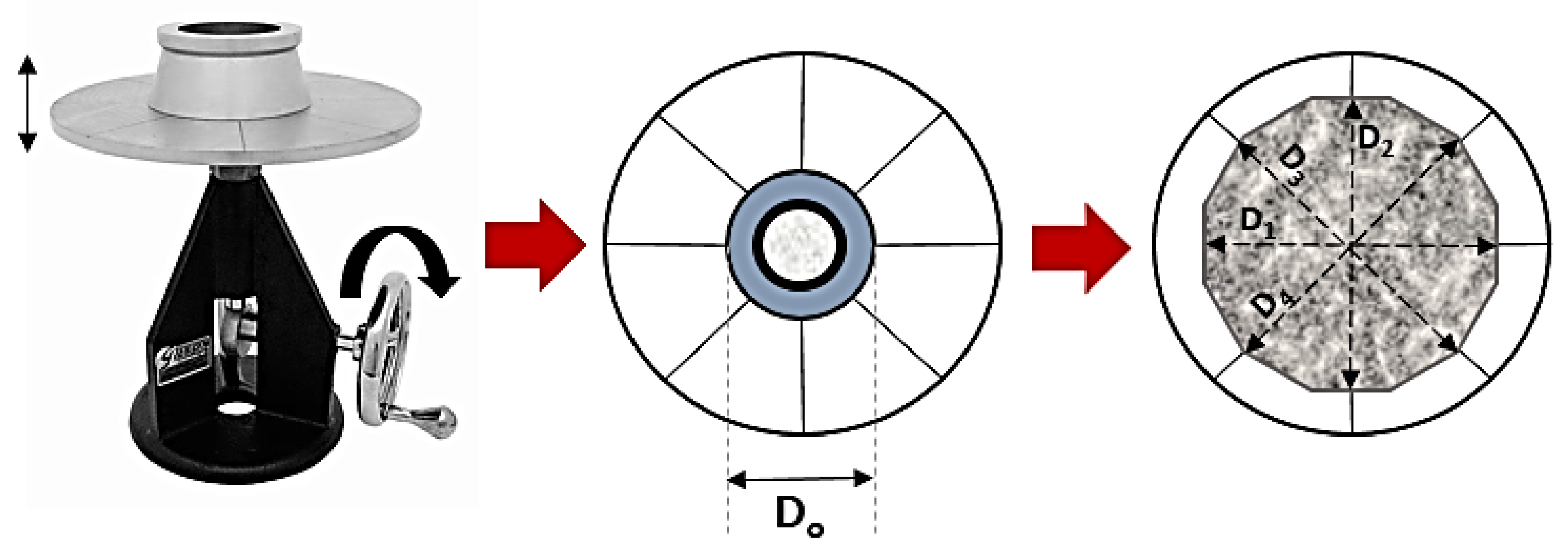
4.1. Flowability and Setting Time
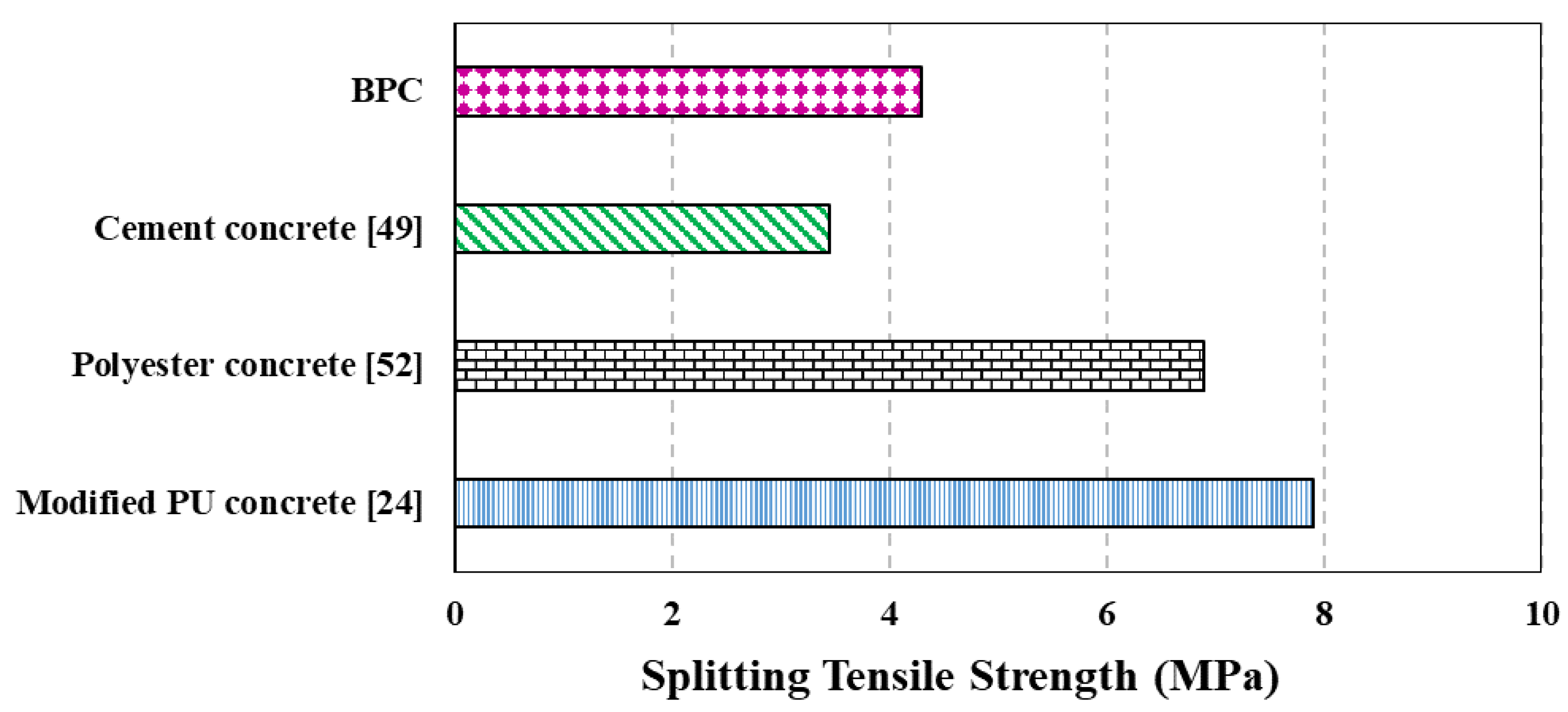
4.2. Mechanical Properties
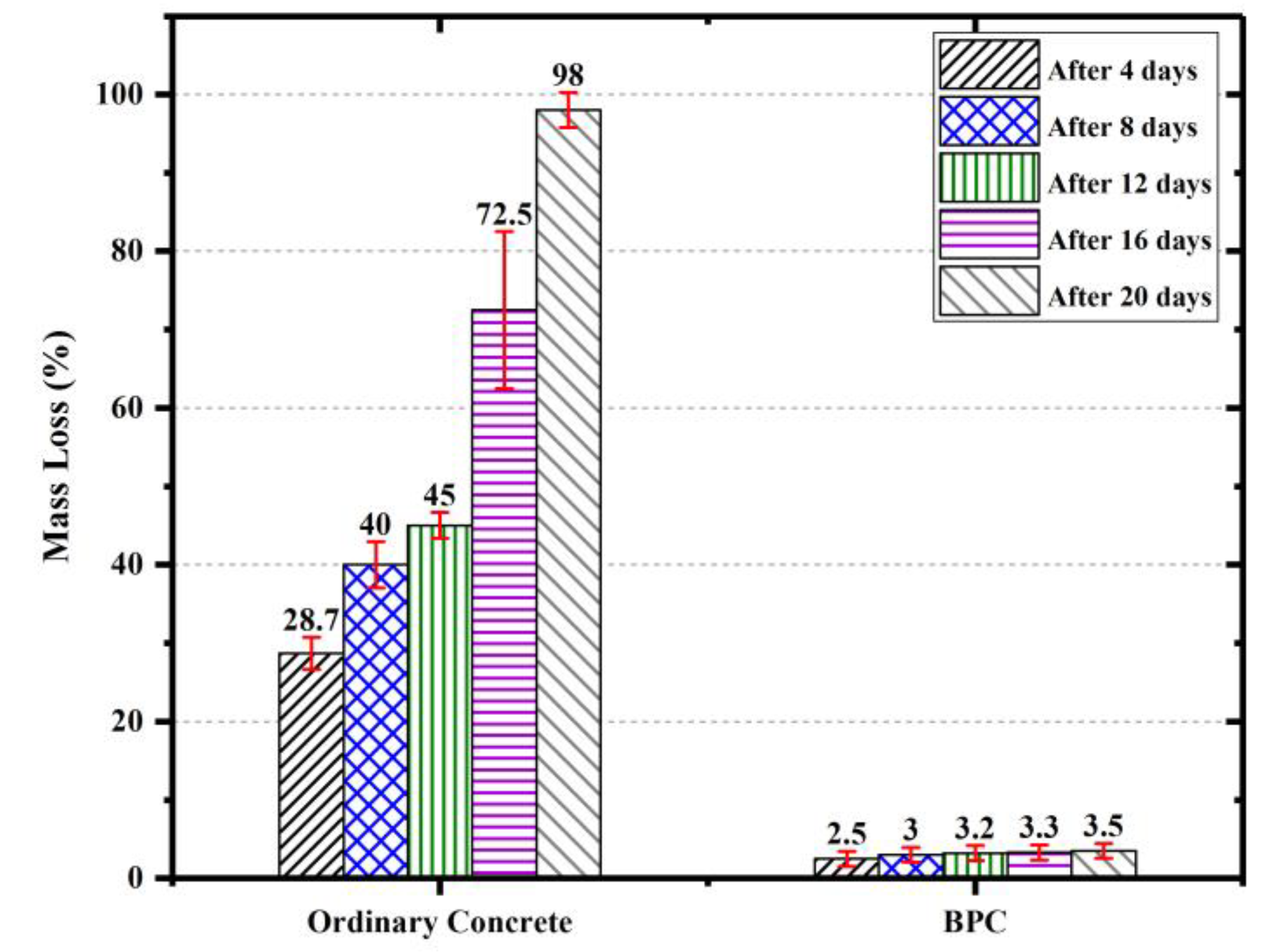
