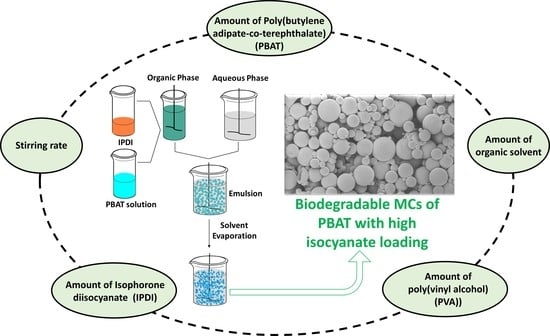
Biodegradable Microcapsules of Poly(Butylene Adipate-co-Terephthalate) (PBAT) as Isocyanate Carriers and the Effect of the Process Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microcapsules (MCs) Production
2.3. Microcapsules’ (MCs) Characterization
2.3.1. Optical Microscopy and Scanning Electron Microscopy (SEM)
2.3.2. Gel Permeation Chromatography (GPC)
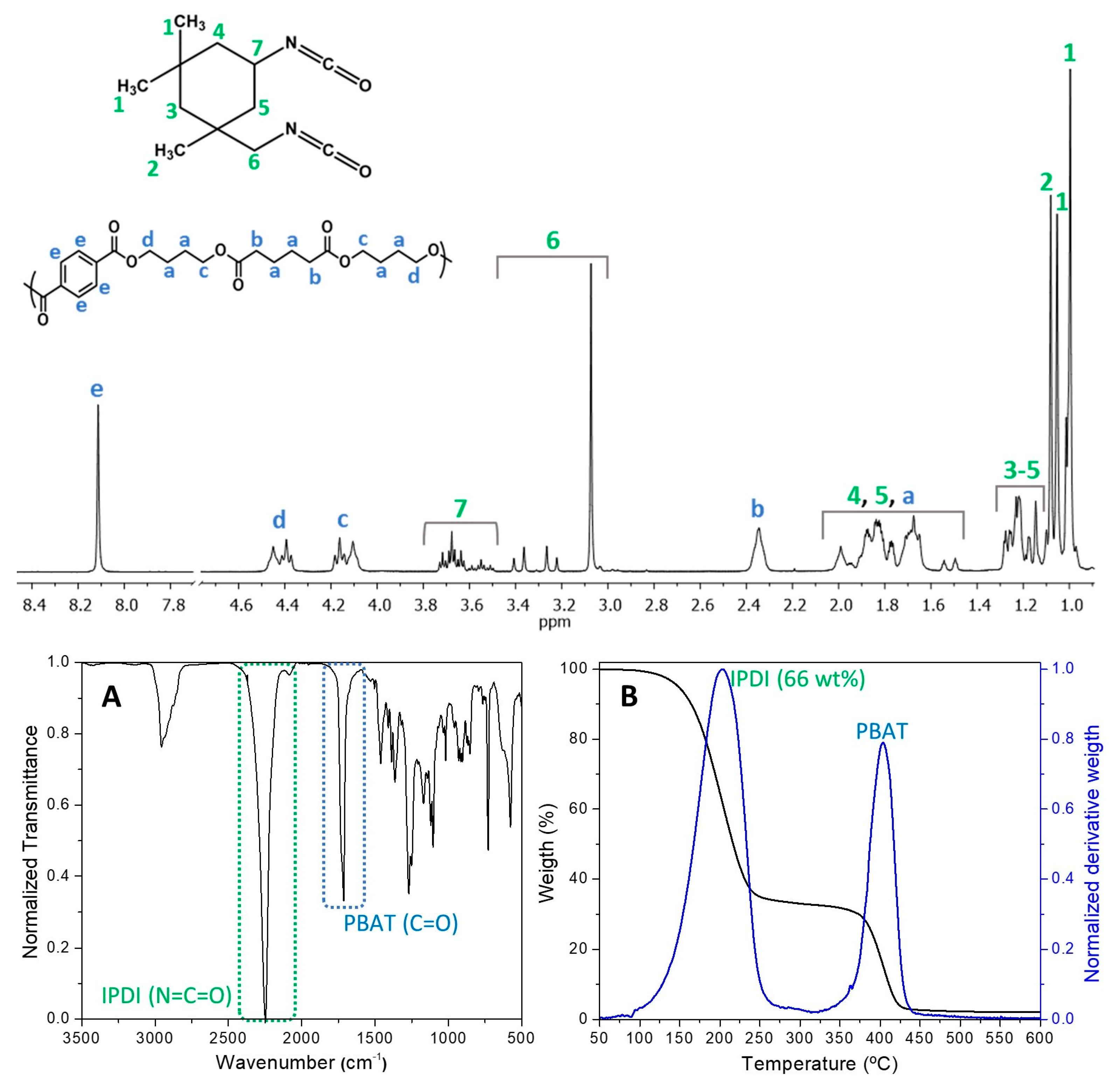
2.3.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.4. Proton Nuclear Magnetic Resonance (1H NMR)
2.3.5. Thermogravimetric Analysis (TGA) and Derivative Thermogravimetry (DTG)
2.3.6. Viscosity Measurements
2.3.7. Statistical Analysis
3. Results and Discussion
3.1. MCs (R-MCs) Production and Characterization
3.1.1. Encapsulation Process
3.1.2. Characterization of R-MCs and IPDI Load
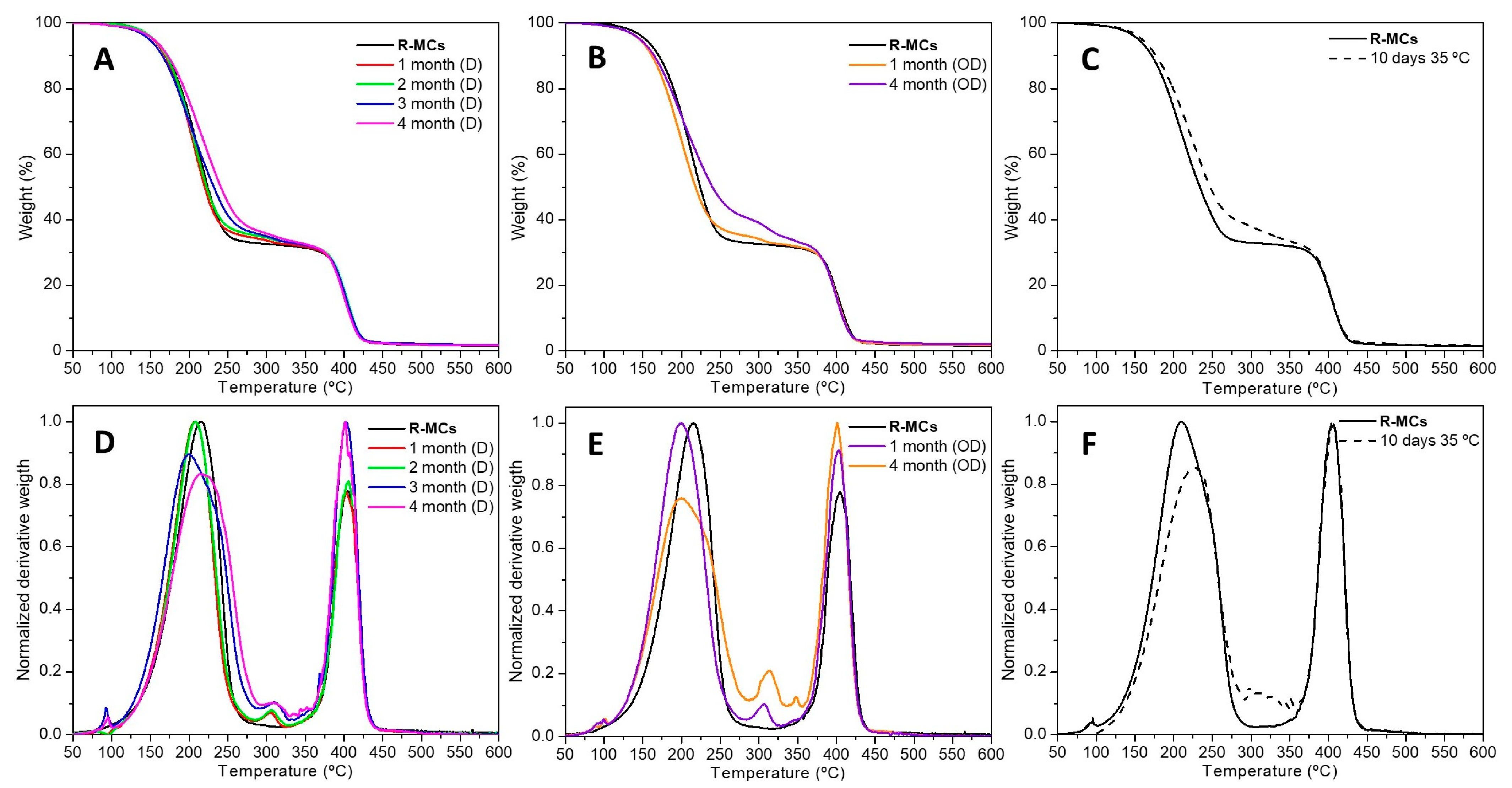
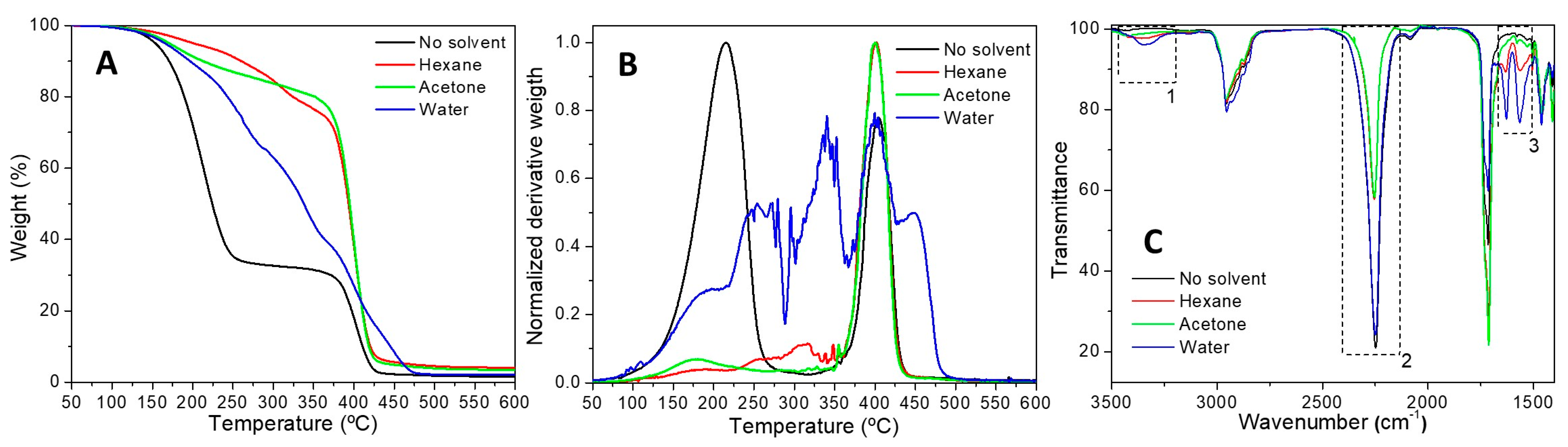
3.1.3. Stability Tests
3.2. Effect of Process Parameters
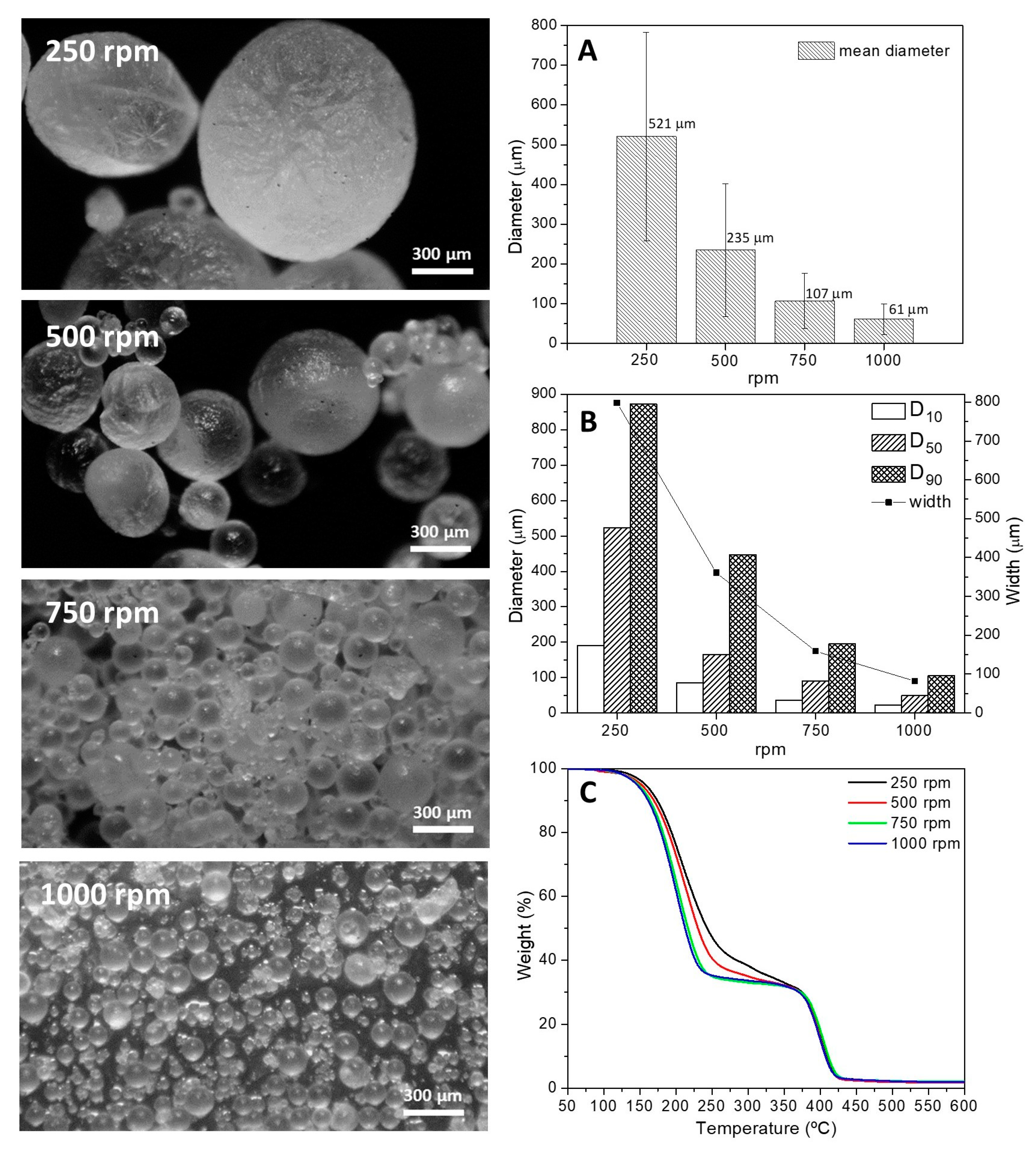
3.2.1. Mechanical Stirring Rate
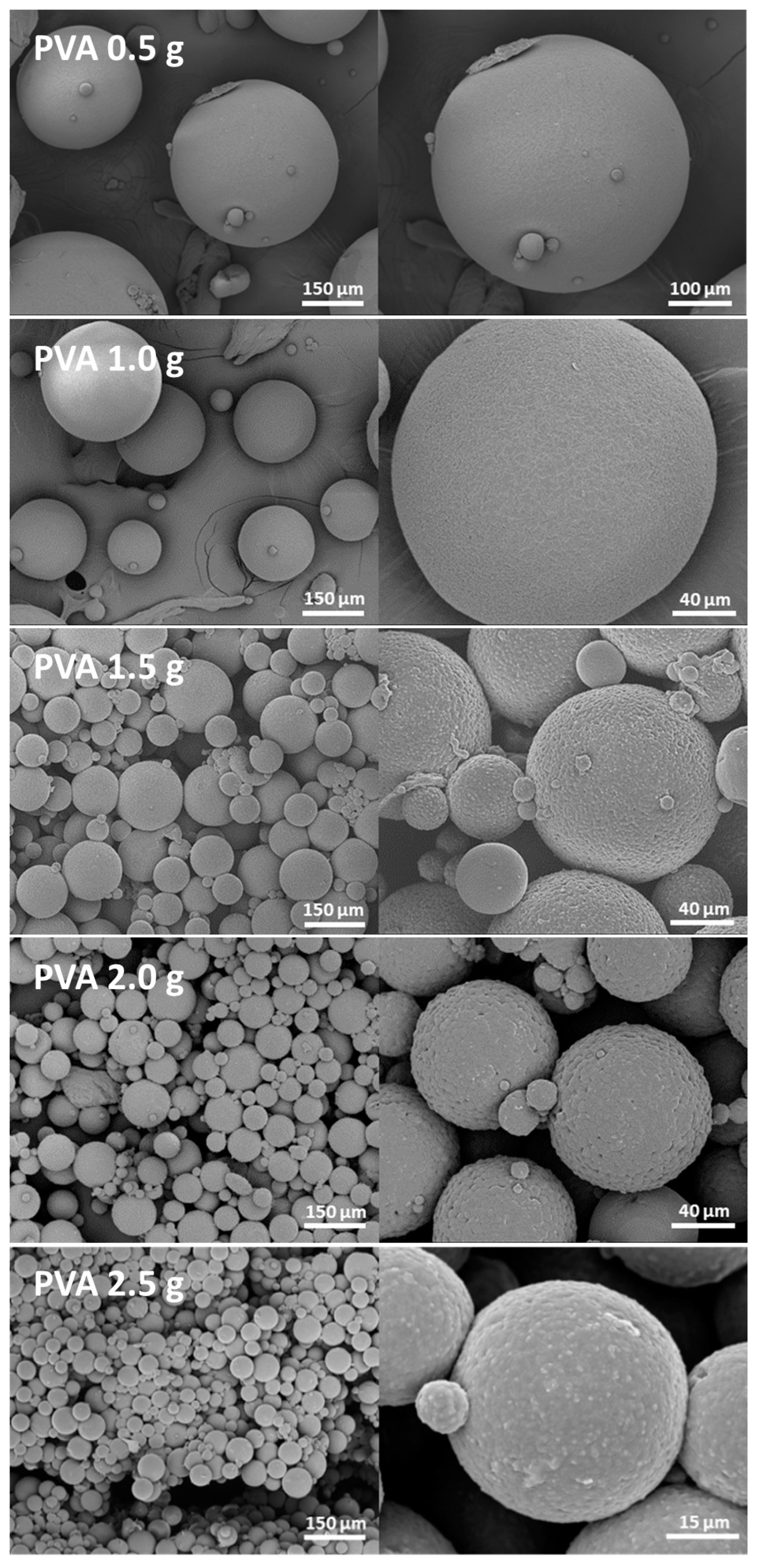
3.2.2. Amount of Poly(Vinyl Alcohol) (PVA)
3.2.3. Amount of Poly(Butylene Adipate-Co-Terephthalate) (PBAT)
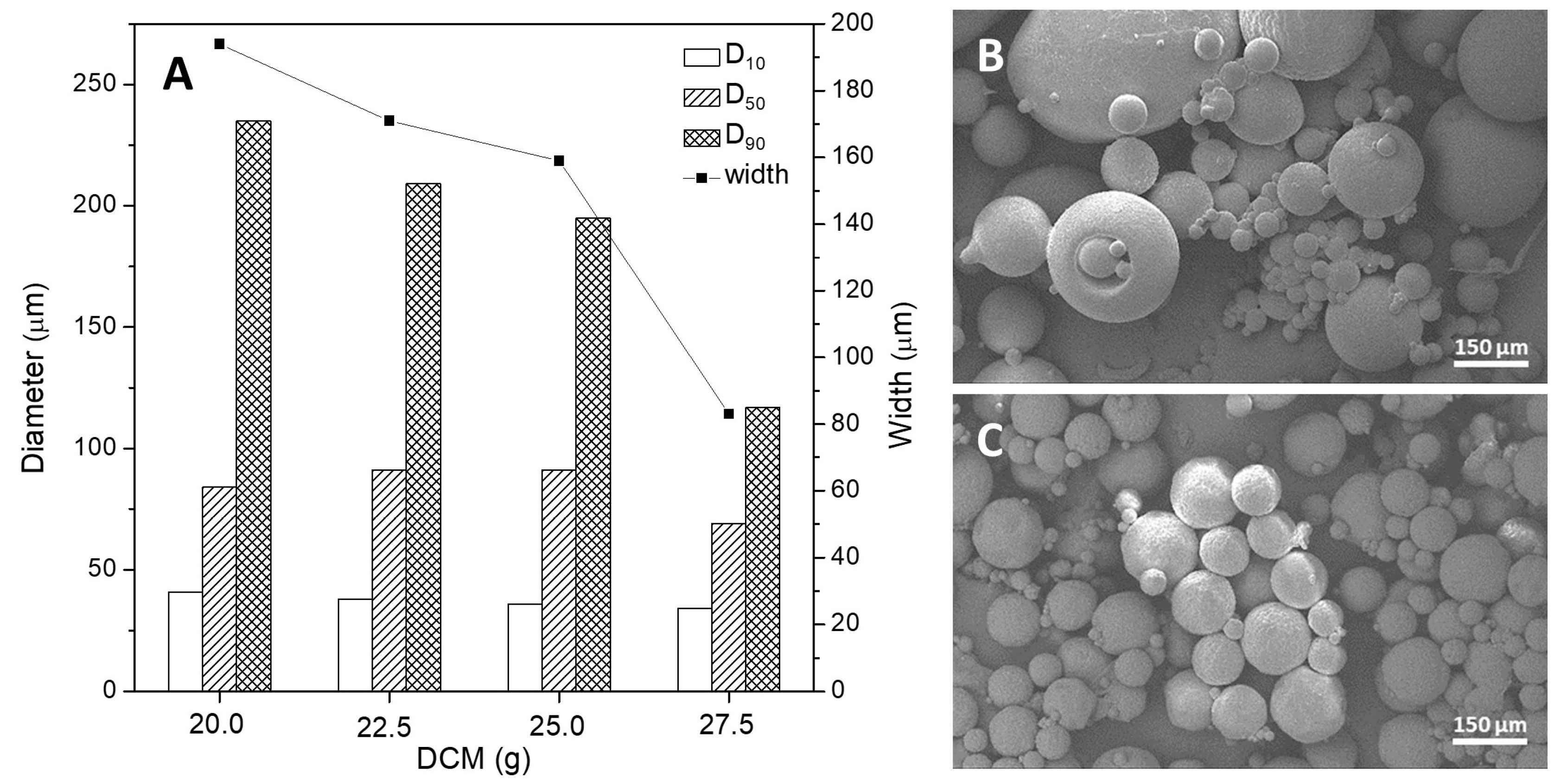
3.2.4. Amount of Dichloromethane (DCM)
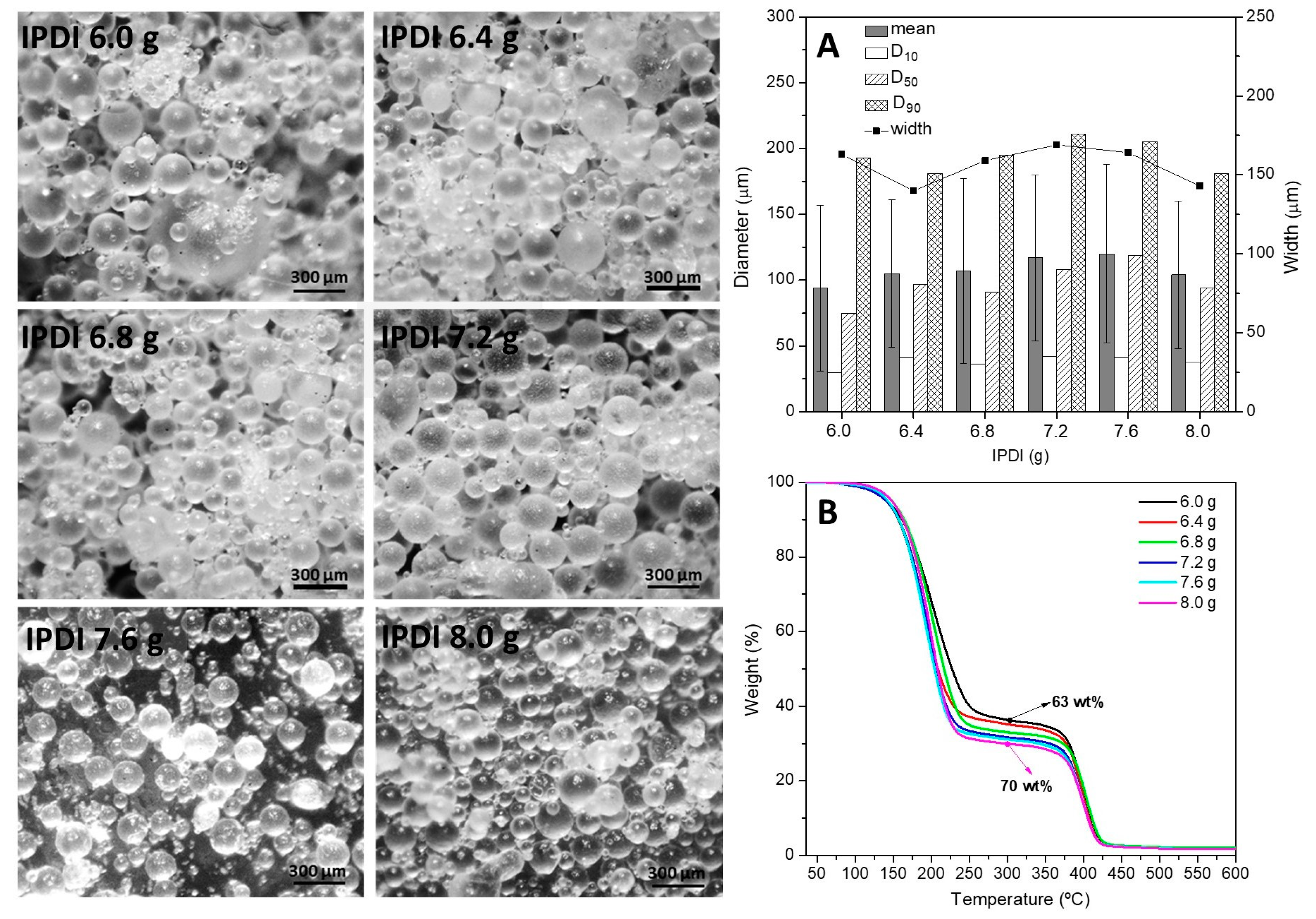
3.2.5. Amount of Isophorone Diisocyanate (IPDI)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szycher, M. 4. Isocyanate chemistry. In Szycher’s Handbook of Polyurethanes, 1st ed.; Taylor & Francis; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Golling, F.E.; Pires, R.; Hecking, A.; Weikard, J.; Richter, F.; Danielmeier, K.; Dijkstra, D. Polyurethanes for coatings and adhesives—Chemistry and applications. Polym. Int. 2019, 68, 848–855. [Google Scholar] [CrossRef]
- Lockey, J.E.; Redlich, C.A.; Streicher, R.; Pfahles-Hutchens, A.; Hakkinen, P.B.J.; Ellison, G.L.; Harber, P.; Utell, M.; Holland, J.; Comai, A.; et al. Isocyanates and human health: Multistakeholder information needs and research priorities. J. Occup. Environ. Med. 2015, 57, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Bakerly, N.D.; Moore, V.C.; Vellore, A.D.; Jaakkola, M.S.; Robertson, A.S.; Burge, P.S. Fifteen-year trends in occupational asthma: Data from the shield surveillance scheme. Occup. Med. 2008, 58, 169–174. [Google Scholar] [CrossRef]
