Effects of Ethylene-Propylene-Diene Monomers (EPDMs) with Different Moony Viscosity on Crystallization Behavior, Structure, and Mechanical Properties of Thermoplastic Vulcanizates (TPVs)
Abstract
:1. Introduction
2. Part of Experiment
2.1. Materials and Sample Preparation
2.2. Thermal Analysis
2.3. Synchrotron SAXS/WAXD Measurements
2.4. Mechanical Testing
2.5. Dynamic Mechanical Analysis (DMA)
3. Results and Discussion
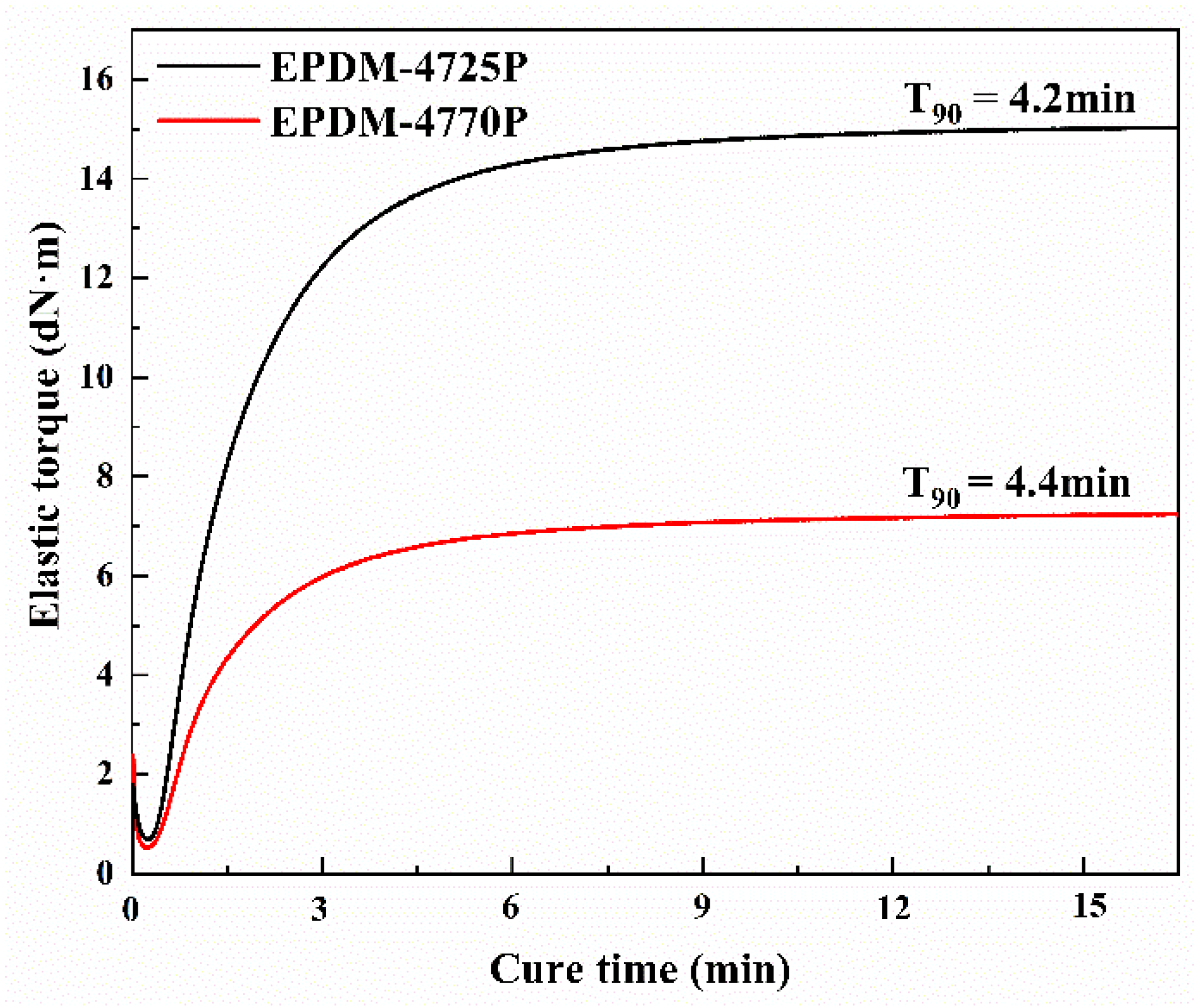
3.1. Crosslinking of EPDMs
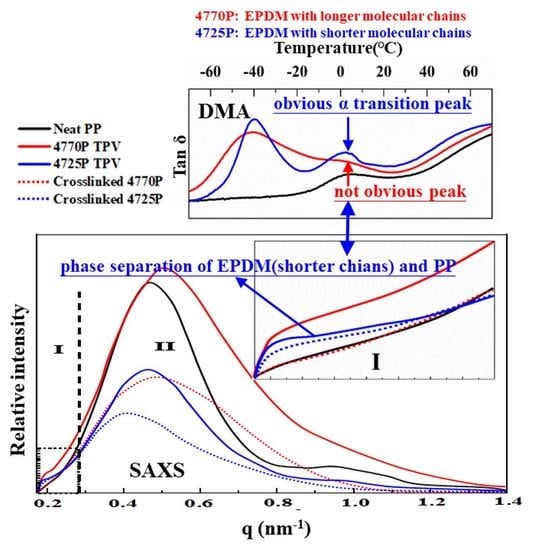
3.2. Structure of the Crosslinked EPDMs and EPDM/PP TPVs
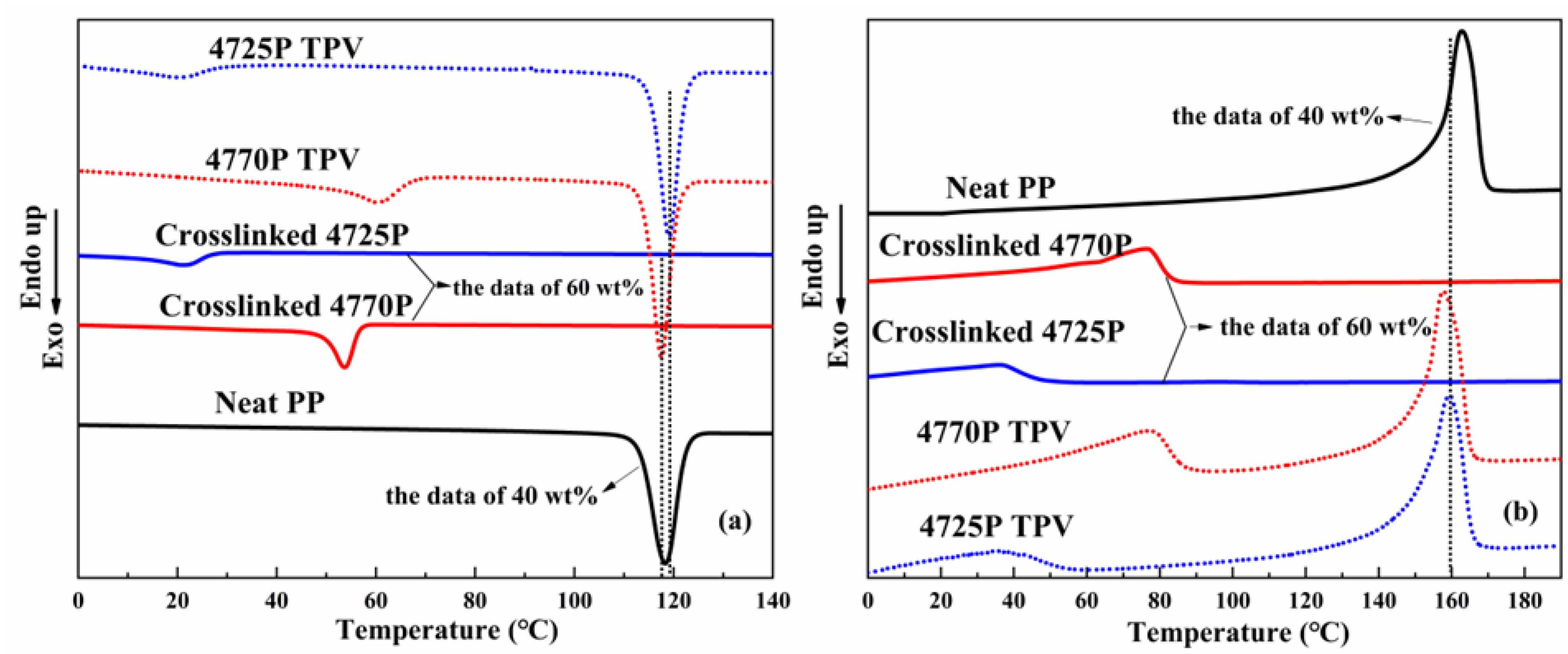
3.3. Non-isothermal Crystallization of the EPDMs, Crosslinked EPDMs, and TPVs, with DSC
3.4. Packing Structure of the EPDMs and TPV
3.5. Dynamic Mechanical Analysis
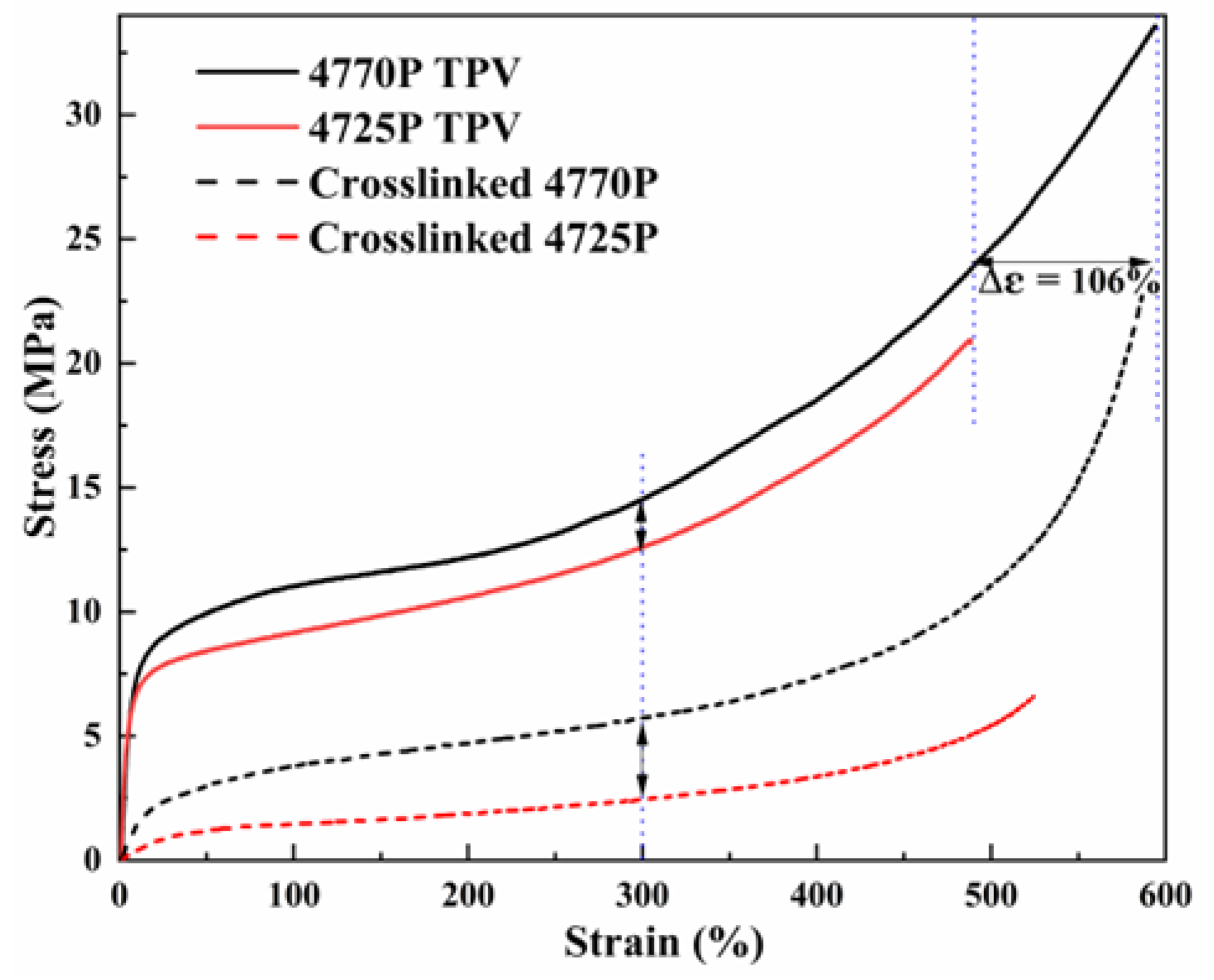
3.6. Different Tensile Properties of the TPVs and the Crosslinked EPDMs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, S.Q.; Tian, H.C.; Wu, H.G.; Ning, N.Y.; Tian, M.; Zhang, L.Q. Coupling effect of molecular weight and crosslinking kinetics on the formation of rubber nanoparticles and their agglomerates in EPDM/PP TPVs during dynamic vulcanization. Soft Matter 2020, 16, 2185–2198. [Google Scholar] [CrossRef]
- Le Hel, C.; Bounor-Legare, V.; Catherin, M.; Lucas, A.; Thevenon, A.; Cassagnau, P. TPV: A New Insight on the Rubber Morphology and Mechanic/Elastic Properties. Polymers 2020, 12, 2315. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Tian, H.; Pan, W.; Feng, J.; Wang, D.; Ning, N.; Tian, M.; Zhang, L. Unexpected Improvement of Both Mechanical Strength and Elasticity of EPDM/PP Thermoplastic Vulcanizates by Introducing β-Nucleating Agents. Macromolecules 2021, 54, 2835–2843. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, D.H.; Zhu, J.H.; Shi, Y.P.; Sun, J.; Ji, Y.B. Morphology, crystallization, and thermal and mechanical properties of dynamic vulcanization EPDM/PP thermoplastic elastomer. J. Thermoplast. Compos. Mater. 2019, 32, 922–935. [Google Scholar] [CrossRef]
