The Role of Dissolution Time on the Properties of All-Cellulose Composites Obtained from Oil Palm Empty Fruit Bunch
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation and Extraction of OPEFB Cellulose
2.2.1. Soda Pulping Process
2.2.2. Holocellulose Production
2.2.3. Bleaching Process
2.3. Preparation of All-Cellulose Composite Film
2.4. X-ray Diffraction
2.5. Fourier Transform Infrared (FTIR) Analysis
2.6. Scanning Electron Microscopy (SEM)
2.7. Tensile Testing
2.8. Differential Scanning Calorimetry (DSC)
2.9. Thermogravimetric Analysis (TGA)
3. Results and Discussion
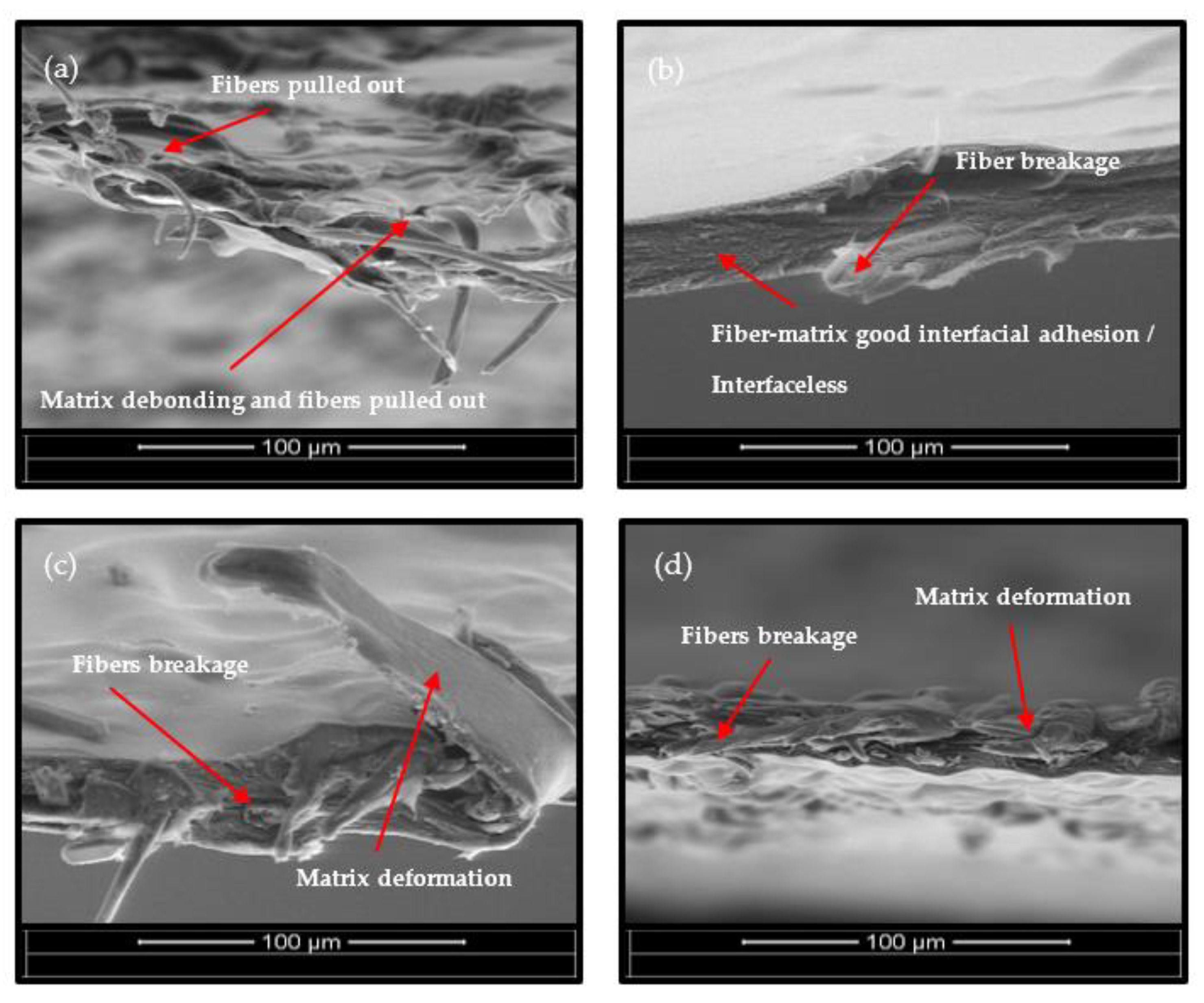
3.1. Tensile Properties
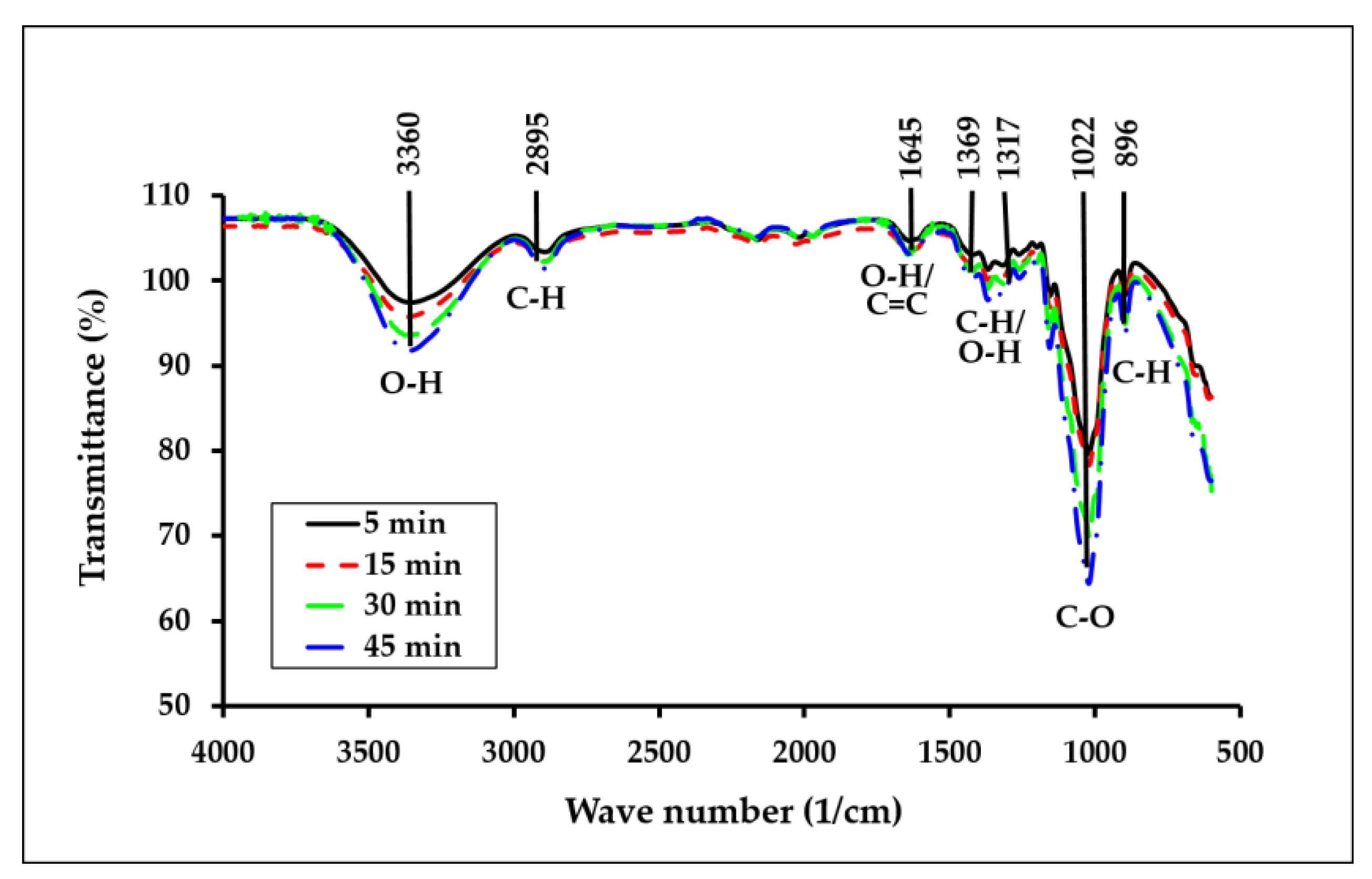
3.2. FTIR Analysis
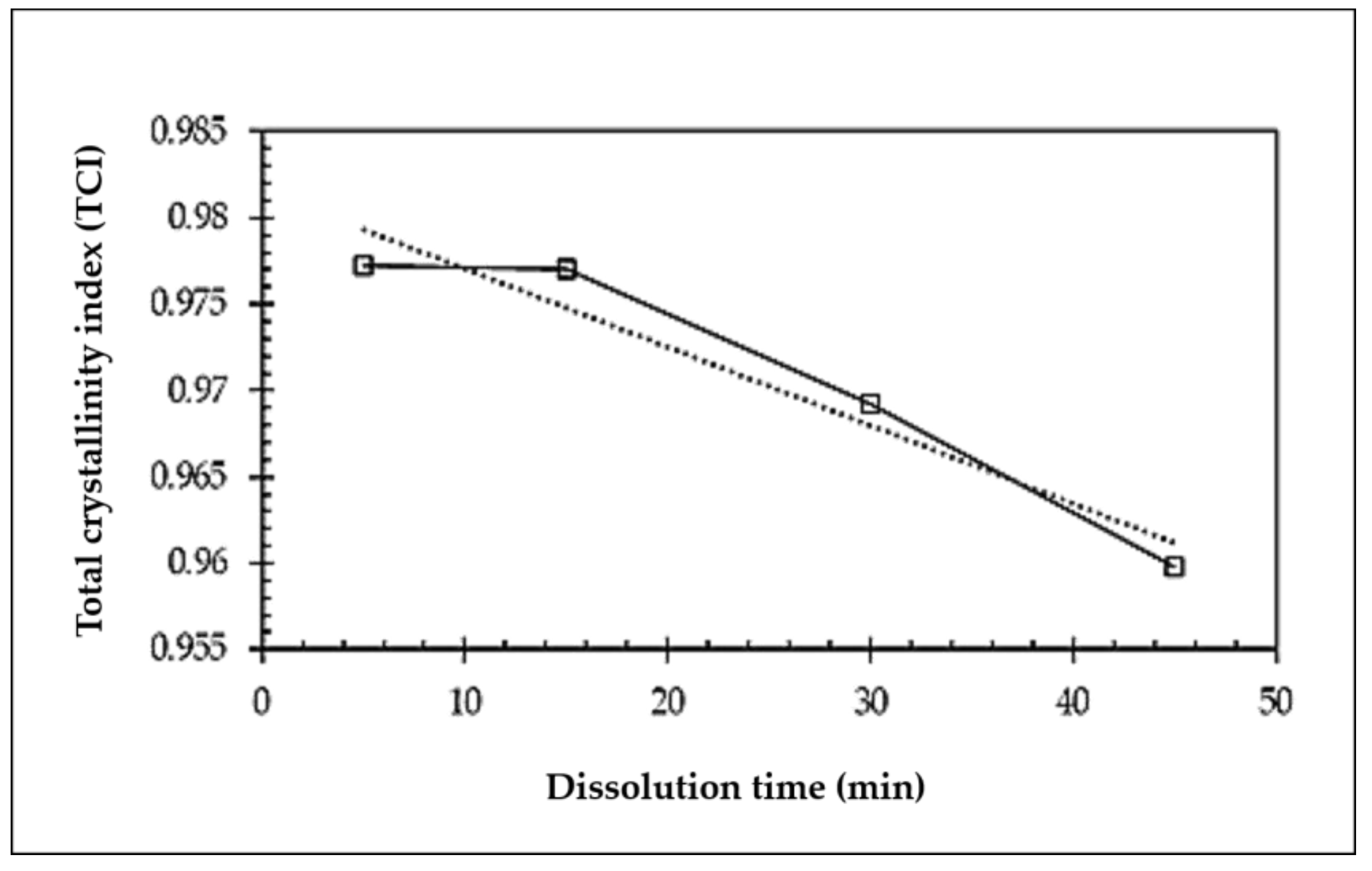
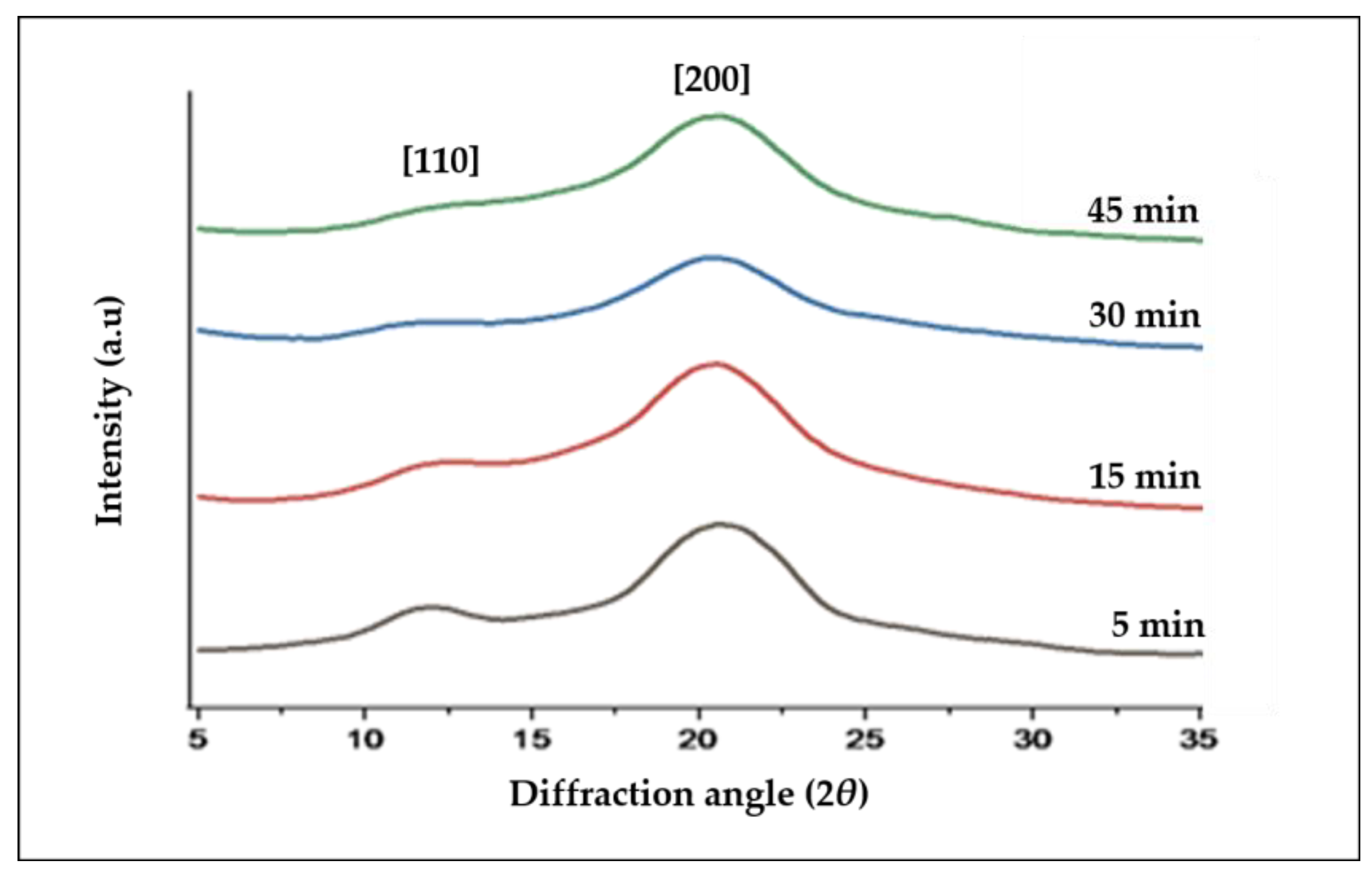
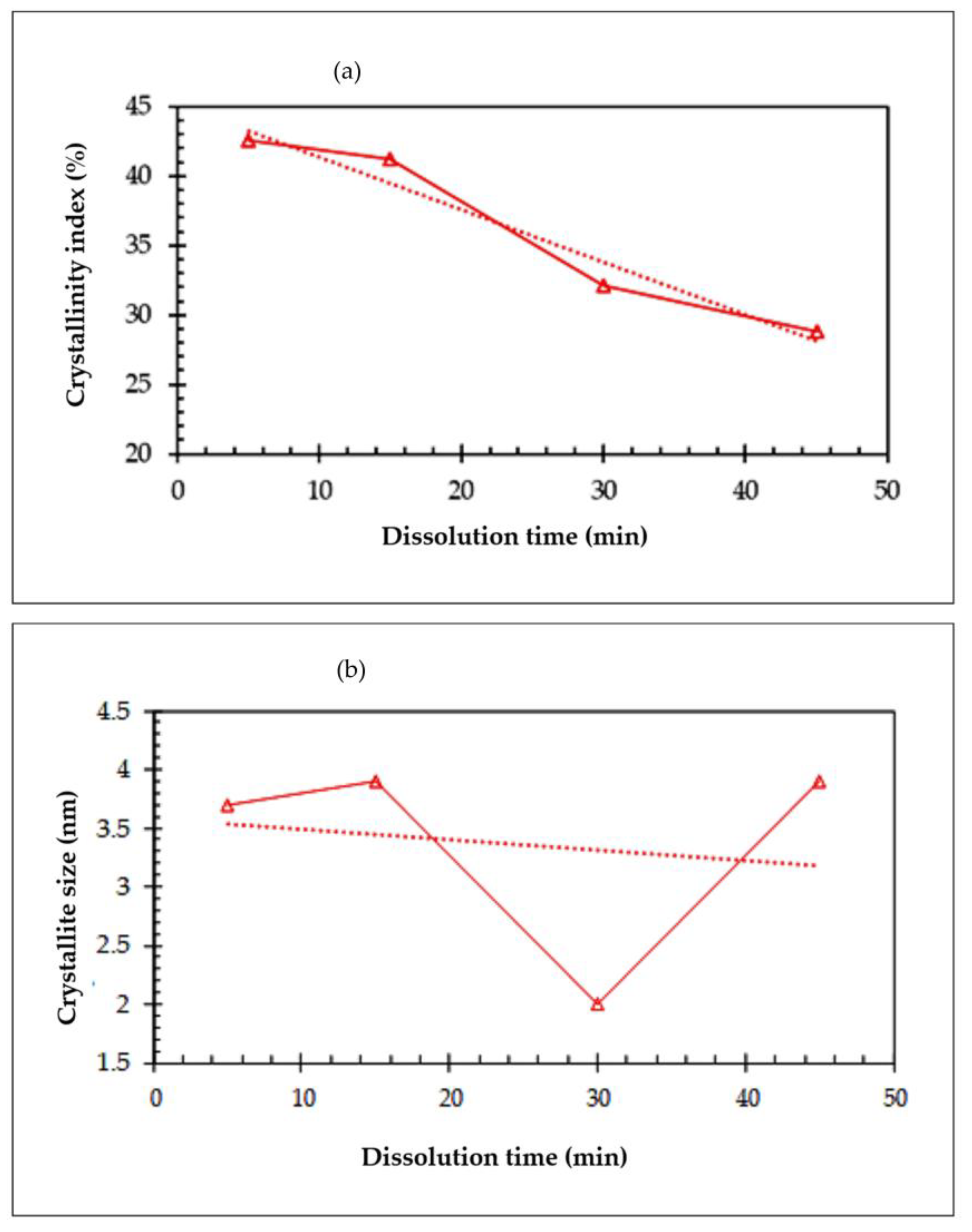