- Bello, D.; Woskie, S.R.; Streicher, R.P.; Liu, Y.; Stowe, M.H.; Eisen, E.A.; Ellenbecker, M.J.; Sparer, J.; Youngs, F.; Cullen, M.R.; et al. Polyisocyanates in occupational environments: A critical review of exposure limits and metrics. Am. J. Ind. Med. 2004, 46, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Song, J.; Jiang, Y.; Zhang, Y.; Gu, J. Preparation of isocyanate microcapsules by interfacial polymerization/in-situ polymerization and their application in wood adhesives. J. Adhes. 2021, 98, 2689–2705. [Google Scholar] [CrossRef]
- Saifullah, M.; Shishir, M.R.I.; Ferdowsi, R.; Tanver Rahman, M.R.; Van Vuong, Q. Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: A critical review. Trends Food Sci. Technol. 2019, 86, 230–251. [Google Scholar] [CrossRef]
- Santos, A.N.B.; Santos, D.J.D.; Carastan, D.J. Microencapsulation of reactive isocyanates for application in self-healing materials: A review. J. Microencapsul. 2021, 38, 338–356. [Google Scholar] [CrossRef] [PubMed]
- Messersmith, R.E.; Bartlett, M.E.; Rose, D.J.; Smith, D.A.; Patchan, M.W.; Benkoski, J.J.; Trexler, M.M.; Hoffman, C.M. Rapid underwater adhesive utilizing crosslinker and amine catalyst-filled microcapsules. ACS Appl. Polym. Mater. 2021, 3, 996–1002. [Google Scholar] [CrossRef]
- Sun, D.; Yan, Z.; Mingzhang, L.; Ziming, W.; Suping, C.; Jinglei, Y. Robust and impermeable metal shell microcapsules for one-component self-healing coatings. Appl. Surf. Sci. 2021, 546, 149114. [Google Scholar] [CrossRef]
- Singh, M.N.; Hemant, K.S.; Ram, M.; Shivakumar, H.G. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar]
- Ghiman, R.; Pop, R.; Rugina, D.; Focsan, M. Recent progress in preparation of microcapsules with tailored structures for bio-medical applications. J. Mol. Struct. 2022, 1248, 131366. [Google Scholar] [CrossRef]
- Casanova, F.; Santos, L. Encapsulation of cosmetic active ingredients for topical application—A review. J. Microencapsul. 2016, 33, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.M.; Barreiro, M.F.; Coelho, M.; Rodrigues, A.E. Microencapsulation of essential oils with biodegradable polymeric carriers for cosmetic applications. Chem. Eng. J. 2014, 245, 191–200. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, microspheres, and microcapsules for advanced drug delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Kaushik, S.C.; Tyagi, S.K.; Akiyama, T. Development of phase change materials based microencapsulated technology for buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1373–1391. [Google Scholar] [CrossRef]
- Ahangaran, F.; Navarchian, A.H.; Picchioni, F. Material encapsulation in poly(methyl methacrylate) shell: A review. J. Appl. Polym. Sci. 2019, 136, 48039. [Google Scholar] [CrossRef]
