Supramolecular Optimization of Sensory Function of a Hemicurcuminoid through Its Incorporation into Phospholipid and Polymeric Polydiacetylenic Vesicles: Experimental and Computational Insight
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Synthesis of the Mixed Vesicles
2.3. Methods
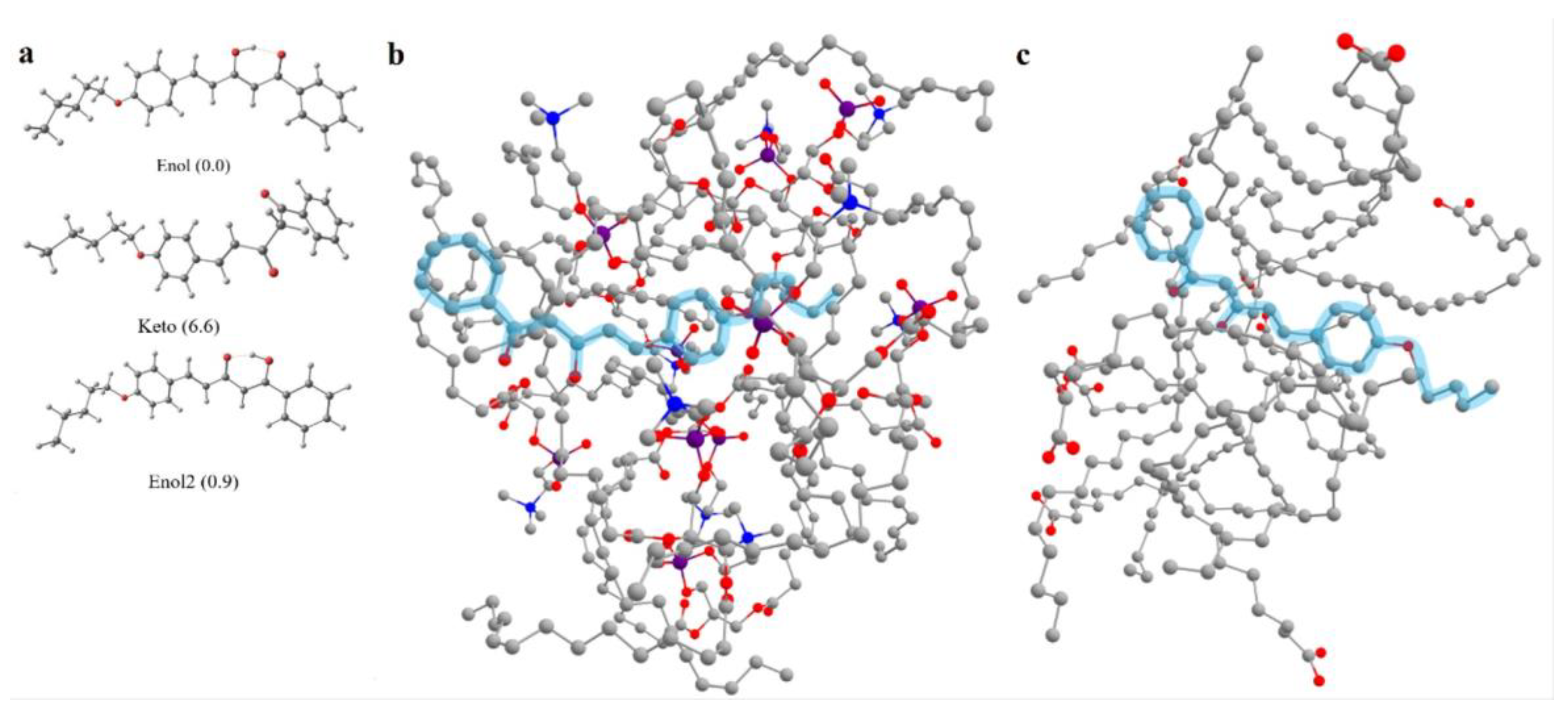
2.4. Computations
3. Results and Discussion
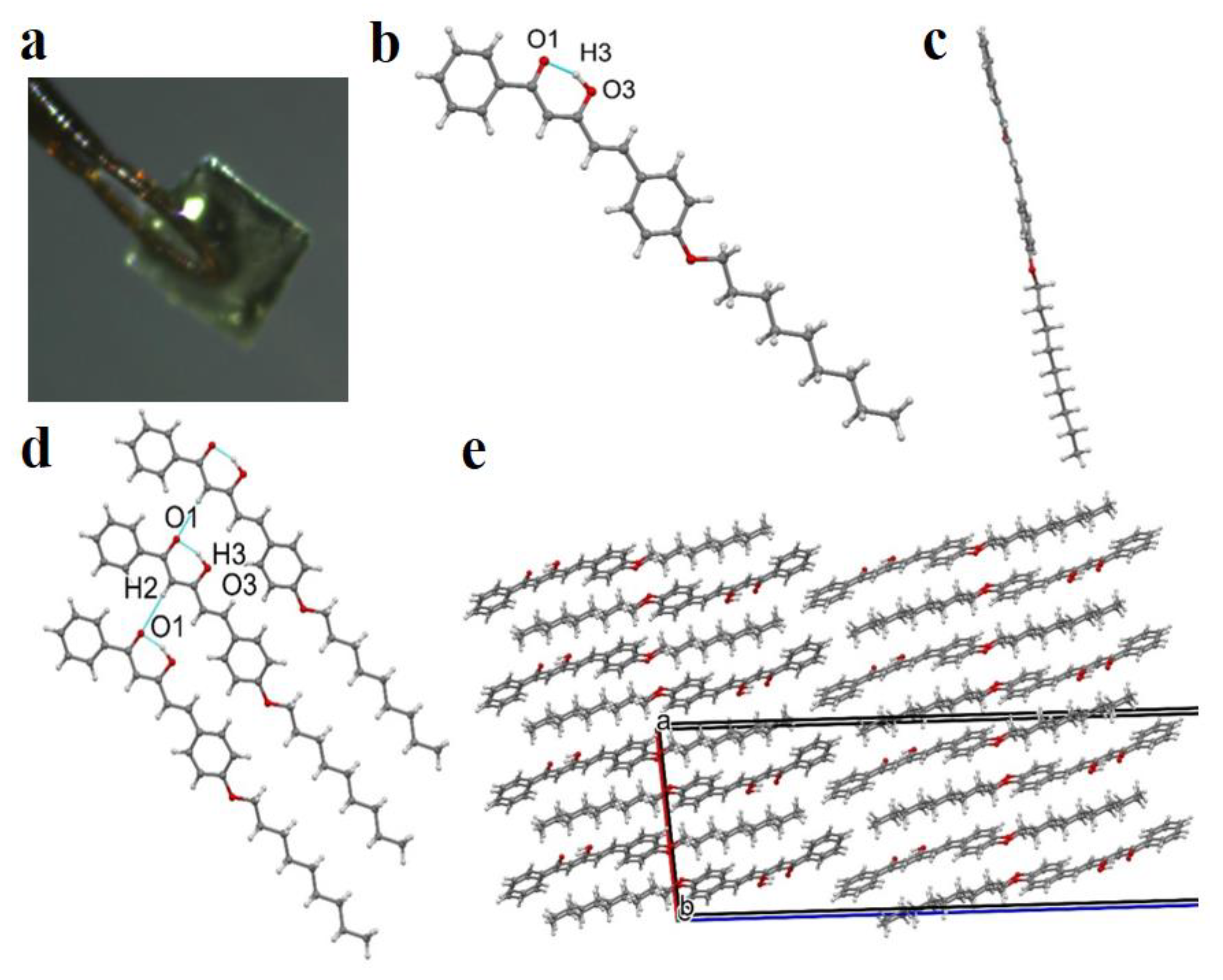
3.1. Synthesis and Structure of Hemicurcuminoids
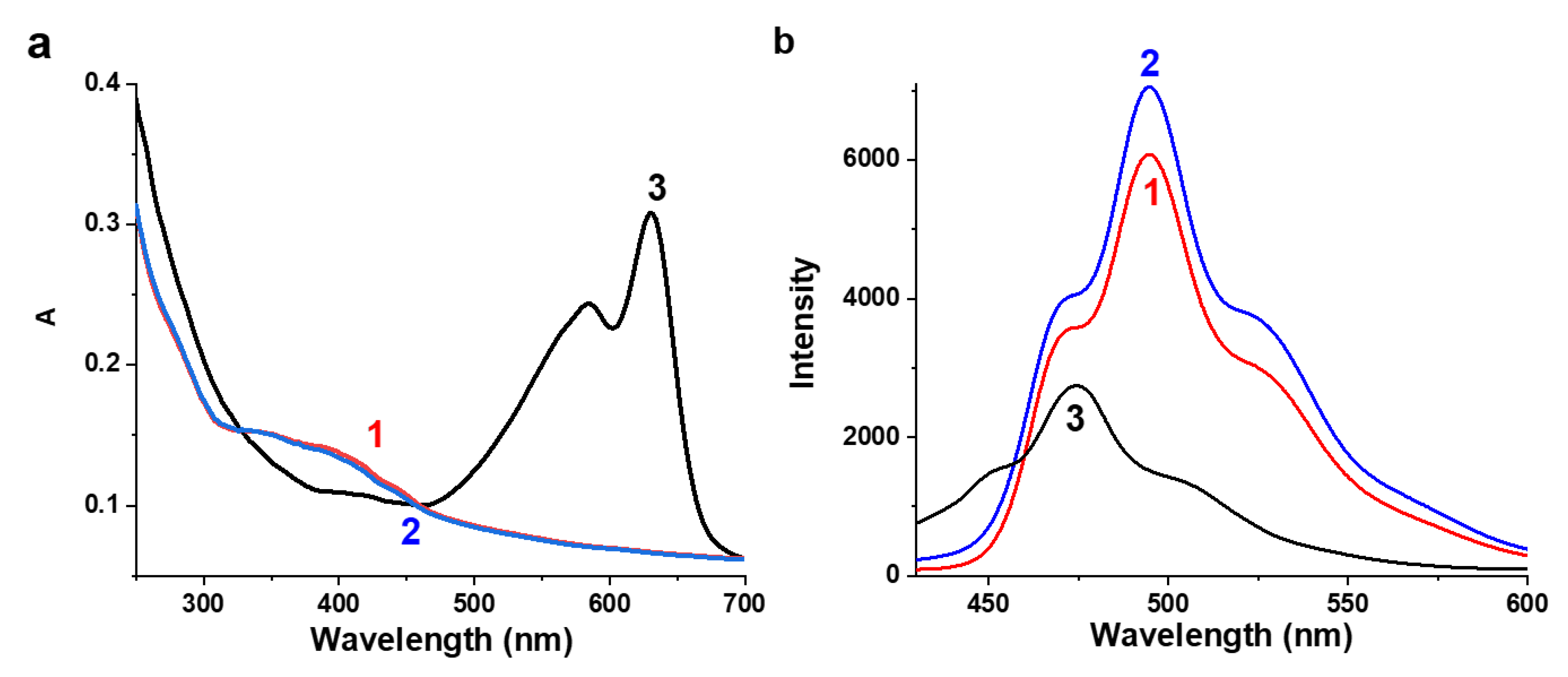
3.2. Synthesis and Spectral Properties of PDA-HCur, PC-HCur, PS-HCur
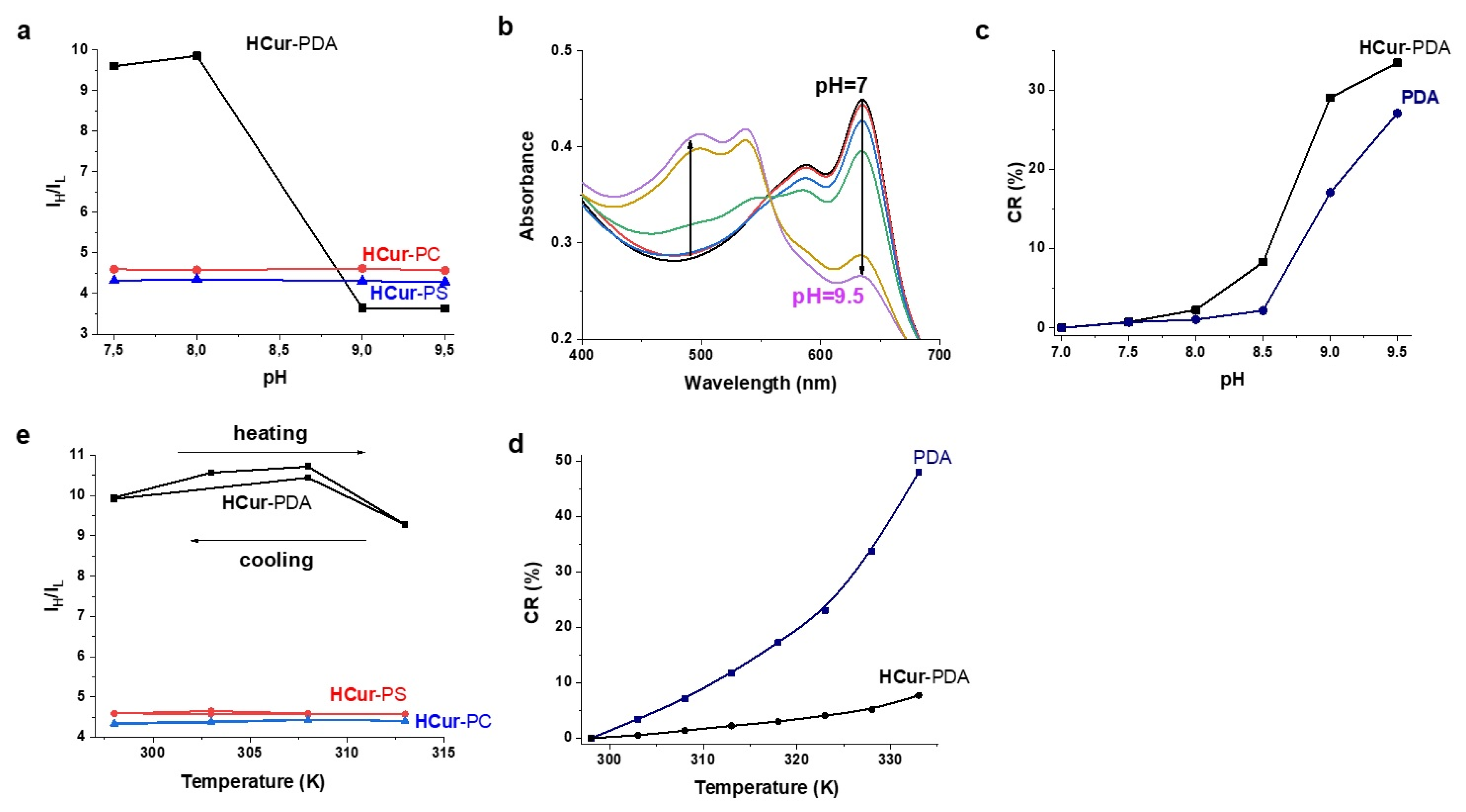
3.3. Dependence of Spectral Behavior of PDA-HCur, PC-HCur, PS-HCur on External Stimuli (pH, Heating, Metal Ions)
3.4. Sensitivity of PDA-HCur Fluorescence to Polyaminoacids and Proteins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ananda, A.; Moreira, R.; Henry, J.; Chowdhury, M.; Cote, G.; Good, T. A bio-sensing strategy for the detection of prions in foods. LWT—Food Sci. Technol. 2005, 38, 849–858. [Google Scholar] [CrossRef]
- Gal, N.; Morag, A.; Kolusheva, S.; Winter, R.; Landau, M.; Jelinek, R. Lipid Bilayers Significantly Modulate Cross-Fibrillation of Two Distinct Amyloidogenic Peptides. J. Am. Chem. Soc. 2013, 135, 13582–13589. [Google Scholar] [CrossRef]