3.3. X-ray Diffraction (XRD)
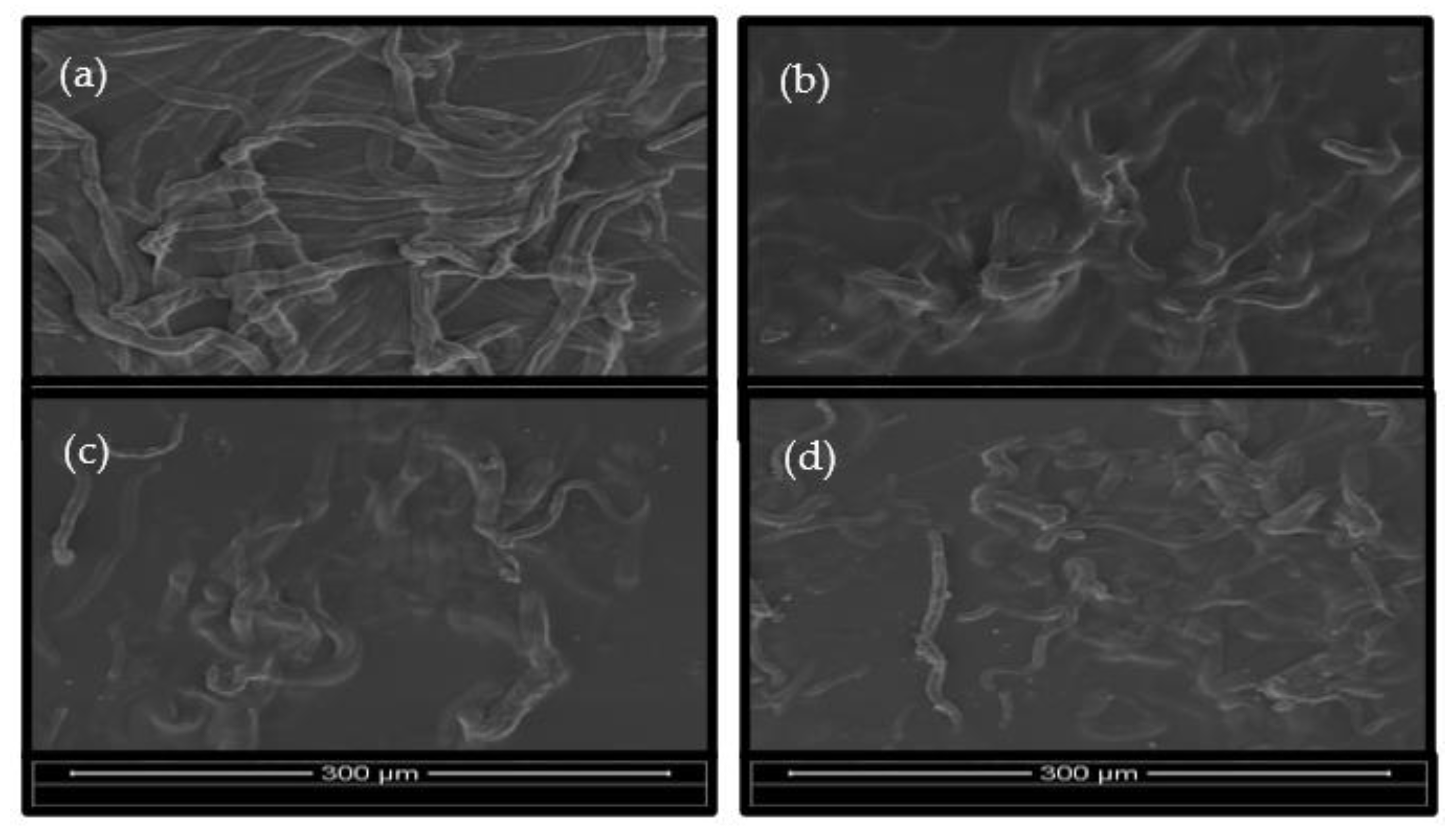
3.4. Scanning Electron Microscopy (SEM)
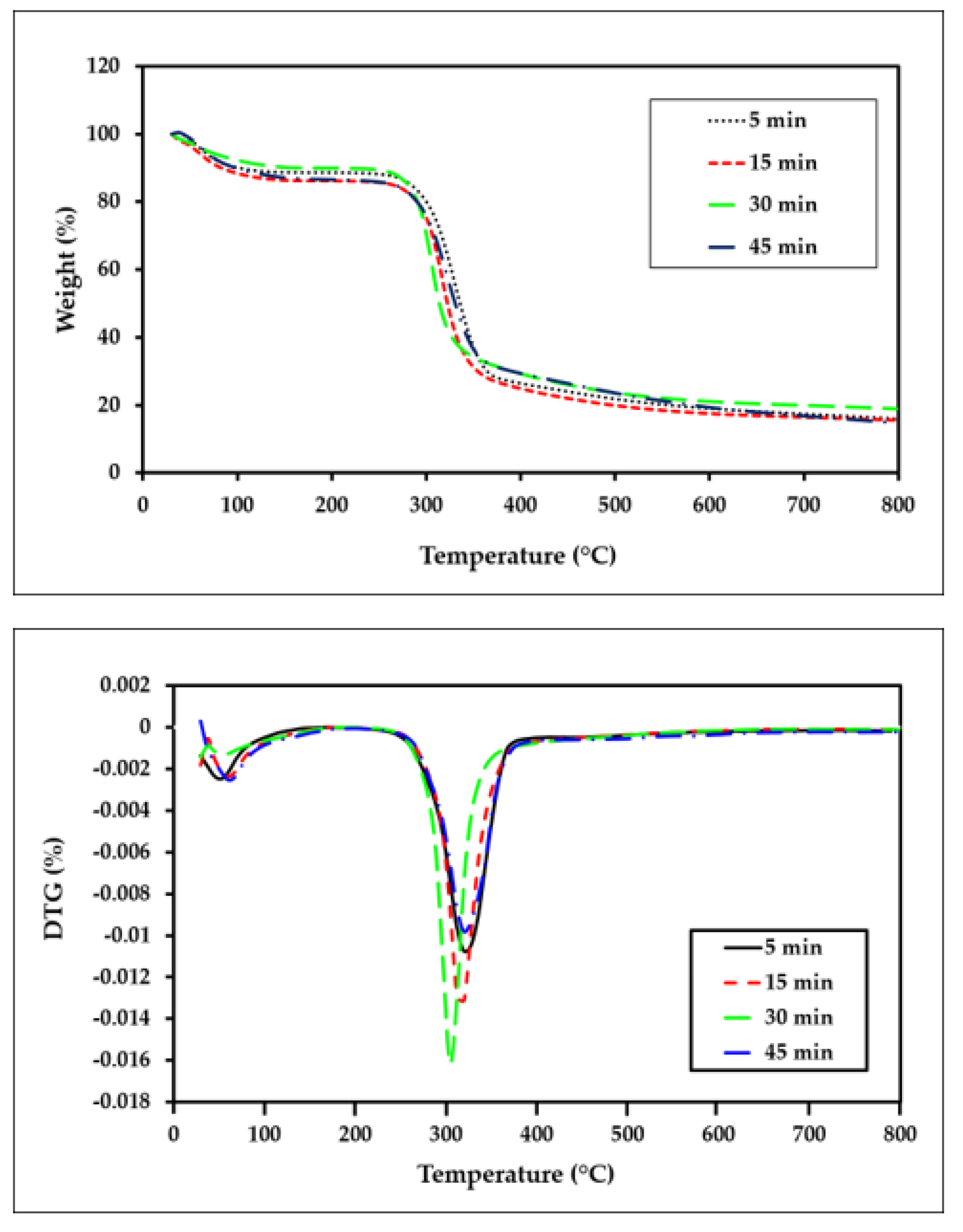
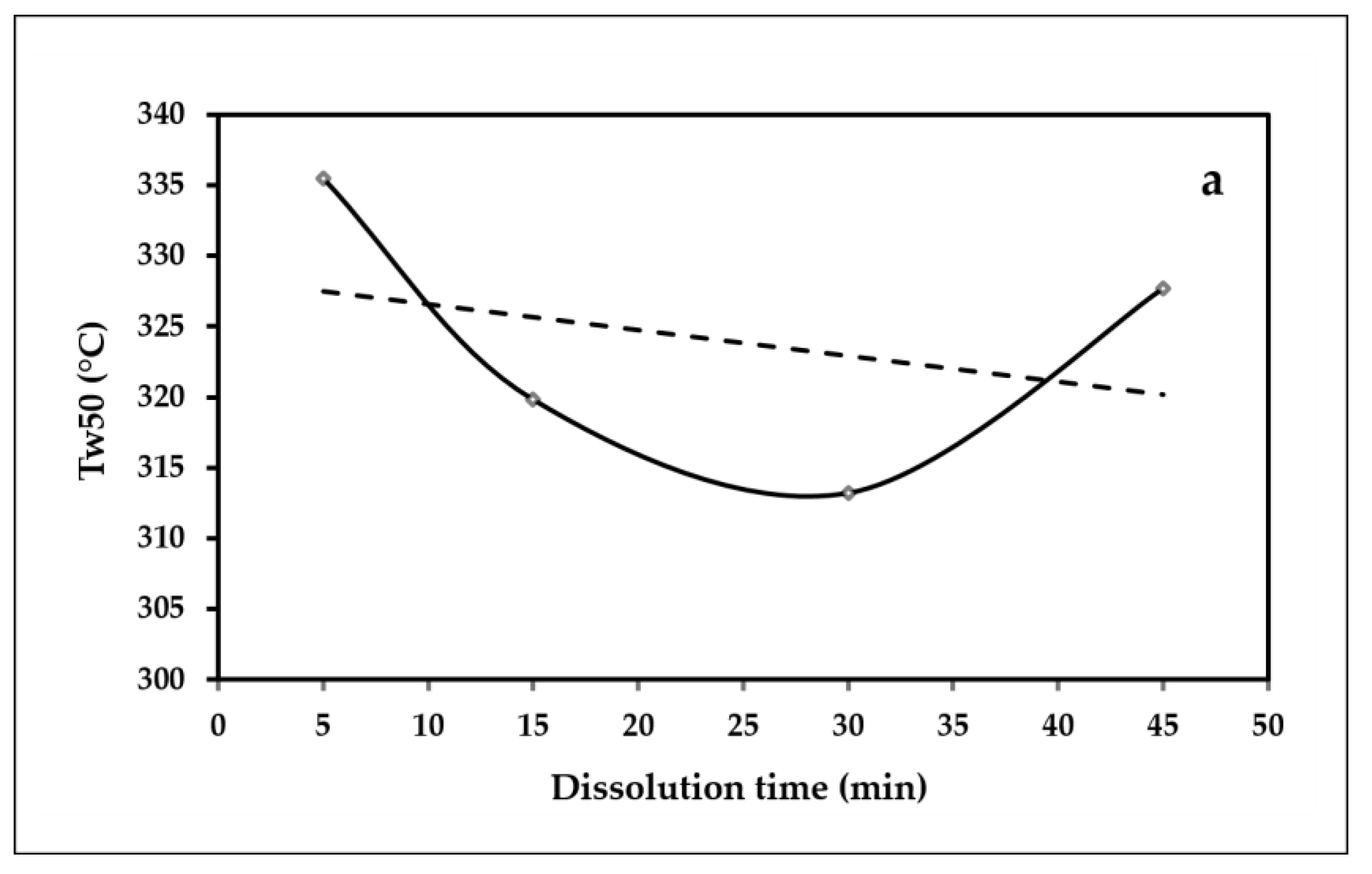
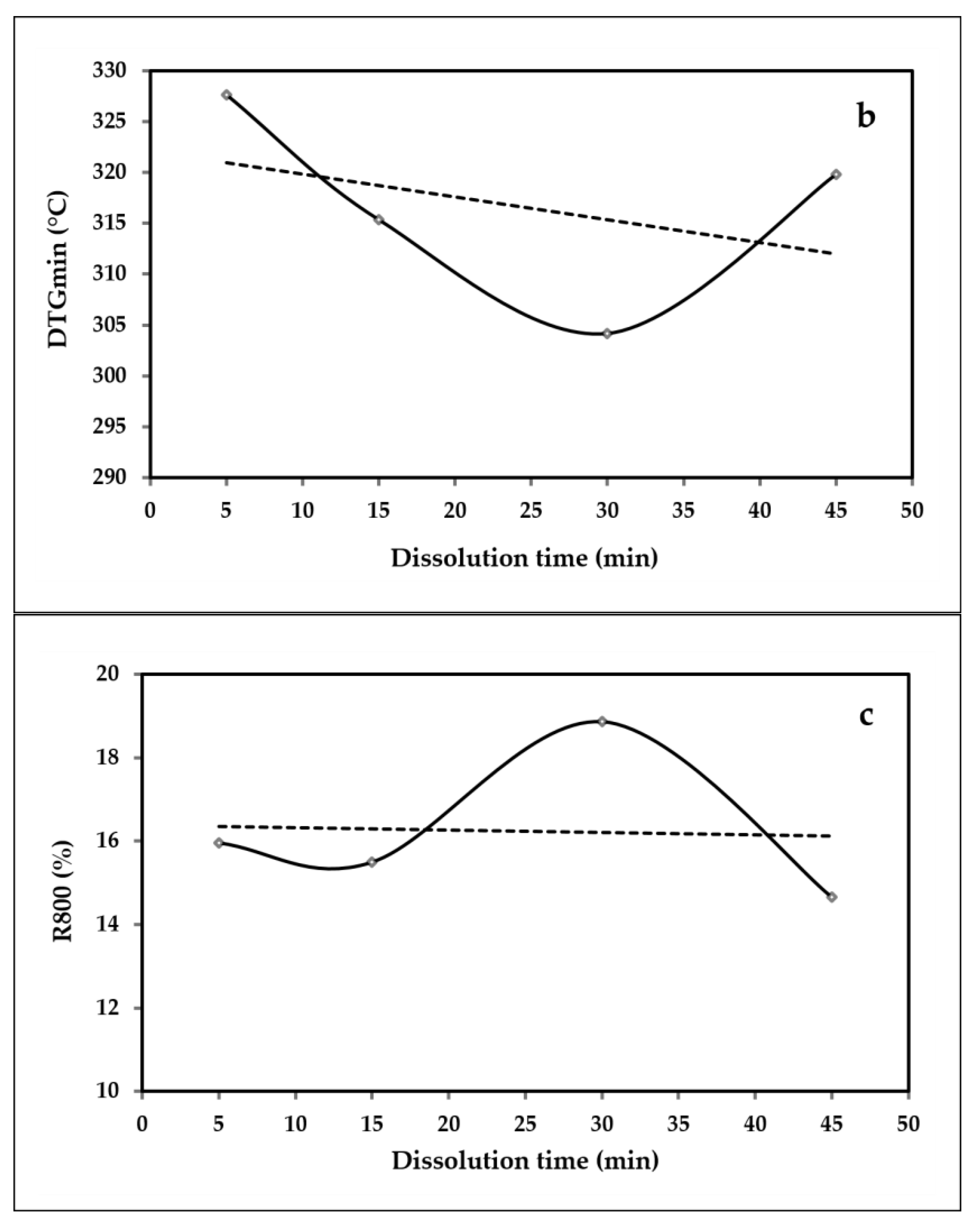
3.5. Thermogravimetric TGA