4.3. Durability
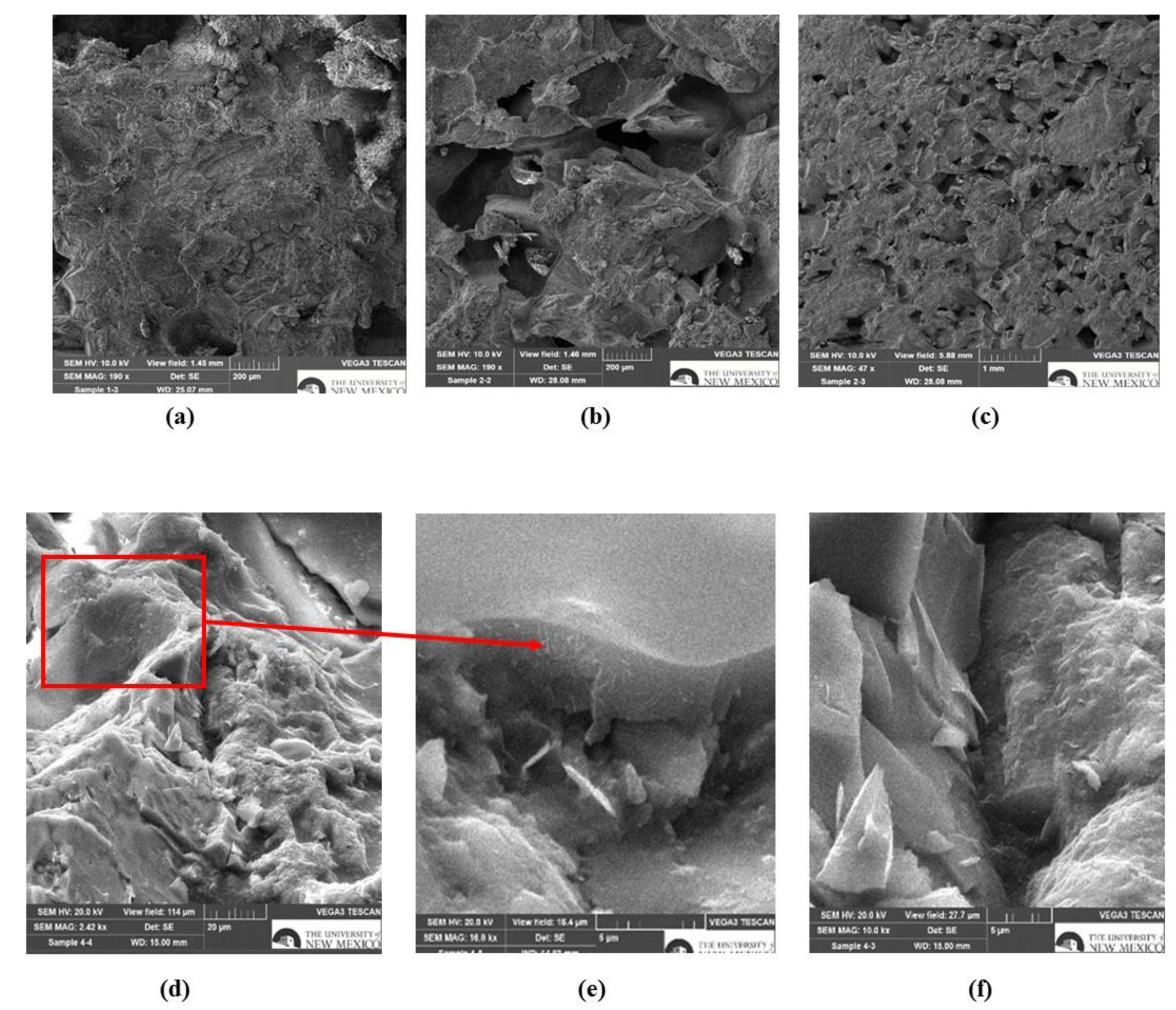
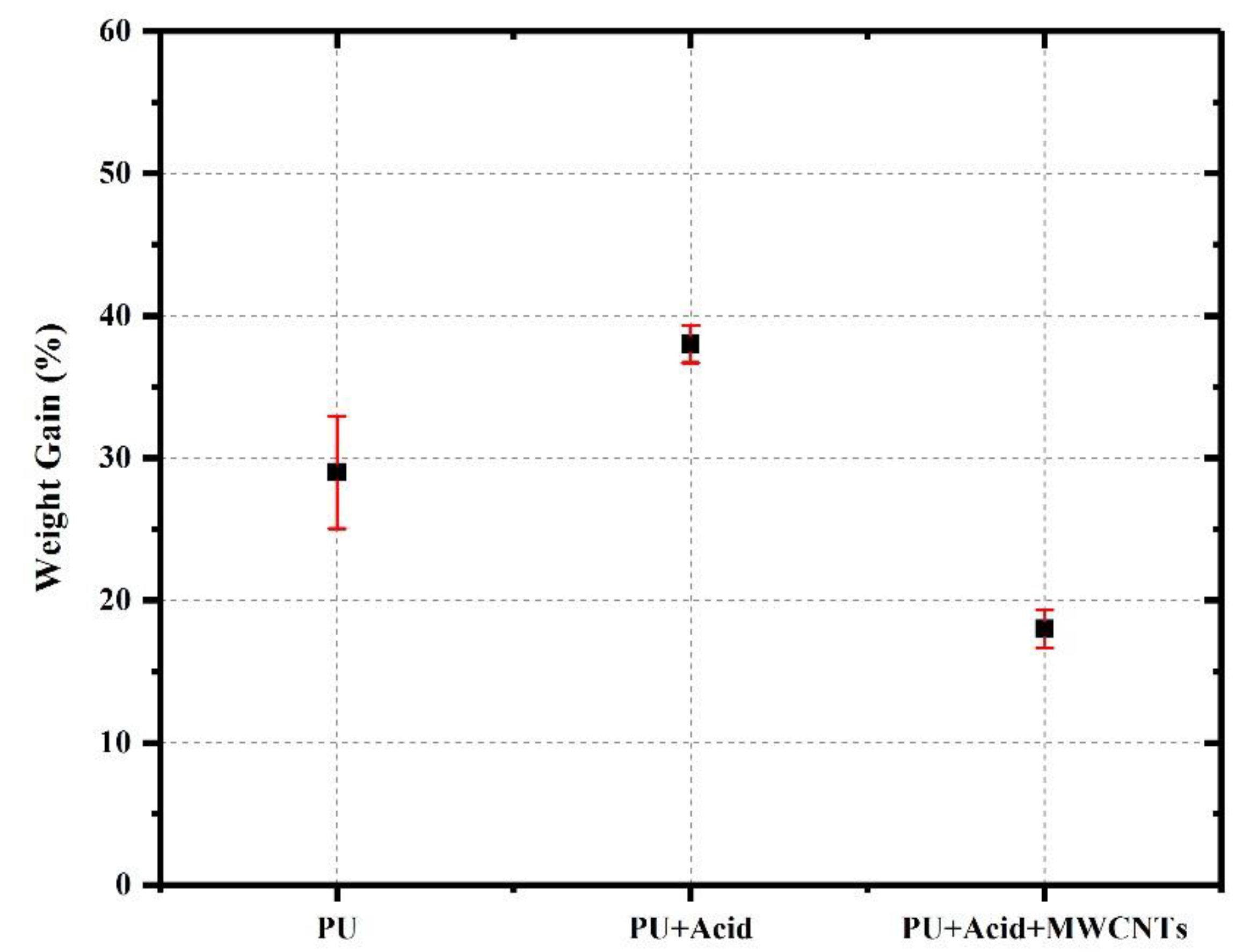
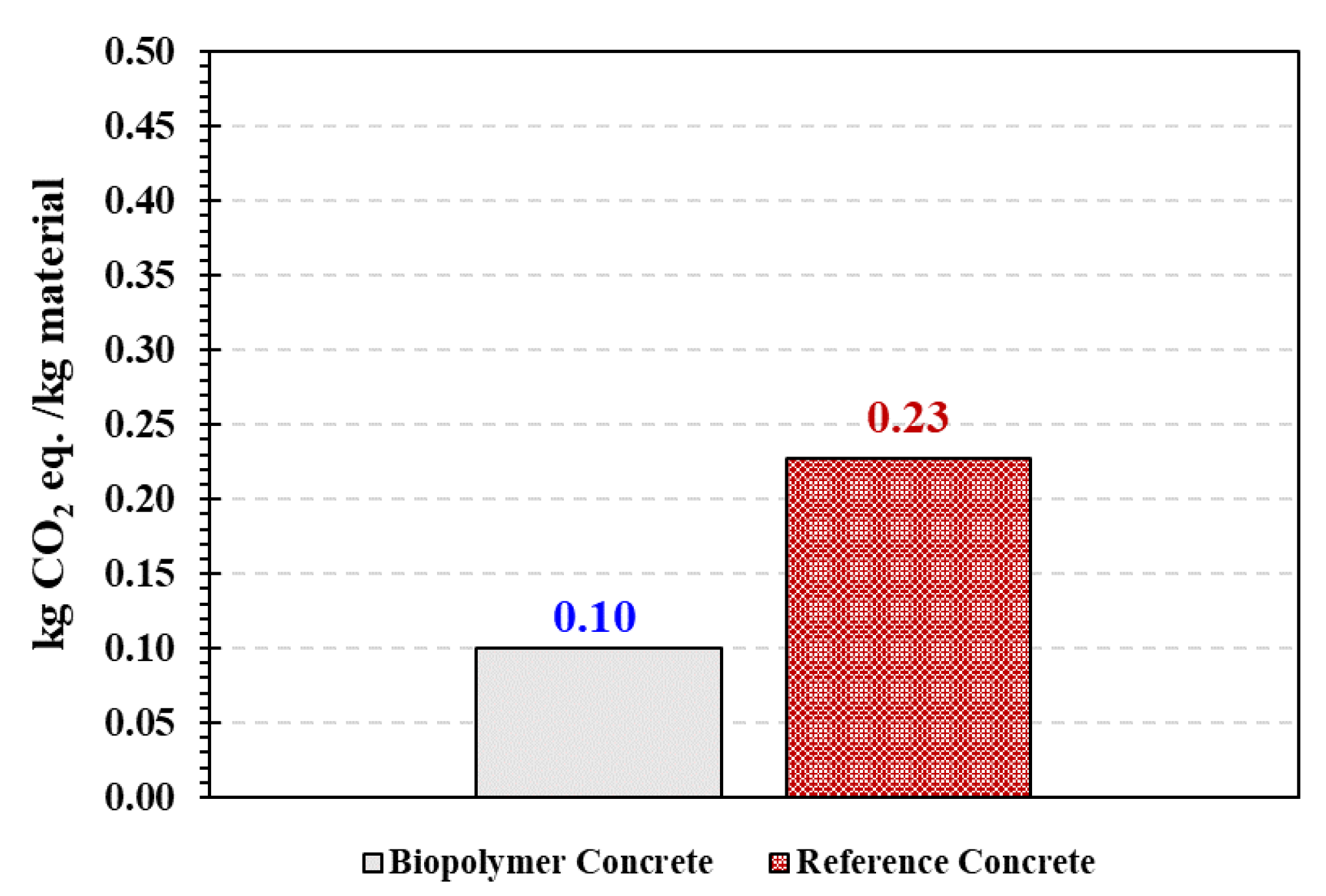
4.4. Microstructural, Chemical and Carbon Footprint Analysis