- Grabowska, I.; Sharma, N.; Vasilescu, A.; Iancu, M.; Badea, G.; Boukherroub, R.; Ogale, S.; Szunerits, S. Electrochemical Aptamer-Based Biosensors for the Detection of Cardiac Biomarkers. ACS Omega 2018, 3, 12010–12018. [Google Scholar] [CrossRef] [Green Version]
- Healey, M.; Sivakumaran, M.; Platt, M. Rapid quantification of prion proteins using resistive pulse sensing. Analyst 2020, 145, 2595–2601. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Z.; Haque, F.; Guo, P. Engineering of protein nanopores for sequencing, chemical or protein sensing and disease diagnosis. Curr. Opin. Biotechnol. 2018, 51, 80–89. [Google Scholar] [CrossRef]
- Tyagi, A.; Liu, X.; Abidi, I.H.; Gao, Z.; Park, B.M.; Zeng, X.; Ou, X.; Cagang, A.A.; Zhuang, M.; Hossain, D.; et al. Modular functionalization of crystalline graphene by recombinant proteins: A nanoplatform for probing biomolecules. Nanoscale 2018, 10, 22572–22582. [Google Scholar] [CrossRef]
- Pandey, S.P.; Awasthi, A.A.; Singh, P.K. Supramolecular tuning of thioflavin-T aggregation hosted by polystyrene sulfonate. Phys. Chem. Chem. Phys. 2021, 23, 14716–14724. [Google Scholar] [CrossRef] [PubMed]
- Goshisht, M.K.; Tripathi, N. Fluorescence-based sensors as an emerging tool for anion detection: Mechanism, sensory materials and applications. J. Mater. Chem. C 2021, 9, 9820–9850. [Google Scholar] [CrossRef]