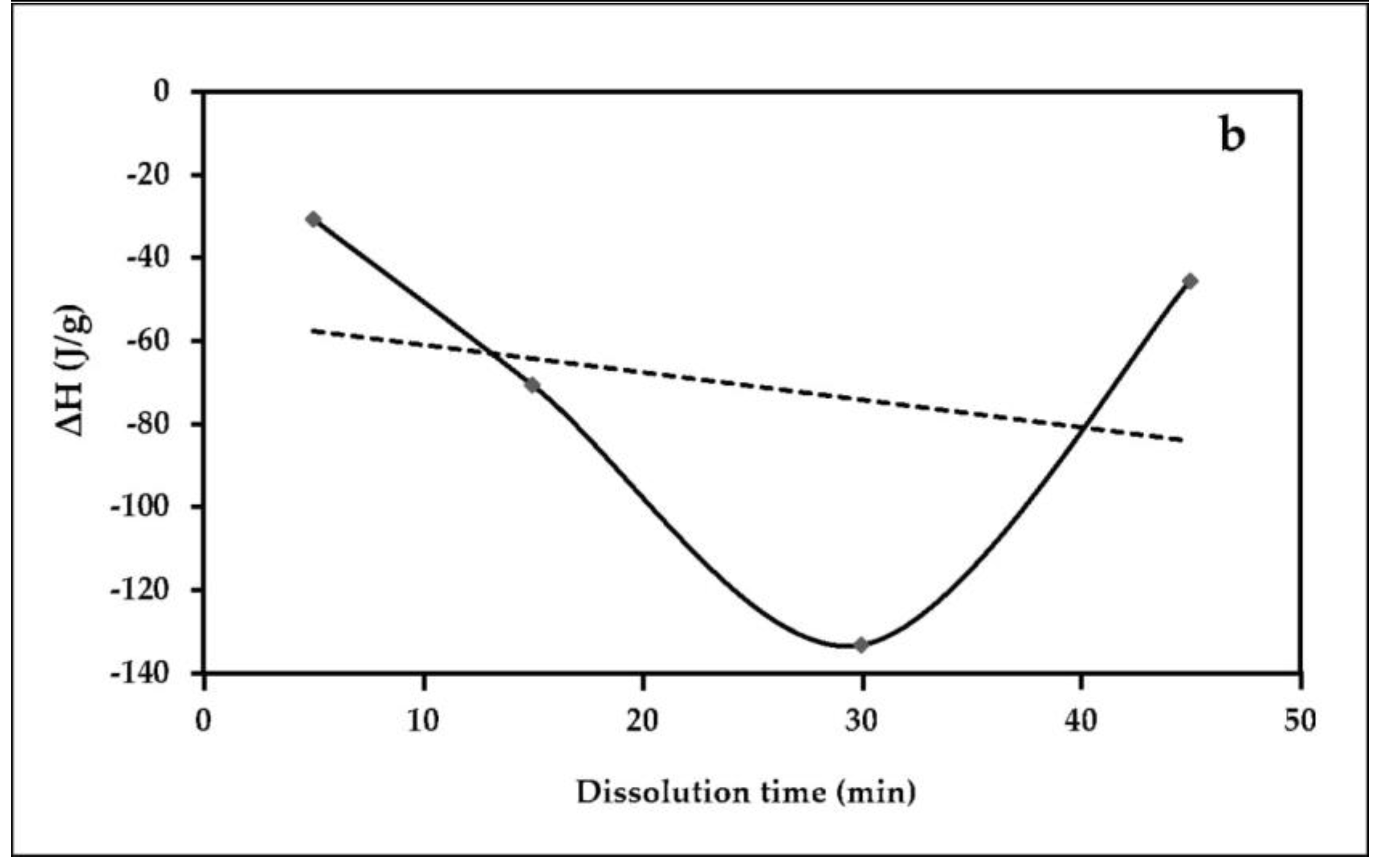
3.6. Differential Scanning Calorimetry (DSC)
4. Conclusions
- The amount of dissolved fiber surfaces is adequate to provide sufficient interfacial adhesion to the composite, while a considerable fraction of the fiber cores remain, reinforcing the material.