5. Conclusions
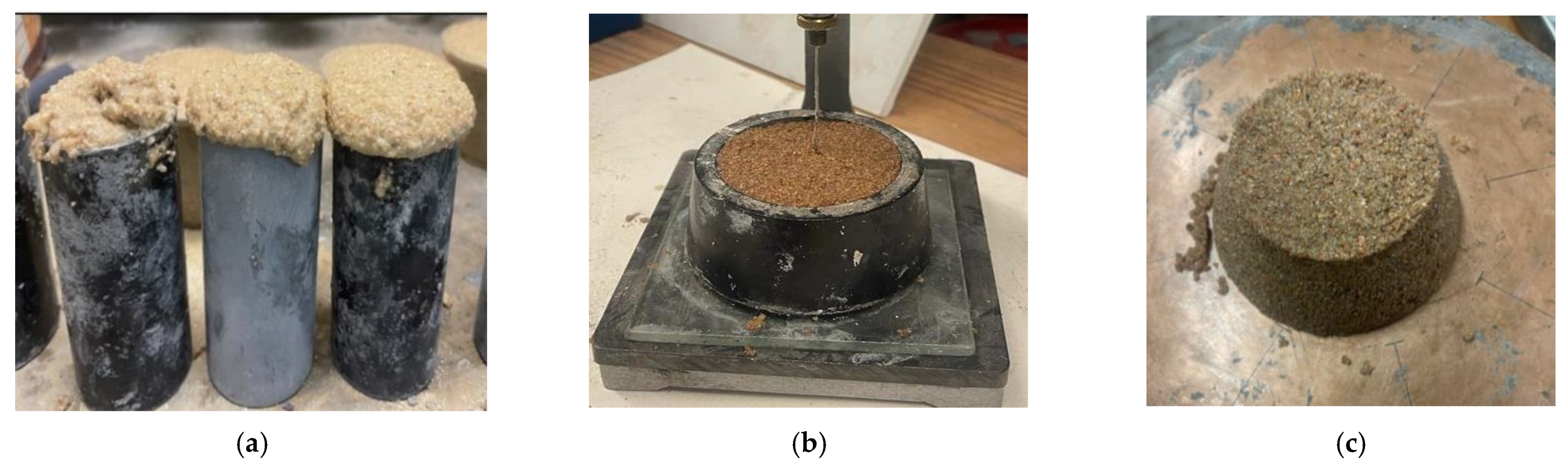
- Due to the unique PU reaction, typical challenges in producing BPC include excessive foaming, fast setting, and low to no flowability.
- Benzoic acid increased the setting time from 5 min to 30 min and eliminated the excessive foaming formation. Yet, the flowability was not improved, and BPC mixes showed 0% flowability.