- Sevim, S.; Sorrenti, A.; Franco, C.; Furukawa, S.; Pané, S.; Demello, A.J.; Puigmartí-Luis, J. Self-assembled materials and supramolecular chemistry within microfluidic environments: From common thermodynamic states to non-equilibrium structures. Chem. Soc. Rev. 2018, 47, 3788–3803. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, M. Supramolecular chemistry-general principles and selected examples from anion recognition and metallosupramolecular chemistry. Naturwissenschaften 2007, 94, 951–966. [Google Scholar] [CrossRef]
- Barooah, N.; Mohanty, J.; Bhasikuttan, A.C. Cucurbituril-Based Supramolecular Assemblies: Prospective on Drug Delivery, Sensing, Separation, and Catalytic Applications. Langmuir 2022, 38, 6249–6264. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Aranda, F.J.; Teruel, J.A.; Ortiz, A. New pH-sensitive liposomes containing phosphatidylethanolamine and a bacterial dirhamnolipid. Chem. Phys. Lipids 2011, 164, 16–23. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Shao, T.; Lian, G.; Hu, K.; Liu, Y.; Zhou, M.; Wang, X.; Huang, L.; Meng, X.; et al. Simple and practical, highly sensitive and responsive recognition of cysteine: Design, synthesis and mechanism study of a novel curcumin fluorescent probe. Arab. J. Chem. 2022, 15, 104087. [Google Scholar] [CrossRef]