- Li, M.; Rouaud, O.; Poncelet, D. Microencapsulation by solvent evaporation: State of the art for process engineering approaches. Int. J. Pharm. 2008, 363, 26–39. [Google Scholar] [CrossRef]
- O’Donnell, P.B.; McGinity, J.W. Preparation of microspheres by the solvent evaporation technique. Adv. Drug Deliv. Rev. 1997, 28, 25–42. [Google Scholar] [CrossRef]
- Sawalha, H.; Schroën, K.; Boom, R. Biodegradable polymeric microcapsules: Preparation and properties. Chem. Eng. J. 2011, 169, 1–10. [Google Scholar] [CrossRef]
- Božič, M.; Elschner, T.; Tkaučič, D.; Bračič, M.; Hribernik, S.; Stana Kleinschek, K.; Kargl, R. Effect of different surface active polysaccharide derivatives on the formation of ethyl cellulose particles by the emulsion-solvent evaporation method. Cellulose 2018, 25, 6901–6922. [Google Scholar] [CrossRef]
- Suri, S.; Ruan, G.; Winter, J.; Schmidt, C.E. Chapter I.2.19—Microparticles and nanoparticles. In Biomaterials Science, 3rd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: Oxford, UK, 2013; pp. 360–388. [Google Scholar]
- Urbaniak, T.; Musiał, W. Influence of solvent evaporation technique parameters on diameter of submicron lamivudine-poly-ε-caprolactone conjugate particles. Nanomaterials 2019, 9, 1240. [Google Scholar] [CrossRef] [PubMed]
- Badulescu, R.; Vivod, V.; Jausovec, D.; Voncina, B. Treatment of cotton fabrics with ethyl cellulose microcapsules. In Medical and Healthcare Textiles; Anand, S.C., Kennedy, J.F., Miraftab, M., Rajendran, S., Eds.; Woodhead Publishing: Cambridge, UK, 2010; pp. 226–235. [Google Scholar]
- Zandi, M.; Pourjavadi, A.; Hashemi, S.A.; Arabi, H. Preparation of ethyl cellulose microcapsules containing perphenazine and polymeric perphenazine based on acryloyl chloride for physical and chemical studies of drug release control. Polym. Int. 1998, 47, 413–418. [Google Scholar] [CrossRef]
- Valle, J.A.B.; Valle, R.D.C.S.C.; Bierhalz, A.C.K.; Bezerra, F.M.; Hernandez, A.L.; Lis Arias, M.J. Chitosan microcapsules: Methods of the production and use in the textile finishing. J. Appl. Polym. Sci. 2021, 138, 50482. [Google Scholar] [CrossRef]
- Ryu, S.-J.; Bae, H.-S. Properties analysis of crosslinked chitosan microcapsules by multiple emulsification method. Fash. Text. 2021, 8, 9. [Google Scholar] [CrossRef]
- Hia, I.L.; Pasbakhsh, P.; Chan, E.-S.; Chai, S.-P. Electrosprayed multi-core alginate microcapsules as novel self-healing containers. Sci. Rep. 2016, 6, 34674. [Google Scholar] [CrossRef] [PubMed]