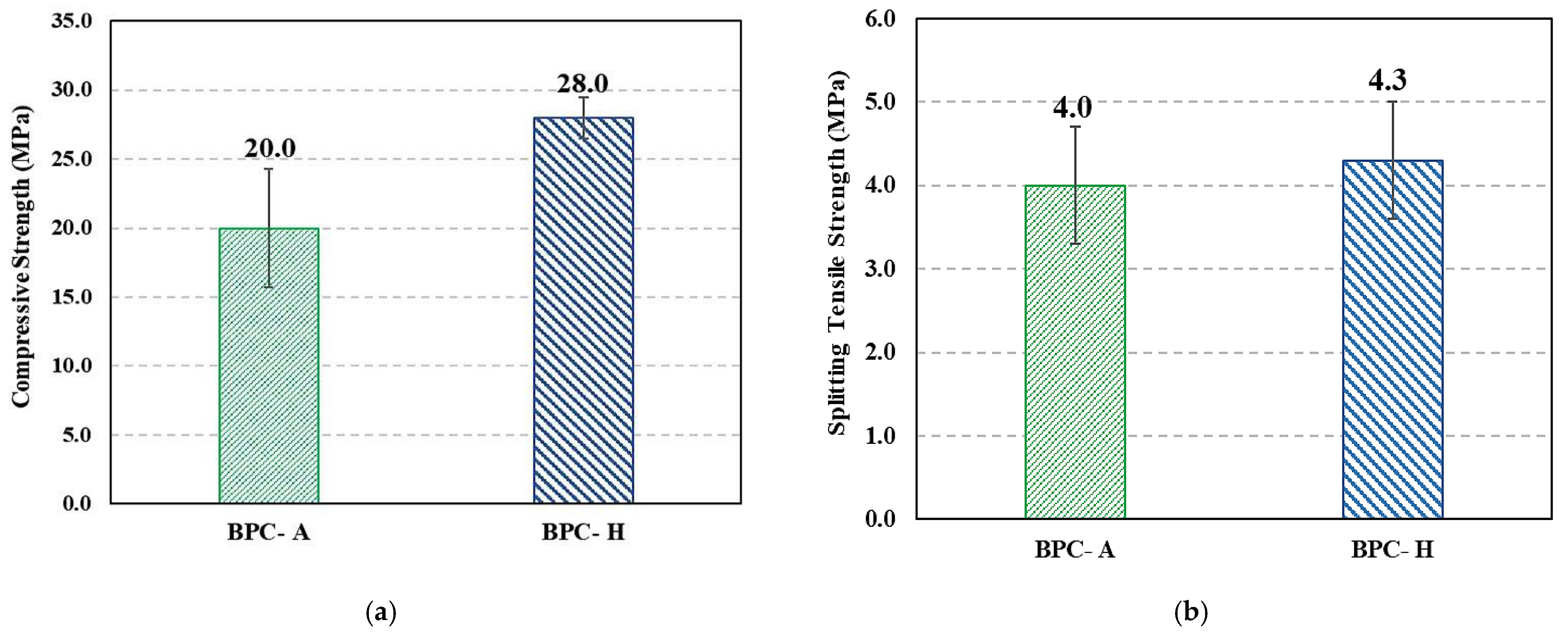
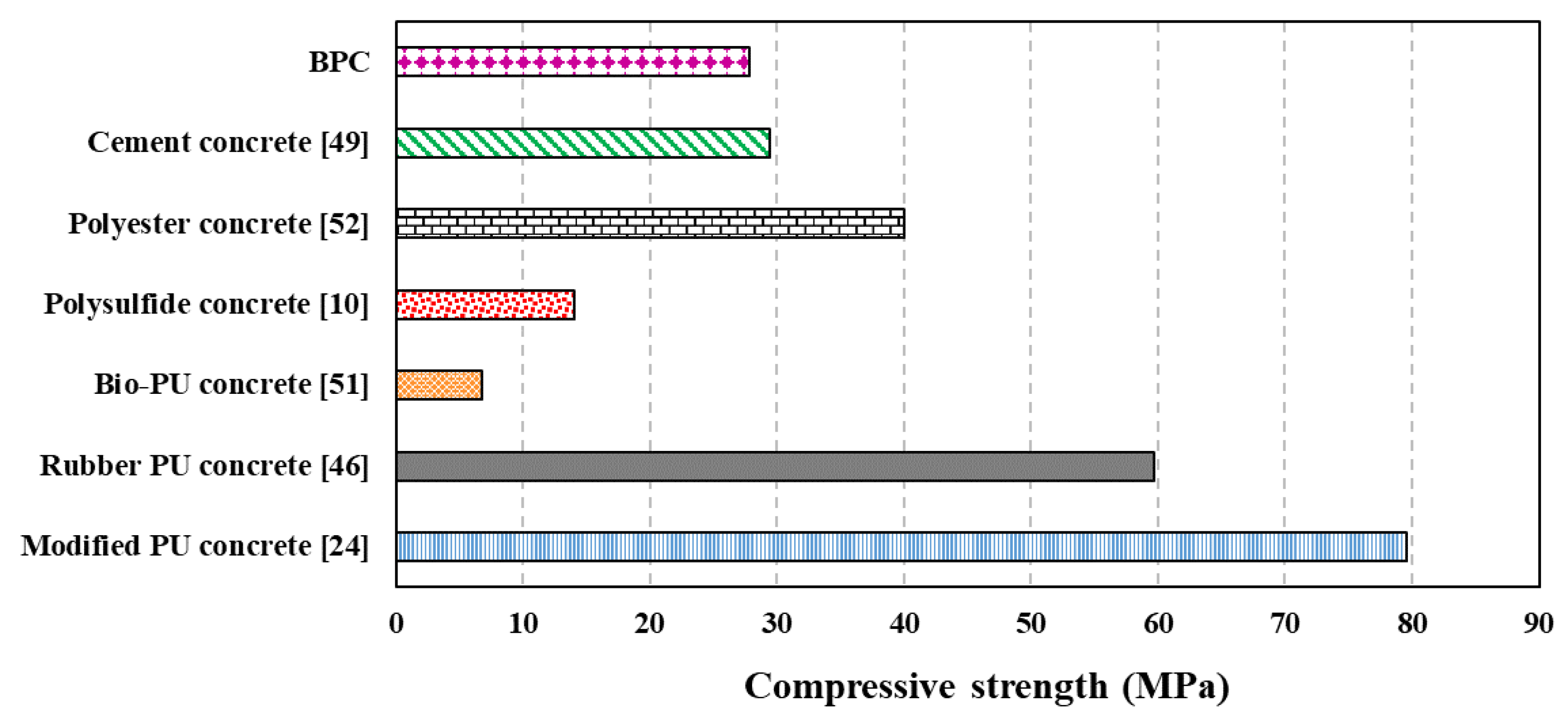
- The new BPC showed relatively low density, appreciable compressive strength ranging from 20–30 MPa based on the method of curing, and good tensile strength of 4 MPa. BPC had a relatively low modulus of elasticity and high Poisson’s ratio compared with cement concrete but in the same range of values reported for other PCs (e.g., polyester concrete). However, BPC outperformed ordinary concrete’s durability against aggressive environments and showed minimum weight loss while serving in highly aggressive acid environment.