- Wang, F.; Huang, W.; Zhang, Y.; Wang, M.; Sun, L.; Tang, B.; Wang, W. Determination of Protein by Fluorescence Enhancement of Curcumin in Lanthanum-Curcumin-Sodium Dodecyl Benzene Sulfonate-Protein System. J. Fluoresc. 2010, 21, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Ran, C.; Xu, X.; Raymond, S.B.; Ferrara, B.J.; Neal, K.; Bacskai, B.J.; Medarova, Z.; Moore, A. Design, Synthesis, and Testing of Difluoroboron-Derivatized Curcumins as Near-Infrared Probes for in Vivo Detection of Amyloid-β Deposits. J. Am. Chem. Soc. 2009, 131, 15257–15261. [Google Scholar] [CrossRef] [Green Version]
- Khorasani, M.Y.; Langari, H.; Sany, S.B.T.; Rezayi, M.; Sahebkar, A. The role of curcumin and its derivatives in sensory applications. Mater. Sci. Eng. C 2019, 103, 109792. [Google Scholar] [CrossRef]
- Zakaria, H.; El Kurdi, R.; Patra, D. Curcumin-PLGA based nanocapsule for the fluorescence spectroscopic detection of dopamine. RSC Adv. 2022, 12, 28245–28253. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [Green Version]
- Lyu, H.; Wang, D.; Cai, L.; Wang, D.-J.; Li, X.-M. Synthesis, photophysical and solvatochromic properties of diacetoxyboron complexes with curcumin derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 220, 117126. [Google Scholar] [CrossRef] [PubMed]