- Mou, C.-L.; Deng, Q.-Z.; Hu, J.-X.; Wang, L.-Y.; Deng, H.-B.; Xiao, G.; Zhan, Y. Controllable preparation of monodisperse alginate microcapsules with oil cores. J. Colloid Interface Sci. 2020, 569, 307–319. [Google Scholar] [CrossRef]
- Zhang, P.; Bai, S.; Chen, S.; Li, D.; Jia, Z.; Zhou, C.; Feng, J.; Yu, L. Preparation of polyacrylamide microspheres with core-shell structure via surface-initiated atom transfer radical polymerization. RSC Adv. 2016, 6, 91463–91467. [Google Scholar] [CrossRef]
- Sabatini, V.; Pellicano, L.; Farina, H.; Pargoletti, E.; Annunziata, L.; Ortenzi, M.A.; Stori, A.; Cappelletti, G. Design of new polyacrylate microcapsules to modify the water-soluble active substances release. Polymers 2021, 13, 809. [Google Scholar] [CrossRef]
- Campini, P.A.L.; Oliveira, É.R.D.; Camani, P.H.; Silva, C.G.D.; Yudice, E.D.C.; Oliveira, S.A.D.; Rosa, D.D.S. Assessing the efficiency of essential oil and active compounds/poly (lactic acid) microcapsules against common foodborne pathogens. Int. J. Biol. Macromol. 2021, 186, 702–713. [Google Scholar] [CrossRef]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(lactic acid)-based microparticles for drug delivery applications: An overview of recent advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef]
- Han, F.Y.; Thurecht, K.J.; Whittaker, A.K.; Smith, M.T. Bioerodable PLGA-based microparticles for producing sustained-release drug formulations and strategies for improving drug loading. Front. Pharmacol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Abulateefeh, S.R.; Alkilany, A.M. Synthesis and characterization of PLGA shell microcapsules containing aqueous cores prepared by internal phase separation. AAPS PharmSciTech 2016, 17, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Cesari, A.; Loureiro, M.V.; Vale, M.; Yslas, E.I.; Dardanelli, M.; Marques, A.C. Polycaprolactone microcapsules containing citric acid and naringin for plant growth and sustainable agriculture: Physico-chemical properties and release behavior. Sci. Total Environ. 2020, 703, 135548. [Google Scholar] [CrossRef] [PubMed]
- Delgado, B.; Carrêlo, H.; Loureiro, M.V.; Marques, A.C.; Borges, J.P.; Cidade, M.T. Injectable hydrogels with two different rates of drug release based on pluronic/water system filled with poly(ε-caprolactone) microcapsules. J. Mater. Sci. 2021, 56, 13416–13428. [Google Scholar] [CrossRef]
- Yang, J.; Keller, M.W.; Moore, J.S.; White, S.R.; Sottos, N.R. Microencapsulation of isocyanates for self-healing polymers. Macromolecules 2008, 41, 9650–9655. [Google Scholar] [CrossRef]