- The low elastic modulus shall allow low tensile stress buildup from restrained shrinkage, which in turn, suppresses the reflective cracking.
- Chemical and microstructural analysis of bio-based PUs and PU concrete revealed that employment of MWCNTs in the polymer matrix compensated the lower crosslinking density due to the addition of benzoic acid to the PU, promoting the formation of urea bond structure, leading to further polymerization/crosslinking, and generated a percolated network structure within the polymer matrix.
- MWCNTs addition led to superior mechanical, thermal, and durability properties for the PU specimen containing benzoic acid and chemically treated MWCNTs.
- The carbon footprint for BPC was 50% lower than the reference cement concrete, which suggests BPC can provide a sustainable concrete alternative in infrastructural applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akindoyo, J.O.; Beg, M.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane Types, Synthesis and Applications—A Review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Ansari, M.A.; Somdee, P.; Marossy, K. Synthesis of Cross-Linked Polyurethane Elastomers with the Inclusion of Polar-Aromatic Moieties (BA, PNBA and 3, 5-DNBA): Electrical and Thermo-Mechanical Properties Analysis. J. Polym. Res. 2021, 28, 1–11. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Jung, Y.C.; Yoo, H.J.; Cho, J.W. Effect of Functionalized Carbon Nanotubes on Molecular Interaction and Properties of Polyurethane Composites. Macromol. Chem. Phys. 2006, 207, 1773–1780. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mani, M.P.; Palaniappan, S.K.; Rathanasamy, R. Fabrication and Characterisation of Nanofibrous Polyurethane Scaffold Incorporated with Corn and Neem Oil Using Single Stage Electrospinning Technique for Bone Tissue Engineering Applications. J. Polym. Res. 2018, 25, 1–12. [Google Scholar] [CrossRef]
- He, Y.; Xie, D.; Zhang, X. The Structure, Microphase-Separated Morphology, and Property of Polyurethanes and Polyureas. J. Mater. Sci. 2014, 49, 7339–7352. [Google Scholar] [CrossRef]
- Noparvar-Qarebagh, A.; Roghani-Mamaqani, H.; Salami-Kalajahi, M. Functionalization of Carbon Nanotubes by Furfuryl Alcohol Moieties for Preparation of Novolac Phenolic Resin Composites with High Carbon Yield Values. Colloid Polym. Sci. 2015, 293, 3623–3631. [Google Scholar] [CrossRef]
- Preparation, Characterization and Properties of Acid Functionalized Multi-Walled Carbon Nanotube Reinforced Thermoplastic Polyurethane Nanocomposites. Mater. Sci. Eng. B 2011, 176, 1435–1447. [CrossRef]
- Tran, L.; Kim, J. A Comparative Study of the Thermoplastic Polyurethane/Carbon Nanotube and Natural Rubber/Carbon Nanotube Composites According to Their Mechanical and Electrical Properties. Fibers Polym. 2018, 19, 1948–1955. [Google Scholar] [CrossRef]
- Vemuganti, S.; Stormont, J.C.; Pyrak-Nolte, L.J.; Dewers, T.; Taha, M.M.R. Cement Sensors with Acoustic Bandgaps Using Carbon Nanotubes. Smart Mater. Struct. 2021, 30, 035011. [Google Scholar] [CrossRef]
- Douba, A.; Emiroglu, M.; Kandil, U.F.; Reda Taha, M.M. Very Ductile Polymer Concrete Using Carbon Nanotubes. Constr. Build. Mater. 2019, 196, 468–477. [Google Scholar] [CrossRef]
- Ferdous, W.; Manalo, A.; Aravinthan, T.; Van Erp, G. Properties of Epoxy Polymer Concrete Matrix: Effect of Resin-to-Filler Ratio and Determination of Optimal Mix for Composite Railway Sleepers. Constr. Build. Mater. 2016, 124, 287–300. [Google Scholar] [CrossRef]
- Daghash, S.M.; Tarefder, R.; Taha, M.M.R. A New Class of Carbon Nanotube: Polymer Concrete with Improved Fatigue Strength. In Nanotechnology in Construction; Sobolev, K., Shah, S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 285–290. ISBN 978-3-319-17087-9. [Google Scholar]
- ACI Committee 548; Guide for the Use of Polymers in Concrete, ACI 548. IR-09. American Concrete Institute: Farmington Hills, MI, USA, 2009.