- Naskar, K.; Gohs, U.; Heinrich, G. Influence of molecular structure of blend components on the performance of thermoplastic vulcanisates prepared by electron induced reactive processing. Polymer 2016, 91, 203–210. [Google Scholar] [CrossRef]
- Parenteau, T.; Bertevas, E.; Ausias, G.; Stocek, R.; Grohens, Y.; Pilvin, P. Characterisation and micromechanical modelling of the elasto-viscoplastic behavior of thermoplastic elastomers. Mech. Mater. 2014, 71, 114–125. [Google Scholar] [CrossRef]
- Nakason, C.; Saiwari, S.; Kaesaman, A. Thermoplastic vulcanizates based on maleated natural rubber/polypropylene blends: Effect of blend ratios on rheological, mechanical, and morphological properties. Polym. Eng. Sci. 2006, 46, 594–600. [Google Scholar] [CrossRef]
- Martin, G.; Barres, C.; Sonntag, P.; Garois, N.; Cassagnau, P. Morphology development in thermoplastic vulcanizates (TPV): Dispersion mechanisms of a pre-crosslinked EPDM phase. Eur. Polym. J. 2009, 45, 3257–3268. [Google Scholar] [CrossRef]
- Antunes, C.F.; van Duin, M.; Machado, A.V. Effect of cross-linking on morphology and phase inversion of EPDM/PP blends. Mater. Chem. Phys. 2012, 133, 410–418. [Google Scholar] [CrossRef]
- Tian, M.; Han, J.B.; Zou, H.; Tian, H.C.; Wu, H.G.; She, Q.Y.; Chen, W.Q.; Zhang, L.Q. Dramatic influence of compatibility on crystallization behavior and morphology of polypropylene in NBR/PP thermoplastic vulcanizates. J. Polym. Res. 2012, 19, 1–13. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Liu, Z.W.; Su, B.; Chen, F.; Fu, Q.; Ning, N.Y.; Tian, M. Property enhancement of PP-EPDM thermoplastic vulcanizates via shear-induced break-up of nano-rubber aggregates and molecular orientation of the matrix. Polymer 2015, 63, 170–178. [Google Scholar] [CrossRef]