- The best dissolution time was discovered to be 15 min, which has the highest tensile strength.
- A decrease in the crystallite size and degree of crystallinity was observed with an increase in the dissolution time in the ACC films. The initial crystallinity of the OPEFB-bleached pulp affected the processing and the properties of the all-cellulose composites.
- The thermal stability of the ACC films shows a declining trend as the dissolution time is increased.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- OECD. Only Nine Percent of Plastic Recycled Worldwide. Available online: https://phys.org/news/2022-02-percent-plastic-recycled-worldwide-oecd.html (accessed on 16 December 2022).
- JEC Group. New Trends in Composites and Plastics Recycling. Available online: https://www.jeccomposites.com/news/new-trends-in-composites-and-plastics-recycling/ (accessed on 16 December 2022).
- Osman, A.F.; Ashafee, A.; Moh. T., L.; Adnan, S.A.; Alakrach, A. Influence of Hybrid Cellulose/Bentonite Fillers on Structure, Ambient, and Low Temperature Tensile Properties of Thermoplastic Starch Composites. Polym. Eng. Sci. 2020, 60, 810–822. [Google Scholar] [CrossRef]
- Chen, F.; Sawada, D.; Hummel, M.; Sixta, H.; Budtova, T. Unidirectional All-Cellulose Composites from Flax via Controlled Impregnation with Ionic Liquid. Polymers 2020, 12, 1010. [Google Scholar] [CrossRef]
- Spörl, J.M.; Batti, F.; Vocht, M.-P.; Raab, R.; Müller, A.; Hermanutz, F.; Buchmeiser, M.R. Ionic Liquid Approach toward Manufacture and Full Recycling of All-Cellulose Composites. Macromol. Mater. Eng. 2017, 303, 1700335. [Google Scholar] [CrossRef]
- Gupta, H.; Kanaujia, K.K.; Abbas, R.S.M.; Shukla, R. A Review on the Mechanical Properties of Natural Fibre Reinforced Polypropylene Composites. Int. Res. J. Eng. Technol. 2019, 6, 337–342. [Google Scholar]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Syafiq, R.; Sherwani, S.F.K. Nanocellulose Reinforced Thermoplastic Starch (TPS), Polylactic Acid (PLA), and Polybutylene Succinate (PBS) for Food Packaging Applications. Front. Chem. 2020, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Jumaidin, R.; Khiruddin, M.A.A.; Asyul Sutan Saidi, Z.; Salit, M.S.; Ilyas, R.A. Effect of Cogon Grass Fibre on the Thermal, Mechanical and Biodegradation Properties of Thermoplastic Cassava Starch Biocomposite. Int. J. Biol. Macromol. 2020, 146, 746–755. [Google Scholar] [CrossRef]
- Sari, N.H.; Pruncu, C.I.; Sapuan, S.M.; Ilyas, R.A.; Catur, A.D.; Suteja, S.; Sutaryono, Y.S.; Pullen, G. The Effect of Water Immersion and Fibre Content on Properties of Corn Husk Fibres Reinforced Thermoset Polyester Composite. Polym. Test. 2020, 91, 106751. [Google Scholar] [CrossRef]
- Amiandamhen, S.O.; Meincken, M.; Tyhoda, L. Natural Fibre Modification and Its Influence on Fibre-Matrix Interfacial Properties in Biocomposite Materials. Fibers Polym. 2020, 21, 677–689. [Google Scholar] [CrossRef]
- Azizi Samir, M.A.S.; Alloin, F.; Dufresne, A. Review of Recent Research into Cellulosic Whiskers, Their Properties and Their Application in Nanocomposite Field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef]
- Bondeson, D.; Syre, P.; Niska, K.O. All Cellulose Nanocomposites Produced by Extrusion. J. Biobased Mater. Bioenergy 2007, 1, 367–371. [Google Scholar] [CrossRef]
- Duchemin, B.J.C.; Newman, R.H.; Staiger, M.P. Structure–Property Relationship of All-Cellulose Composites. Compos. Sci. Technol. 2009, 69, 1225–1230. [Google Scholar] [CrossRef]
- Huber, T.; Müssig, J.; Curnow, O.; Pang, S.; Bickerton, S.; Staiger, M.P. A Critical Review of All-Cellulose Composites. J. Mater. Sci. 2011, 47, 1171–1186. [Google Scholar] [CrossRef]
- Baghaei, B.; Skrifvars, M. All-Cellulose Composites: A Review of Recent Studies on Structure, Properties and Applications. Molecules 2020, 25, 2836. [Google Scholar] [CrossRef]
- Capiati, N.J.; Porter, R.S. The Concept of One Polymer Composites Modelled with High Density Polyethylene. J. Mater. Sci. 1975, 10, 1671–1677. [Google Scholar] [CrossRef]
- Nishino, T.; Matsuda, I.; Hirao, K. All-Cellulose Composite. Macromolecules 2004, 37, 7683–7687. [Google Scholar] [CrossRef]
- Nishino, T.; Arimoto, N. All-Cellulose Composite Prepared by Selective Dissolving of Fiber Surface. Biomacromolecules 2007, 8, 2712–2716. [Google Scholar] [CrossRef]
- Gindl, W.; Keckes, J. All-Cellulose Nanocomposite. Polymer 2005, 46, 10221–10225. [Google Scholar] [CrossRef]
- Gindl, W.; Martinschitz, K.J.; Boesecke, P.; Keckes, J. Structural Changes during Tensile Testing of an All-Cellulose Composite by in Situ Synchrotron X-Ray Diffraction. Compos. Sci. Technol. 2006, 66, 2639–2647. [Google Scholar] [CrossRef]
- Gindl, W.; Schöberl, T.; Keckes, J. Structure and Properties of a Pulp Fibre-Reinforced Composite with Regenerated Cellulose Matrix. Appl. Phys. A 2006, 83, 19–22. [Google Scholar] [CrossRef]
- Li, J.; Nawaz, H.; Wu, J.; Zhang, J.; Wan, J.; Mi, Q.; Yu, J.; Zhang, J. All-Cellulose Composites Based on the Self-Reinforced Effect. Compos. Commun. 2018, 9, 42–53. [Google Scholar] [CrossRef]
- Cheng, G.; Zhu, P.; Li, J.; Cheng, F.; Lin, Y.; Zhou, M. All-Cellulose Films with Excellent Strength and Toughness via a Facile Approach of Dissolution-Regeneration. J. Appl. Polym. Sci. 2018, 136, 46925. [Google Scholar] [CrossRef]
- Arévalo, R.; Picot, O.T.; Wilson, R.M.; Soykeabkaew, N.; Peijs, T. All-Cellulose Composites by Partial Dissolution of Cotton Fibres. J. Biobased Mater. Bioenergy 2010, 4, 129–138. [Google Scholar] [CrossRef]