- Ding, H.; Sun, Q.; Wang, Y.; Jia, D.; Li, C.; Ji, C.; Feng, Y. Flexural Behavior of Polyurethane Concrete Reinforced by Carbon Fiber Grid. Materials 2021, 14, 5421. [Google Scholar] [CrossRef]
- Ren, S.; Hu, X. Fatigue Properties and Its Prediction of Polymer Concrete for the Repair of Asphalt Pavements. Polymers 2022, 14, 2941. [Google Scholar] [CrossRef]
- Ferdous, W.; Manalo, A.; Khennane, A.; Kayali, O. Geopolymer Concrete-Filled Pultruded Composite Beams—Concrete Mix Design and Application. Cem. Concr. Compos. 2015, 58, 1–13. [Google Scholar] [CrossRef]
- El-Hawary, M.; Al-Khaiat, H.; Fereig, S. Performance of Epoxy-Repaired Concrete in a Marine Environment. Cem. Concr. Res. 2000, 30, 259–266. [Google Scholar] [CrossRef]
- Al-Eis, K.A.; LaBarca, I.K. Evaluation of the Uretek Method of Pavement Lifting; Wisconsin Department of Transportation, Division of Transportation Systems Development, Bureau of Technical Services, Materials Management Section: Madison, WI, USA, 2007. [Google Scholar]
- Wang, H.; Liu, H.X.; Zhuang, C.H. Study on the Polyurethane Concrete for the Rapid Repairment of Highway Pavement. Appl. Mech. Mater. 2012, 193–194, 762–769. [Google Scholar] [CrossRef]
- Hussain, H.K.; Zhang, L.Z.; Liu, G.W. An Experimental Study on Strengthening Reinforced Concrete T-Beams Using New Material Poly-Urethane-Cement (PUC). Constr. Build. Mater. 2013, 40, 104–117. [Google Scholar] [CrossRef]
- Hussain, H.K.; Liu, G.W.; Yong, Y.W. Experimental Study to Investigate Mechanical Properties of New Material Polyurethane–Cement Composite (PUC). Constr. Build. Mater. 2014, 50, 200–208. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, Q. Experimental Study of Reinforced Concrete T-Beams Strengthened with a Composite of Prestressed Steel Wire Ropes Embedded in Polyurethane Cement (PSWR–PUC). Int. J. Civ. Eng. 2018, 16, 1109–1123. [Google Scholar] [CrossRef]
- Hu, Z.-X.; Hu, X.-M.; Cheng, W.-M.; Zhao, Y.-Y.; Wu, M.-Y. Performance Optimization of One-Component Polyurethane Healing Agent for Self-Healing Concrete. Constr. Build. Mater. 2018, 179, 151–159. [Google Scholar] [CrossRef]
- Lei, J.; Feng, F.; Xu, S.; Wen, W.; He, X. Study on Mechanical Properties of Modified Polyurethane Concrete at Different Temperatures. Appl. Sci. 2022, 12, 3184. [Google Scholar] [CrossRef]
- Xu, S.; Xu, M.; Zhang, Y.; Guo, Y.; Peng, G.; Xu, Y. An Indoor Laboratory Simulation and Evaluation on the Aging Resistance of Polyether Polyurethane Concrete for Bridge Deck Pavement. Front. Mater. 2020, 7, 237. [Google Scholar] [CrossRef]
- Jiang, Z.; Tang, C.; Yang, J.; You, Y.; Lv, Z. A Lab Study to Develop Polyurethane Concrete for Bridge Deck Pavement. Int. J. Pavement Eng. 2022, 23, 1404–1412. [Google Scholar] [CrossRef]
- Gao, H.; Sun, Q. Study on Fatigue Test and Life Prediction of Polyurethane Cement Composite (PUC) under High or Low Temperature Conditions. Adv. Mater. Sci. Eng. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Hong, B.; Lu, G.; Gao, J.; Wang, D. Evaluation of Polyurethane Dense Graded Concrete Prepared Using the Vacuum Assisted Resin Transfer Molding Technology. Constr. Build. Mater. 2021, 269, 121340. [Google Scholar] [CrossRef]
- Li, L.; Yu, T. Curing Comparison and Performance Investigation of Polyurethane Concrete with Retarders. Constr. Build. Mater. 2022, 326, 126883. [Google Scholar] [CrossRef]
- Mohd Sari, K.A.; Mat, S.; Haji Badri, K.; Mohd Zain, M.F. A Study on the Characteristics of Palm-Based Polyurethane as a Lightweight Aggregate In Concrete Mix. Sains Malays. 2015, 44, 771–778. [Google Scholar] [CrossRef]
- Raman, S.N.; Somarathna, H.M.C.C.; Mutalib, A.A.; Badri, K.H.; Taha, M.R. Bio-Based Polyurethane Elastomer for Strengthening Application of Concrete Structures Under Dynamic Loadings. In Proceedings of the International Congress on Polymers in Concrete (ICPIC 2018); Taha, M.M.R., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 751–757. [Google Scholar]
- Allami, T.; Alamiery, A.; Nassir, M.H.; Kadhum, A.H. Investigating Physio-Thermo-Mechanical Properties of Polyurethane and Thermoplastics Nanocomposite in Various Applications. Polymers 2021, 13, 2467. [Google Scholar] [CrossRef]
- Rodríguez, R.; Pérez, B.; Flórez, S. Effect of Different Nanoparticles on Mechanical Properties and Curing Behavior of Thermoset Polyurethane Adhesives. J. Adhes. 2014, 90, 848–859. [Google Scholar] [CrossRef]
- Thiele, L.; Okamoto, O.K.; Juettner, W.; Henkel AG and Co KGaA. Two-component polyurethane composition with delayed crosslinking. US Patent US 9,567,499, 2017. [Google Scholar]
- Daghash, S. New Generation Polymer Concrete Incorporating Carbon Nanotubes. Master’s Thesis, University of New Mexico, Albuquerque, NM, USA, 2014. [Google Scholar]
- ASTM C230/C230M; Standard Specification for Flow Table for Use in Tests of Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM C191, C09 Committee; Standard Test Method for Time of Setting of Hydraulic Cement by Vicat Needle. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM C469/C469M-17b; Standard Test Method for Static Modulus of Elasticity and Poisson’s Ratio of Concrete in Compression. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM C39-17b, C09 Committee; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM C496/C496M; Standard Test Method for Splitting Tensile Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D543-14; Standard Practices for Evaluating the Resistance of Plastics to Chemical Reagents. ASTM International: West Conshohocken, PA, USA, 2014.
- CCaLC • CCaLC2 for Windows Carbon Footprinting Tool. Available online: http://www.ccalc.org.uk/ccalc2.php (accessed on 12 November 2022).
- Xia, Z.; Cunningham, D.; Wohlgemuth, J. A New Method for Measuring Cross-Link Density in Ethylene Vinyl Acetate-Based Encapsulant. Photovolt. Int. 2009, 5, 150–159. [Google Scholar]
- Hong, B.; Xian, G.; Li, H. Effects of Water or Alkali Solution Immersion on the Water Uptake and Physicomechanical Properties of Polyurethane. Polym. Eng. Sci. 2018, 58, 2276–2287. [Google Scholar] [CrossRef]
- Rao, R.R.; Mondy, L.A.; Long, K.N.; Celina, M.C.; Wyatt, N.; Roberts, C.C.; Soehnel, M.M.; Brunini, V.E. The Kinetics of Polyurethane Structural Foam Formation: Foaming and Polymerization. AIChE J. 2017, 63, 2945–2957. [Google Scholar] [CrossRef]
- Jia, Z.; Jia, D.; Sun, Q.; Wang, Y.; Ding, H. Preparation and Mechanical-Fatigue Properties of Elastic Polyurethane Concrete Composites. Materials 2021, 14, 3839. [Google Scholar] [CrossRef] [PubMed]
- Alzaydi, A.A.; Shihata, S.A.; Alp, T. The Compressive Strength of a New Ureaformaldehyde-Based Polymer Concrete. J. Mater. Sci. 1990, 25, 2851–2856. [Google Scholar] [CrossRef]
- Hyun, S.-H.; Yeon, J.H. Strength Development Characteristics of UP-MMA Based Polymer Concrete with Different Curing Temperature. Constr. Build. Mater. 2012, 37, 387–397. [Google Scholar] [CrossRef]
- McGraw-Hill Encyclopedia of Science and Technology Volumes 1-20 11th Edition: McGraw Hill: 9780071792738: Amazon.Com: Books. Available online: https://www.amazon.com/McGraw-Hill-Encyclopedia-Science-Technology-Volumes/dp/0071792732 (accessed on 30 September 2022).