- Bhattacharya, A.B.; Chatterjee, T.; Naskar, K. Automotive applications of thermoplastic vulcanizates. J. Appl. Polym. Sci. 2020, 137, 49181. [Google Scholar] [CrossRef]
- Chen, Y.K.; Xu, C.H.; Cao, L.M.; Wang, Y.P.; Cao, X.D. PP/EPDM-based dynamically vulcanized thermoplastic olefin with zinc dimethacrylate: Preparation, rheology, morphology, crystallization and mechanical properties. Polym. Test. 2012, 31, 728–736. [Google Scholar] [CrossRef]
- Cao, L.M.; Zheng, A.X.; Cao, X.W.; Yuan, D.S.; Xu, C.H.; Chen, Y.K. Morphology and non-isothermal crystallization of dynamically vulcanized PP/EPDM blends in situ compatibilized via magnesium dimethacrylate. Polym. Test. 2017, 62, 68–78. [Google Scholar] [CrossRef]
- Bhattacharya, A.B.; Raju, A.T.; Chatterjee, T.; Naskar, K. Development and characterizations ofultra-high molecular weight EPDM/PP based TPV nanocomposites for automotive applications. Polym. Compos. 2020, 41, 4950–4962. [Google Scholar] [CrossRef]
- Xu, D.H.; Zhou, Y.; He, W.D.; Wu, H.M.; Yu, J. Influence of Rubber Ratio and Crosslinking Agent on Mechanical Properties, Crystallization and Rheological Behaviors of EPDM/PP Thermoplastic Elastomer. Int. Polym. Process. 2019, 34, 457–466. [Google Scholar] [CrossRef]
- Ma, L.F.; Bao, R.Y.; Liu, Z.Y.; Yang, W.; Yang, M.B. Effect of cross-linking degree of EPDM phase on the electrical properties and formation of dual networks of thermoplastic vulcanizate composites based on isotactic polypropylene (iPP)/ethylene-propylene-diene rubber (EPDM) blends. Rsc Adv. 2016, 6, 74567–74574. [Google Scholar] [CrossRef]