- Yousefi, H.; Faezipour, M.; Nishino, T.; Shakeri, A.; Ebrahimi, G. All-Cellulose Composite and Nanocomposite Made from Partially Dissolved Micro-and Nanofibers of Canola Straw. Polym. J. 2011, 43, 559–564. [Google Scholar] [CrossRef]
- Ghaderi, M.; Mousavi, M.; Yousefi, H.; Labbafi, M. All-Cellulose Nanocomposite Film Made from Bagasse Cellulose Nanofibers for Food Packaging Application. Carbohydr. Polym. 2014, 104, 59–65. [Google Scholar] [CrossRef]
- Tanpichai, S.; Witayakran, S. All-Cellulose Composites from Pineapple Leaf Microfibers: Structural, Thermal, and Mechanical Properties. Polym. Compos. 2016, 39, 895–903. [Google Scholar] [CrossRef]
- Senthil Muthu Kumar, T.; Rajini, N.; Obi Reddy, K.; Varada Rajulu, A.; Siengchin, S.; Ayrilmis, N. All-Cellulose Composite Films with Cellulose Matrix and Napier Grass Cellulose Fibril Fillers. Int. J. Biol. Macromol. 2018, 112, 1310–1315. [Google Scholar] [CrossRef]
- Gindl-Altmutter, W.; Keckes, J.; Plackner, J.; Liebner, F.; Englund, K.; Laborie, M.-P. All-Cellulose Composites Prepared from Flax and Lyocell Fibres Compared to Epoxy–Matrix Composites. Compos. Sci. Technol. 2012, 72, 1304–1309. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Ariffin, H.; Hassan, M.A.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Nishida, H. Utilisation of Superheated Steam in Oil Palm Biomass Pretreatment Process for Reduced Chemical Use and Enhanced Cellulose Nanofibre Production. Int. J. Nanotechnol. 2019, 16, 668. [Google Scholar] [CrossRef]
- Suriani, M.J.; Radzi, F.S.M.; Ilyas, R.A.; Petrů, M.; Sapuan, S.M.; Ruzaidi, C.M. Flammability, Tensile, and Morphological Properties of Oil Palm Empty Fruit Bunches Fiber/Pet Yarn-Reinforced Epoxy Fire Retardant Hybrid Polymer Composites. Polymers 2021, 13, 1282. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Marliana, M.M.; Issam, A.M.; Bakare, I.O. Exploring Isolated Lignin Material from Oil Palm Biomass Waste in Green Composites. Mater. Des. 2011, 32, 2604–2610. [Google Scholar] [CrossRef]
- Isroi; Cifriadi, A.; Panji, T.; Wibowo, N.A.; Syamsu, K. Bioplastic Production from Cellulose of Oil Palm Empty Fruit Bunch. IOP Conf. Ser. Earth Environ. Sci. 2017, 65, 012011. [Google Scholar] [CrossRef]
- Zailuddin, N.L.I.; Osman, A.F.; Husseinsyah, S.; Ariffin, Z.; Badrun, F.H. Mechanical Properties and X-Ray Diffraction of Oil Palm Empty Fruit Bunch All-Nanocellulose Composite Films. In Proceedings of the Second International Conference on the Future of ASEAN (ICoFA) 2017—Volume 2; Springer: Singapore, 2018; pp. 505–514. [Google Scholar] [CrossRef]
- Zailuddin, N.L.I.; Osman, A.F.; Rahman, R. Morphology, Mechanical Properties, and Biodegradability of All-Cellulose Composite Films from Oil Palm Empty Fruit Bunch. SPE Polym. 2020, 1, 4–14. [Google Scholar] [CrossRef]
- Zailuddin, N.L.I.; Osman, A.F.; Rahman, R. Effects of Formic Acid Treatment on Properties of Oil Palm Empty Fruit Bunch (OPEFB)-Based All Cellulose Composite (ACC) Films. J. Eng. Sci. 2020, 16, 75–95. [Google Scholar] [CrossRef]
- Gea, S.; Andita, D.; Rahayu, S.; Nasution, D.Y.; Rahayu, S.U.; Piliang, A.F. Preliminary Study on the Fabrication of Cellulose Nanocomposite Film from Oil Palm Empty Fruit Bunches Partially Solved into Licl/Dmac with the Variation of Dissolution Time. J. Phys. Conf. Ser. 2018, 1116, 042012. [Google Scholar] [CrossRef]
- Gea, S.; Panindia, N.; Piliang, A.F.; Sembiring, A.; Hutapea, Y.A. All-Cellulose Composite Isolated from Oil Palm Empty Fruit Bunch. J. Phys. Conf. Ser. 2018, 1116, 042013. [Google Scholar] [CrossRef]
- Husseinsyah, S.; Zailuddin, N.L.I.; Osman, A.F.; Li Li, C.; Alrashdi, A.A.; Alakrach, A. Methyl Methacrylate (MMA) Treatment of Empty Fruit Bunch (EFB) to Improve the Properties of Regenerated Cellulose Biocomposite Films. Polymers 2020, 12, 2618. [Google Scholar] [CrossRef] [PubMed]
- Rosli, W.D.W.; Leh, C.P.; Zainuddin, Z.; Tanaka, R. Optimisation of Soda Pulping Variables for Preparation of Dissolving Pulps from Oil Palm Fibre. Holzforschung 2003, 57, 106–113. [Google Scholar] [CrossRef]
- Soykeabkaew, N.; Arimoto, N.; Nishino, T.; Pejis, T. All-Cellulose Composites by Surface Selective Dissolution of Aligned Ligno-Cellulosic Fibres. Compos. Sci. Technol. 2008, 68, 2201–2207. [Google Scholar] [CrossRef]
- Soykeabkaew, N.; Sian, C.; Gea, S.; Nishino, T.; Peijs, T. All-Cellulose Nanocomposites by Surface Selective Dissolution of Bacterial Cellulose. Cellulose 2009, 16, 435–444. [Google Scholar] [CrossRef]
- Korhonen, O.; Sawada, D.; Budtova, T. All-Cellulose Composites via Short-Fiber Dispersion Approach Using NaOH–Water Solvent. Cellulose 2019, 26, 4881–4893. [Google Scholar] [CrossRef]
- Duchemin, B.; Le Corre, D.; Leray, N.; Dufresne, A.; Staiger, M.P. All-Cellulose Composites Based on Microfibrillated Cellulose and Filter Paper via a NaOH-Urea Solvent System. Cellulose 2015, 23, 593–609. [Google Scholar] [CrossRef]
- Shahmoradi, A.R.; Talebibahmanbigloo, N.; Javidparvar, A.A.; Bahlakeh, G.; Ramezanzadeh, B. Studying the Adsorption/Inhibition Impact of the Cellulose and Lignin Compounds Extracted from Agricultural Waste on the Mild Steel Corrosion in HCl Solution. J. Mol. Liq. 2020, 304, 112751. [Google Scholar] [CrossRef]
- Wei, Q.Y.; Lin, H.; Yang, B.; Li, L.; Zhang, L.Q.; Huang, H.D.; Zhong, G.J.; Xu, L.; Li, Z.M. Structure and Properties of All-Cellulose Composites Prepared by Controlling the Dissolution Temperature of a NaOH/Urea Solvent. Ind. Eng. Chem. Res. 2020, 59, 10428–10435. [Google Scholar] [CrossRef]
- Perez, S.; Samain, D. Structure and Engineering of Celluloses. Adv. Carbohydr. Chem. Biochem. 2010, 1, 25–116. [Google Scholar]