- Reda Taha, M.M.; Genedy, M.; Ohama, Y. 17-Polymer Concrete. In Developments in the Formulation and Reinforcement of Concrete, 2nd ed.; Mindess, S., Ed.; Woodhead Publishing Series in Civil and Structural Engineering; Woodhead Publishing: Sawston, UK, 2019; pp. 391–408. ISBN 978-0-08-102616-8. [Google Scholar]
- Lu, G.; Liu, P.; Wang, Y.; Faßbender, S.; Wang, D.; Oeser, M. Development of a Sustainable Pervious Pavement Material Using Recycled Ceramic Aggregate and Bio-Based Polyurethane Binder. J. Clean. Prod. 2019, 220, 1052–1060. [Google Scholar] [CrossRef]
- Carrión, F.; Montalbán, L.; Real, J.I.; Real, T. Mechanical and Physical Properties of Polyester Polymer Concrete Using Recycled Aggregates from Concrete Sleepers. Sci. World J. 2014, 2014, 526346. [Google Scholar] [CrossRef]
- Svenum, I.-H.; Ringdalen, I.G.; Bleken, F.L.; Friis, J.; Höche, D.; Swang, O. Structure, Hydration, and Chloride Ingress in C-S-H: Insight from DFT Calculations. Cem. Concr. Res. 2020, 129, 105965. [Google Scholar] [CrossRef]
- Li, Y.; Duan, L.; Cheng, L.; Yang, Y.; Li, Y.; Cheng, Y.; Song, D. Thermal Analysis and Crystallization Kinetics of Polyurethane. J. Therm. Anal. Calorim. 2019, 135, 2843–2848. [Google Scholar] [CrossRef]
- Han, Q.; Urban, M.W. Kinetics and Mechanisms of Catalyzed and Noncatalyzed Reactions of OH and NCO in Acrylic Polyol–1,6-Hexamethylene Diisocyanate (HDI) Polyurethanes. VI. J. Appl. Polym. Sci. 2002, 86, 2322–2329. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Winey, K.I. Polymer Nanocomposites Containing Carbon Nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Mondal, S.; Hu, J.L. Thermal Degradation Study of Functionalized MWNT Reinforced Segmented Polyurethane Membrane. J. Elastomers Plast. 2006, 38, 261–271. [Google Scholar] [CrossRef]
- Xia, H.; Song, M. Preparation and Characterisation of Polyurethane Grafted Single-Walled Carbon Nanotubes and Derived Polyurethane Nanocomposites. J. Mater. Chem. 2006, 16, 1843–1851. [Google Scholar] [CrossRef]






| MWCNT Properties | Measurements |
|---|---|
| Outer diameter, nm | 10–20 |
| Inner diameter, nm | 3–5 |
| MWCNTs ash, wt.% | <0.1 |
| Purity, wt.% | >99.9 |
| Length, µm | 10–30 |
| Specific surface area, m2/g | 100 |
| Bulk density, g/cm3 | 2.1 |
| Electrical conductivity, S/cm | >10 |
| MIX ID | Polyether Polyol | Polyisocyanate | Aggregate | Benzoic Acid | MWCNTs |
|---|---|---|---|---|---|
| BPC | 92.0 | 84.6 | 2250.3 | 7.1 | 0.88 |
| Fresh Properties | |
|---|---|
| Flowability (%) | 0 |
| Setting time (mins) | 30 |
| Mechanical Properties | BPC-A | BPC-H | ||
|---|---|---|---|---|
| Mean | STDV. (COV%) | Mean | STDV. (COV%) | |
| Hardened density, ρ (kg/m3) | 1830.0 | 45.0 (2.5) | 1858.0 | 36.0 (1.9) |
| Modulus of elasticity, E (GPa) | 9.3 | 0.9 (10.1) | 9.9 | 0.9 (8.8) |
| Poisson’s ratio, ν | 0.24 | 0.0 (9.8) | 0.26 | 0.0 (4.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murcia, D.H.; Al Shanti, S.; Hamidi, F.; Rimsza, J.; Yoon, H.; Gunawan, B.; Abdellatef, M.; Taha, M.R. Development and Characterization of a Sustainable Bio-Polymer Concrete with a Low Carbon Footprint. Polymers 2023, 15, 628. https://doi.org/10.3390/polym15030628
Murcia DH, Al Shanti S, Hamidi F, Rimsza J, Yoon H, Gunawan B, Abdellatef M, Taha MR. Development and Characterization of a Sustainable Bio-Polymer Concrete with a Low Carbon Footprint. Polymers. 2023; 15(3):628. https://doi.org/10.3390/polym15030628
Chicago/Turabian StyleMurcia, Daniel Heras, Siham Al Shanti, Fatemeh Hamidi, Jessica Rimsza, Hongkyu Yoon, Budi Gunawan, Mohammed Abdellatef, and Mahmoud Reda Taha. 2023. "Development and Characterization of a Sustainable Bio-Polymer Concrete with a Low Carbon Footprint" Polymers 15, no. 3: 628. https://doi.org/10.3390/polym15030628
APA StyleMurcia, D. H., Al Shanti, S., Hamidi, F., Rimsza, J., Yoon, H., Gunawan, B., Abdellatef, M., & Taha, M. R. (2023). Development and Characterization of a Sustainable Bio-Polymer Concrete with a Low Carbon Footprint. Polymers, 15(3), 628. https://doi.org/10.3390/polym15030628