- Peng, T.; Lv, F.; Gong, Z.; Cao, L.M.; Yan, X.S.; Ge, L.; Abubakar, S.; Chen, Y.K. Design of PP/EPDM/NBR TPVs with tunable mechanical properties via regulating the core-shell structure. Polym. Test. 2020, 90, 106767. [Google Scholar] [CrossRef]
- Ning, N.; Li, S.; Wu, H.; Tian, H.; Yao, P.; Hu, G.-H.; Tian, M.; Zhang, L. Preparation, microstructure, and microstructure-properties relationship of thermoplastic vulcanizates (TPVs): A review. Prog. Polym. Sci. 2018, 79, 61–97. [Google Scholar] [CrossRef]
- Avalos, B.F.; Mendoza, P.C.; Ortiz, C.J.; Ramos, V. Effect of the Ethylene Content on the Properties m-Polypropylene/EPDM Blends. MOJ Polym. Sci. 2017, 1, 5–9. [Google Scholar]
- Wu, H.G.; Tian, M.; Zhang, L.; Tian, H.; Wu, Y.; Ning, N. New understanding of microstructure formation of the rubber phase in thermoplastic vulcanizates (TPV). Soft Matter 2014, 10, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Wang, K.; Ning, N.Y.; Geng, C.Z.; Deng, H.; Chen, F.; Fu, Q.; Qian, Y.Y.; Zheng, D. Dependence of mechanical properties on β-form content and crystalline morphology for β-nucleated isotactic polypropylene. Polym. Adv. Technol. 2011, 22, 2044–2054. [Google Scholar] [CrossRef]
- Tang, X.G.; Bao, R.Y.; Wei, Y.; Yang, W.; Xie, B.H.; Hou, M. Effect of β-phase on the fracture behavior of dynamically vulcanized PP/EPDM blends studied by the essential work of fracture approach. Eur. Polym. J. 2009, 45, 1448–1453. [Google Scholar] [CrossRef]
- Wang, F.; Du, H.; Liu, H.; Zhang, Y.; Zhang, X.; Zhang, J. The synergistic effects of β-nucleating agent and ethylene–octene copolymer on toughening isotactic polypropylene. Polym. Test. 2015, 45, 1–11. [Google Scholar] [CrossRef]