- Loureiro, M.V.; Attaei, M.; Rocha, S.; Vale, M.; Bordado, J.C.; Simões, R.; Pinho, I.; Marques, A.C. The role played by different active hydrogen sources in the microencapsulation of a commercial oligomeric diisocyanate. J. Mater. Sci. 2020, 55, 4607–4623. [Google Scholar] [CrossRef]
- Loureiro, M.V.; Vale, M.; Galhano, R.; Matos, S.; Bordado, J.C.; Pinho, I.; Marques, A.C. Microencapsulation of isocyanate in biodegradable poly(ε-caprolactone) capsules and application in monocomponent green adhesives. ACS Appl. Polym. Mater. 2020, 2, 4425–4438. [Google Scholar] [CrossRef]
- Jiang, L.; Wolcott, M.P.; Zhang, J. Study of biodegradable polylactide/poly(butylene adipate-co-terephthalate) blends. Biomacromolecules 2006, 7, 199–207. [Google Scholar] [CrossRef]
- Barbosa, R.F.D.S.; Yudice, E.D.C.; Mitra, S.K.; Rosa, D.D.S. Characterization of Rosewood and Cinnamon Cassia essential oil polymeric capsules: Stability, loading efficiency, release rate and antimicrobial properties. Food Control 2021, 121, 107605. [Google Scholar] [CrossRef]
- Attaei, M.; Calado, L.M.; Taryba, M.G.; Morozov, Y.; Shakoor, R.A.; Kahraman, R.; Marques, A.C.; Montemor, M.F. Autonomous self-healing in epoxy coatings provided by high efficiency isophorone diisocyanate (IPDI) microcapsules for protection of carbon steel. Prog. Org. Coat. 2020, 139, 105445. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Mendez Torrecillas, C.; Halbert, G.W.; Lamprou, D.A. A novel methodology to study polymodal particle size distributions produced during continuous wet granulation. Int. J. Pharm. 2017, 519, 230–239. [Google Scholar] [CrossRef] [PubMed]
- The Jamovi Project 2021 Jamovi Version 2.3.21 Computer Software. Available online: https://www.jamovi.org (accessed on 27 December 2022).
- Wang, Y.; Guo, B.-H.; Wan, X.; Xu, J.; Wang, X.; Zhang, Y.-P. Janus-like polymer particles prepared via internal phase separation from emulsified polymer/oil droplets. Polymer 2009, 50, 3361–3369. [Google Scholar] [CrossRef]
- Loxley, A.; Vincent, B. Preparation of poly(methylmethacrylate) microcapsules with liquid cores. J. Colloid Interface Sci. 1998, 208, 49–62. [Google Scholar] [CrossRef]
- Cai, Y.; Lv, J.; Feng, J. Spectral characterization of four kinds of biodegradable plastics: Poly (lactic acid), poly (butylenes adipate-co-terephthalate), poly (hydroxybutyrate-co-hydroxyvalerate) and poly (butylenes succinate) with FTIR and Raman spectroscopy. J. Polym. Environ. 2013, 21, 108–114. [Google Scholar] [CrossRef]
- Giri, J.; Lach, R.; Le, H.H.; Grellmann, W.; Saiter, J.-M.; Henning, S.; Radusch, H.-J.; Adhikari, R. Structural, thermal and mechanical properties of composites of poly(butylene adipate-co-terephthalate) with wheat straw microcrystalline cellulose. Polym. Bull. 2021, 78, 4779–4795. [Google Scholar] [CrossRef]