- Carrillo, F.; Colom, X.; Suñol, J.J.; Saurina, J. Structural FTIR Analysis and Thermal Characterisation of Lyocell and Viscose-Type Fibres. Eur. Polym. J. 2004, 40, 2229–2234. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of Certain Infrared Bands to Cellulose Crystallinity and Crystal Latticed Type. Part, I. Spectra of Lattice Types I, II, III and of Amorphous Cellulose. J. Appl. Polym. Sci. 1964, 8, 1311–1324. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of Certain Infrared Bands to Cellulose Crystallinity and Crystal Lattice Type. Part II. A New Infrared Ratio for Estimation of Crystallinity in Celluloses I and II. J. Appl. Polym. Sci. 1964, 8, 1325–1341. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Sun, L.; Chen, J.Y.; Jiang, W.; Lynch, V. Crystalline Characteristics of Cellulose Fiber and Film Regenerated from Ionic Liquid Solution. Carbohydr. Polym. 2015, 118, 150–155. [Google Scholar] [CrossRef]
- Pang, J.; Wu, M.; Zhang, Q.; Tan, X.; Xu, F.; Zhang, X.; Sun, R. Comparison of Physical Properties of Regenerated Cellulose Films Fabricated with Different Cellulose Feedstocks in Ionic Liquid. Carbohydr. Polym. 2015, 121, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, G.; Zhang, H.; Gao, X.; Li, M.; Zhang, S. Facile Preparation of All-Cellulose Composites from Softwood, Hardwood, and Agricultural Straw Cellulose by a Simple Route of Partial Dissolution. Carbohydr. Polym. 2021, 256, 117591. [Google Scholar] [CrossRef] [PubMed]
- Roshanghias, A.; Sodeifian, G.; Javidparvar, A.A.; Tarashi, S. Construction of a Novel Polytetrafluoroethylene-Based Sealant Paste: The Effect of Polyvinyl Butyral (PVB) and Nano-Alumina on the Sealing Performance and Construction Formulations. Results Eng. 2022, 14, 100460. [Google Scholar] [CrossRef]
- Jiang, Z.; Tang, L.; Gao, X.; Zhang, W.; Ma, J.; Zhang, L. Solvent Regulation Approach for Preparing Cellulose-Nanocrystal-Reinforced Regenerated Cellulose Fibers and Their Properties. ACS Omega 2019, 4, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous Cellulose—Structure and Characterization. Cellul. Chem. Technol. 2011, 45, 13. [Google Scholar]
- Yeng, L.C.; Wahit, M.U.; Othman, N. Thermal and Flexural Properties of Regenerated Cellulose (RC)/Poly(3-Hydroxybutyrate) (PHB) Biocomposites. J. Teknol. 2015, 75, 107–112. [Google Scholar] [CrossRef]
- Miranda, M.I.G.; Bica, C.I.D.; Nachtigall, S.M.B.; Rehman, N.; Rosa, S.M.L. Kinetical Thermal Degradation Study of Maize Straw and Soybean Hull Celluloses by Simultaneous DSC–TGA and MDSC Techniques. Thermochim. Acta 2013, 565, 65–71. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Li, Z.; Lu, X.; Zhang, X.; Zhang, S.; Zhou, K. Characterization of the Regenerated Cellulose Films in Ionic Liquids and Rheological Properties of the Solutions. Mater. Chem. Phys. 2011, 128, 220–227. [Google Scholar] [CrossRef]




| Samples | Dissolution Time, td (min) | Initial Cellulose Concentration, C (%) |
|---|---|---|
| ACC5 | 5 | 1 |
| ACC15 | 15 | 1 |
| ACC30 | 30 | 1 |
| ACC45 | 45 | 1 |
| ACC Films | Tensile Strength (MPa) | Young’s Modulus (GPa) | Elongation at Break (%) |
|---|---|---|---|
| ACC5 | 11.96 2.7 | 0.73 2.1 | 5.66 |
| ACC15 | 35.78 4.7 | 2.63 2.2 | 19.22 4.7 |
| ACC30 | 34.72 6.3 | 2.94 4.1 | 11.53 6.9 |
| ACC45 | 31.72 5.2 | 2.75 1.2 | 6.46 2.2 |
| Wave Number (cm–1) | Bond and Motion |
|---|---|
| 3600–3000 2850 1420 1370 1310 1255 1160 1025 895 | O(3)H–O(5) intramolecular hydrogen bond stretching vibration of C–H bonds in methyl and methylene groups methyl group deformation and the lignin aromatic ring vibrations –OH in-plane bending of crystalline form of cellulose CH2 wagging motion C–O stretching in guaiacyl ring C–O–C asymmetric bridge stretching C–O–C pyranose ring skeletal vibration β-glucosidic linkage |
| Sample | Degree of Crystallinity (%) | Crystallite Size (nm) |
|---|---|---|
| ACC5 | 42.62 | 3.7 |
| ACC15 | 41.21 | 3.9 |
| ACC30 | 32.08 | 2 |
| ACC45 | 28.8 | 3.9 |
| Sample | Degradation Temperature, °C Tw50 | DTGmin, °C | Residual Char at 800 °C, % R800°C |
|---|---|---|---|
| ACC5 | 335.5 | 327.66 | 15.95 |
| ACC15 | 319.83 | 315.34 | 15.49 |
| ACC30 | 312 | 304.17 | 18.86 |
| ACC45 | 327.66 | 319.83 | 14.66 |
| Sample | Maximum Point Temperature, °C Tmax | Enthalpy, (J/g) ∆H |
|---|---|---|
| ACC5 | 301.09 | −30.79 |
| ACC15 | 300.91 | −70.53 |
| ACC30 | 307.32 | −123.24 |
| ACC45 | 301.07 | −45.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaafar, M.Z.; Mohd Ridzuan, F.F.; Mohamad Kassim, M.H.; Abu, F. The Role of Dissolution Time on the Properties of All-Cellulose Composites Obtained from Oil Palm Empty Fruit Bunch. Polymers 2023, 15, 691. https://doi.org/10.3390/polym15030691
Jaafar MZ, Mohd Ridzuan FF, Mohamad Kassim MH, Abu F. The Role of Dissolution Time on the Properties of All-Cellulose Composites Obtained from Oil Palm Empty Fruit Bunch. Polymers. 2023; 15(3):691. https://doi.org/10.3390/polym15030691
Chicago/Turabian StyleJaafar, Mohd Zaim, Farah Fazlina Mohd Ridzuan, Mohamad Haafiz Mohamad Kassim, and Falah Abu. 2023. "The Role of Dissolution Time on the Properties of All-Cellulose Composites Obtained from Oil Palm Empty Fruit Bunch" Polymers 15, no. 3: 691. https://doi.org/10.3390/polym15030691
APA StyleJaafar, M. Z., Mohd Ridzuan, F. F., Mohamad Kassim, M. H., & Abu, F. (2023). The Role of Dissolution Time on the Properties of All-Cellulose Composites Obtained from Oil Palm Empty Fruit Bunch. Polymers, 15(3), 691. https://doi.org/10.3390/polym15030691