- Ma, L.F.; Wei, X.F.; Zhang, Q.; Wang, W.K.; Gu, L.; Yang, W.; Xie, B.H.; Yang, M.B. Toughening of polyamide 6 with β-nucleated thermoplastic vulcanizates based on polypropylene/ethylene–propylene–diene rubber grafted with maleic anhydride blends. Mater. Des. 2012, 33, 104–110. [Google Scholar] [CrossRef]
- Yang, B.; Hu, L.; Xia, R.; Chen, F.; Zhao, S.C.; Deng, Y.L.; Cao, M.; Qian, J.S.; Chen, P. Effect of different nanofillers on non-isothermal crystallization kinetics and electric conductivity of dynamically-vulcanized PP-EPDM Blends. Macromol. Res. 2016, 24, 74–82. [Google Scholar] [CrossRef]
- Cao, L.; Jiang, X.; Ding, J.; Chen, Y. Effect of zinc dimethacrylate on compatibilization and reinforcement of peroxide dynamically cured PP/EPDM TPVs. Bulg. Chem. Commun. 2015, 47, 691–698. [Google Scholar]
- Wang, Y.X.; Wang, C.C.; Shi, Y.; Liu, L.Z.; Bai, N.; Song, L.F. Effects of Dynamic Crosslinking on Crystallization, Structure and Mechanical Property of Ethylene-Octene Elastomer/EPDM Blends. Polymers 2022, 14, 139. [Google Scholar] [CrossRef]
- Wang, Y.X.; Shi, Y.; Wang, C.C.; Cheng, J.H.; Wang, Y.; Shao, W.J.; Liu, L.Z. Crystallization, structure, and enhanced mechanical property of ethylene-octene elastomer cross-linked with dicumyl peroxide. J. Appl. Polym. Sci. 2021, 138, 50651. [Google Scholar] [CrossRef]
- Aldosari, H. Investigation the Phase Separation in Metallocene Linear Low Density Polyethylene/Polypropylene Blends. Adv. Mater. Res. 2020, 1159, 1–18. [Google Scholar] [CrossRef]
- Antunes, C.F.; van Duin, M.; Machado, A.V. Morphology and phase inversion of EPDM/PP blends-Effect of viscosity and elasticity. Polym. Test. 2011, 30, 907–915. [Google Scholar] [CrossRef]
- Prut, E.V.; Solomatin, D.V.; Kuznetsova, O.P. Rheological behaviors of blends based on polypropylene and EPDM rubber powder. Mendeleev Commun. 2017, 27, 318–320. [Google Scholar] [CrossRef]