- Koh, E.; Kim, N.-K.; Shin, J.; Kim, Y.-W. Polyurethane microcapsules for self-healing paint coatings. RSC Adv. 2014, 4, 16214–16223. [Google Scholar] [CrossRef]
- Yuan, L.; Liang, G.; Xie, J.; Li, L.; Guo, J. Preparation and characterization of poly(urea-formaldehyde) microcapsules filled with epoxy resins. Polymer 2006, 47, 5338–5349. [Google Scholar] [CrossRef]
- Li, Q.; Mishra, A.K.; Kim, N.H.; Kuila, T.; Lau, K.-T.; Lee, J.H. Effects of processing conditions of poly(methylmethacrylate) encapsulated liquid curing agent on the properties of self-healing composites. Compos. B Eng. 2013, 49, 6–15. [Google Scholar] [CrossRef]
- Vysloužil, J.; Doležel, P.; Kejdušová, M.; Mašková, E.; Mašek, J.; Lukáč, R.; Košťál, V.; Vetchý, D.; Dvořáčková, K. Influence of different formulations and process parameters during the preparation of drug-loaded PLGA microspheres evaluated by multivariate data analysis. Acta Pharm. 2014, 64, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Hoa, L.T.M.; Chi, N.T.; Nguyen, L.H.; Chien, D.M. Preparation and characterisation of nanoparticles containing ketoprofen and acrylic polymers prepared by emulsion solvent evaporation method. J. Exp. Nanosci. 2012, 7, 189–197. [Google Scholar] [CrossRef]
- Ibraheem, D.; Iqbal, M.; Agusti, G.; Fessi, H.; Elaissari, A. Effects of process parameters on the colloidal properties of polycaprolactone microparticles prepared by double emulsion like process. Colloids Surf. A Physicochem. Eng. Asp. 2014, 445, 79–91. [Google Scholar] [CrossRef]
- Grundy, M.M.L.; McClements, D.J.; Ballance, S.; Wilde, P.J. Influence of oat components on lipid digestion using an in vitro model: Impact of viscosity and depletion flocculation mechanism. Food Hydrocoll. 2018, 83, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Budd, M.E.; Stephens, R.; Afsar, A.; Salimi, S.; Hayes, W. Exploiting thermally-reversible covalent bonds for the controlled release of microencapsulated isocyanate crosslinkers. React. Funct. Polym. 2019, 135, 23–31. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, S.; Dong, X.; Liang, Y.; Lu, W.; He, Z.; Qi, G. Optimized synthesis of isocyanate microcapsules for self-healing application in epoxy composites. High Perform. Polym. 2020, 32, 669–680. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Liu, X.; Gu, J. Synthesis of isocyanate microcapsules as functional crosslinking agent for wood adhesive. J. Adhes. 2021, 97, 38–52. [Google Scholar] [CrossRef]