- Mazidi, M.M.; Aghjeh, M.K.R. Effects of blend composition and compatibilization on the melt rheology and phase morphology of binary and ternary PP/PA6/EPDM blends. Polym. Bull. 2015, 72, 1975–2000. [Google Scholar] [CrossRef]
- Mazidi, M.M.; Razavi Aghjeh, M.K.; Khonakdar, H.A.; Reuter, U. Structure–property relationships in super-toughened polypropylene-based ternary blends of core–shell morphology. RSC Adv. 2016, 6, 1508–1526. [Google Scholar] [CrossRef]
- Uthaipan, N.; Junhasavasdikul, B.; Vennemann, N.; Nakason, C.; Thitithammawong, A. Investigation of surface properties and elastomeric behaviors of EPDM/EOC/PP thermoplastic vulcanizates with different octene contents. J. Appl. Polym. Sci. 2017, 134, 44857. [Google Scholar] [CrossRef]




| EPDM | Mooney Viscosity (ML1+4, 125 °C)) (MU) | Ethylene Content (wt%) | Propylene Content (wt%) | Ethylidene Norbornene (ENB) Content (wt%) |
|---|---|---|---|---|
| NORDEL™ IP 4770P | 70 | 70.0 | 25.0 | 5.0 |
| NORDEL™ IP 4725P | 25 | 70.0 | 25.0 | 5.0 |
| Samples | Relative Amount by Weight (wt%) | ||||
|---|---|---|---|---|---|
| EPDM4770P | EPDM4725P | PP | DCP | Antioxidant 1010 | |
| Neat PP | 0 | 0 | 100 | 0 | 0 |
| Crosslinked 4770P | 100 | 0 | 0 | 2 | 0 |
| Crosslinked 4725P | 0 | 100 | 0 | 2 | 0 |
| 4770P TPV | 60 | 0 | 40 | 1.2 | 1 |
| 4725P TPV | 0 | 60 | 40 | 1.2 | 1 |
| Sample | Tc (°C) | △Hf (J/g) | Tm (°C) | Crystallinity of Components (wt%) |
|---|---|---|---|---|
| Neat PP | 118 | 98.1 | 165 | 46.9 |
| Crosslinked 4770P | 54 | 66.6 | 77 | 22.7 |
| Crosslinked 4725P | 21 | 37.7 | 36 | 12.7 |
| Sample | TC | △Hf | Tm | Crystallinity of Components (wt%) | ||||
|---|---|---|---|---|---|---|---|---|
| Tc1 (°C) | Tc2 (°C) | △Hf1 (J/g) | △Hf2 (J/g) | Tm1 (°C) | Tm2 (°C) | Crystallinity of EPDM (wt%) | Crystallinity of PP (wt%) | |
| 4770P TPV | 60 | 118 | 26.2 | 40.8 | 77 | 158 | 14.9 | 48.8 |