- He, Z.; Jiang, S.; An, N.; Li, X.; Li, Q.; Wang, J.; Zhao, Y.; Kang, M. Self-healing isocyanate microcapsules for efficient restoration of fracture damage of polyurethane and epoxy resins. J. Mater. Sci. 2019, 54, 8262–8275. [Google Scholar] [CrossRef]
- Di Credico, B.; Levi, M.; Turri, S. An efficient method for the output of new self-repairing materials through a reactive isocyanate encapsulation. Eur. Polym. J. 2013, 49, 2467–2476. [Google Scholar] [CrossRef]
- Sun, D.; Chong, Y.B.; Chen, K.; Yang, J. Chemically and thermally stable isocyanate microcapsules having good self-healing and self-lubricating performances. Chem. Eng. J. 2018, 346, 289–297. [Google Scholar] [CrossRef]
- Yi, H.; Yang, Y.; Gu, X.; Huang, J.; Wang, C. Multilayer composite microcapsules synthesized by pickering emulsion templates and their application in self-healing coating. J. Mater. Chem. A 2015, 3, 13749–13757. [Google Scholar] [CrossRef]
- Nguyen, L.-T.T.; Hillewaere, X.K.D.; Teixeira, R.F.A.; van den Berg, O.; Du Prez, F.E. Efficient microencapsulation of a liquid isocyanate with in situ shell functionalization. Polym. Chem. 2015, 6, 1159–1170. [Google Scholar] [CrossRef]
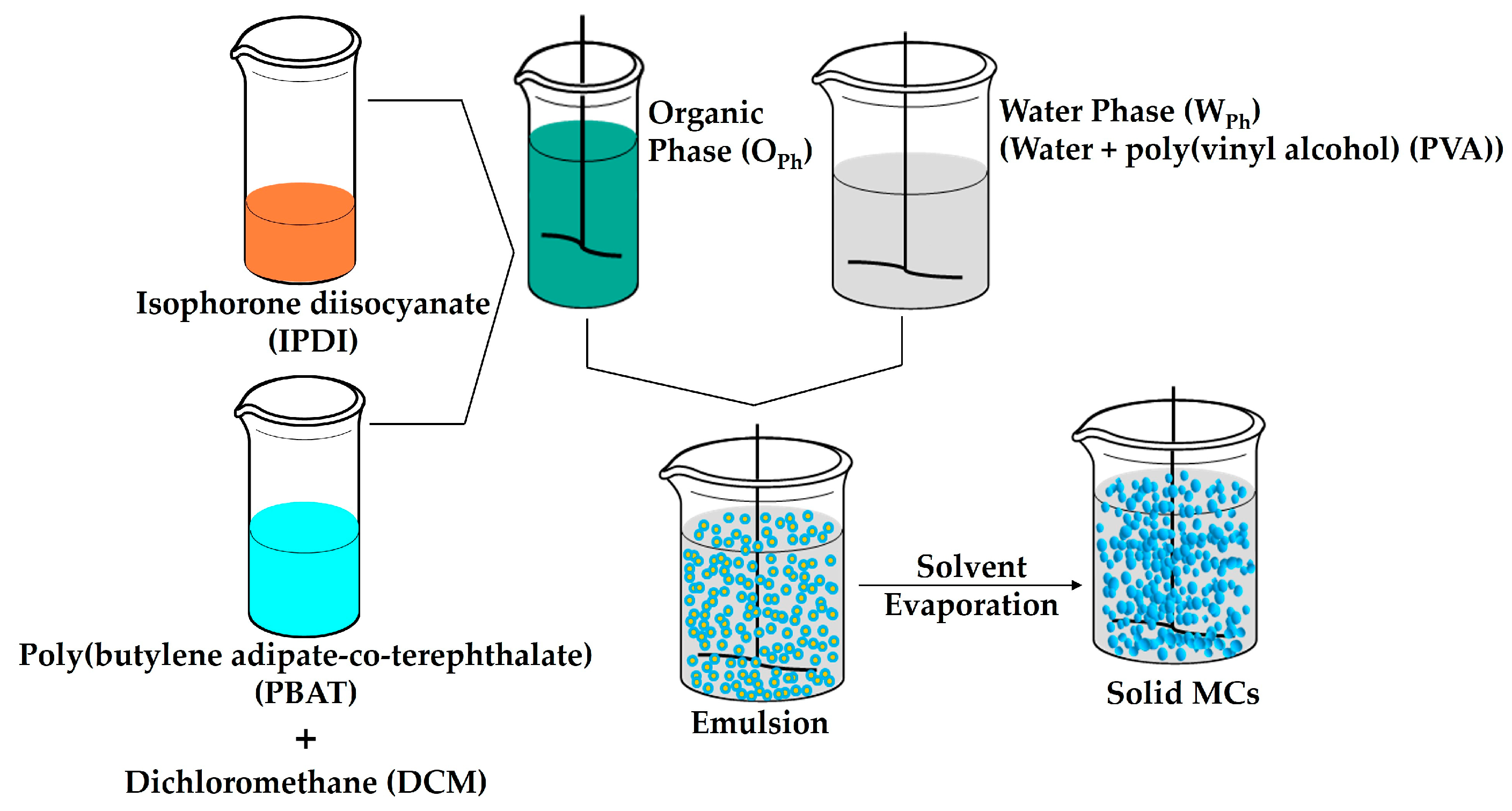




| Stirring Speed (rpm) | PVA (g) | m WPh (g) | m PBAT (g) | m DCM (g) | m IPDI (g) | m OPh (g) |
|---|---|---|---|---|---|---|
| 750 | 1.5 | 50 | 3.25 | 25 | 6.8 | 35.05 |
| Parameter | Stirring Speed (rpm) | PVA (g) | m PBAT (g) | m DCM (g) | m IPDI (g) | Yield (%) |
|---|---|---|---|---|---|---|
| Stirring speed | 250 | 1.5 | 3.25 | 25 | 6.80 | 86.7 ± 2.1 |
| 500 | 1.5 | 3.25 | 25 | 6.80 | 85.7 ± 2.1 | |
| 750 | 1.5 | 3.25 | 25 | 6.80 | 79.3 ± 3.7 | |
| 1000 | 1.5 | 3.25 | 25 | 6.80 | 71.7 ± 1.7 | |
| Amount of PVA | 750 | 0 | 3.25 | 25 | 6.80 | 0 ± 0 |
| 750 | 0.5 | 3.25 | 25 | 6.80 | 78.7 ± 3.7 | |
| 750 | 1 | 3.25 | 25 | 6.80 | 78.0 ± 3.7 | |
| 750 | 1.5 | 3.25 | 25 | 6.80 | 79.3 ± 3.7 | |
| 750 | 2 | 3.25 | 25 | 6.80 | 80.6 ± 0.5 | |
| 750 | 2.5 | 3.25 | 25 | 6.80 | 78.3 ± 0.5 | |
| Amount of PBAT | 750 | 1.5 | 1.60 | 25 | 6.80 | 52.0 ± 9.6 |
| 750 | 1.5 | 2.00 | 25 | 6.80 | 65.7 ± 3.9 | |
| 750 | 1.5 | 2.50 | 25 | 6.80 | 69.7 ± 0.5 | |
| 750 | 1.5 | 3.25 | 25 | 6.80 | 79.3 ± 3.7 | |
| 750 | 1.5 | 4.00 | 25 | 6.80 | 82.3 ± 1.9 | |
| 750 | 1.5 | 4.75 | 25 | 6.80 | 76.7 ± 0.5 | |
| Amount of DCM | 750 | 1.5 | 3.25 | 20.0 | 6.80 | 75.7 ± 1.7 |
| 750 | 1.5 | 3.25 | 22.5 | 6.80 | 77.7 ± 0.5 | |
| 750 | 1.5 | 3.25 | 25.0 | 6.80 | 79.3 ± 3.7 | |
| 750 | 1.5 | 3.25 | 27.5 | 6.80 | 85.7 ± 1.2 | |
| Amount of IPDI | 750 | 1.5 | 3.25 | 25 | 6.00 | 83.7 ± 0.5 |
| 750 | 1.5 | 3.25 | 25 | 6.40 | 82.7 ± 2.5 | |
| 750 | 1.5 | 3.25 | 25 | 6.80 | 79.3 ± 3.7 | |
| 750 | 1.5 | 3.25 | 25 | 7.20 | 70.3 ± 5.3 | |
| 750 | 1.5 | 3.25 | 25 | 7.60 | 77.0 ± 1.6 | |
| 750 | 1.5 | 3.25 | 25 | 8.00 | 83.7 ± 0.9 |
| PVA (g) | PVA Solution Viscosity (cP) | Emulsion Viscosity (cP) | Mean Size (µm) |
|---|---|---|---|
| 0.5 | 2.290 ± 0.08 | 25.76 ± 1.50 | 171 ± 121 |
| 1 | 4.840 ± 0.060 | 31.91 ± 0.64 | 149 ± 89 |
| 1.5 | 10.60 ± 0.15 | 61.35 ± 0.71 | 107 ± 70 |
| 2 | 24.08 ± 1.09 | 79.43 ± 1.24 | 81 ± 30 |
| 2.5 | 36.11 ± 0.50 | 99.48 ± 1.21 | 37 ± 14 |
| PBAT (g) | PBAT Solution Viscosity (cP) | Emulsion Viscosity (cP) | Mean Size (µm) |
|---|---|---|---|
| 1.60 | 61.73 ± 0.09 | 39.31 ± 0.23 | 36 ± 16 |
| 2.00 | 85.81 ± 0.38 | 45.62 ± 0.59 | 49 ± 22 |
| 2.50 | 114.5 ± 0.38 | 51.40 ± 0.20 | 86 ± 53 |
| 3.25 | 208.4 ± 0.69 | 61.35 ± 0.71 | 107 ± 70 |
| 4.00 | 321.25 ± 0.48 | 81.88 ± 0.36 | 143 ± 99 |
| 4.75 | 461.61 ± 0.38 | 103.4 ± 1.8 | 195 ± 125 |
| DCM (g) | PBAT Solution Viscosity (cP) | Emulsion Viscosity (cP) | Mean Size (µm) | IPDI (wt%) |
|---|---|---|---|---|
| 20.0 | 327.0 ± 0.2 | 123.5 ± 1.3 | 112 ± 88 | 64 |
| 22.5 | 259.0 ± 2.3 | 85.17 ± 0.89 | 109 ± 71 | 64 |
| 25.0 | 208.4 ± 0.7 | 61.35 ± 0.71 | 107 ± 70 | 66 |
| 27.5 | 159.3 ± 0.1 | 47.25 ± 0.22 | 74 ± 32 | 66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguiar, A.; Mariquito, A.; Gonçalves, D.; Pinho, I.; Marques, A.C. Biodegradable Microcapsules of Poly(Butylene Adipate-co-Terephthalate) (PBAT) as Isocyanate Carriers and the Effect of the Process Parameters. Polymers 2023, 15, 665. https://doi.org/10.3390/polym15030665
Aguiar A, Mariquito A, Gonçalves D, Pinho I, Marques AC. Biodegradable Microcapsules of Poly(Butylene Adipate-co-Terephthalate) (PBAT) as Isocyanate Carriers and the Effect of the Process Parameters. Polymers. 2023; 15(3):665. https://doi.org/10.3390/polym15030665
Chicago/Turabian StyleAguiar, António, António Mariquito, Diogo Gonçalves, Isabel Pinho, and Ana C. Marques. 2023. "Biodegradable Microcapsules of Poly(Butylene Adipate-co-Terephthalate) (PBAT) as Isocyanate Carriers and the Effect of the Process Parameters" Polymers 15, no. 3: 665. https://doi.org/10.3390/polym15030665
APA StyleAguiar, A., Mariquito, A., Gonçalves, D., Pinho, I., & Marques, A. C. (2023). Biodegradable Microcapsules of Poly(Butylene Adipate-co-Terephthalate) (PBAT) as Isocyanate Carriers and the Effect of the Process Parameters. Polymers, 15(3), 665. https://doi.org/10.3390/polym15030665