| 4725P TPV | 21 | 119 | 19.4 | 39.7 | 36 | 159 | 11.0 | 47.5 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Tensile Stress at 100% (MPa) | Tensile Stress at 300% (MPa) | Permanent Tensile Deformation (%) |
|---|---|---|---|---|---|
| Crosslinked 4770P | 22.7 | 587.1 | 3.7 | 5.0 | 250 |
| Crosslinked 4725P | 6.6 | 524.2 | 1.5 | 2.5 | 65 |
| 4770P TPV | 33.6 | 594.2 | 11.0 | 14.5 | 375 |
| 4725P TPV | 20.9 | 488.3 | 9.2 | 12.6 | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.-F.; Bai, N.; Shi, Y.; Wang, Y.-X.; Song, L.-X.; Liu, L.-Z. Effects of Ethylene-Propylene-Diene Monomers (EPDMs) with Different Moony Viscosity on Crystallization Behavior, Structure, and Mechanical Properties of Thermoplastic Vulcanizates (TPVs). Polymers 2023, 15, 642. https://doi.org/10.3390/polym15030642
Song L-F, Bai N, Shi Y, Wang Y-X, Song L-X, Liu L-Z. Effects of Ethylene-Propylene-Diene Monomers (EPDMs) with Different Moony Viscosity on Crystallization Behavior, Structure, and Mechanical Properties of Thermoplastic Vulcanizates (TPVs). Polymers. 2023; 15(3):642. https://doi.org/10.3390/polym15030642
Chicago/Turabian StyleSong, Li-Fu, Nan Bai, Ying Shi, Yuan-Xia Wang, Li-Xin Song, and Li-Zhi Liu. 2023. "Effects of Ethylene-Propylene-Diene Monomers (EPDMs) with Different Moony Viscosity on Crystallization Behavior, Structure, and Mechanical Properties of Thermoplastic Vulcanizates (TPVs)" Polymers 15, no. 3: 642. https://doi.org/10.3390/polym15030642
APA StyleSong, L.-F., Bai, N., Shi, Y., Wang, Y.-X., Song, L.-X., & Liu, L.-Z. (2023). Effects of Ethylene-Propylene-Diene Monomers (EPDMs) with Different Moony Viscosity on Crystallization Behavior, Structure, and Mechanical Properties of Thermoplastic Vulcanizates (TPVs). Polymers, 15(3), 642. https://doi.org/10.3390/polym15030642