- Reppy, M.A. Enhancing the Emission of Polydiacetylene Sensing Materials Through Fluorophore Addition and Energy Transfer. J. Fluoresc. 2008, 18, 461–471. [Google Scholar] [CrossRef]
- Sansee, A.; Kamphan, A.; Traiphol, R.; Kielar, F. Embedding luminescent iridium complex into polydiacetylene vesicles as a means of development of responsive luminescent system for imaging applications. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 497, 362–369. [Google Scholar] [CrossRef]
- Jose, D.A.; Stadlbauer, S.; König, B. Polydiacetylene-Based Colorimetric Self-Assembled Vesicular Receptors for Biological Phosphate Ion Recognition. Chem.—A Eur. J. 2009, 15, 7404–7412. [Google Scholar] [CrossRef]
- Mounica, A.; Balachandran, C.; Gopalakrishnan, D.; Sivasakthi, P.; Prakash, M.; Aoki, S.; Ganeshpandian, M. Synthesis and antiproliferative activity of novel organometallic cobalt(III) complex encapsulated in polydiacetylene-phospholipid nanoformulation. Inorganica Chim. Acta 2022, 530, 120701. [Google Scholar] [CrossRef]
- Akhmadeev, B.; Gerasimova, T.; Gilfanova, A.; Katsyuba, S.; Islamova, L.; Fazleeva, G.; Kalinin, A.; Daminova, A.; Fedosimova, S.; Amerhanova, S.; et al. Temperature-sensitive emission of dialkylaminostyrylhetarene dyes and their incorporation into phospholipid aggregates: Applicability for thermal sensing and cellular uptake behavior. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 268, 120647. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, G.; Peng, X.; Yoon, J. Biosensors and chemosensors based on the optical responses of polydiacetylenes. Chem. Soc. Rev. 2012, 41, 4610–4630. [Google Scholar] [CrossRef] [PubMed]
- Carpick, R.W.; Sasaki, D.Y.; Marcus, M.S.; Eriksson, M.A.; Burns, A.R. Polydiacetylene films: A review of recent investigations into chromogenic transitions and nanomechanical properties. J. Phys. Condens. Matter 2004, 16, R679–R697. [Google Scholar] [CrossRef] [Green Version]
- Reppy, M.A.; Pindzola, B.A. Biosensing with polydiacetylene materials: Structures, optical properties and applications. Chem. Commun. 2007, 42, 4317–4338. [Google Scholar] [CrossRef]
- Champaiboon, T.; Tumcharern, G.; Potisatityuenyong, A.; Wacharasindhu, S.; Sukwattanasinitt, M. A polydiacetylene multilayer film for naked eye detection of aromatic compounds. Sens. Actuators B Chem. 2009, 139, 532–537. [Google Scholar] [CrossRef]
- Su, Y.-L.; Li, J.-R.; Jiang, L. A study on the interactions of surfactants with phospholipid/polydiacetylene vesicles in aqueous solutions. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 257-258, 25–30. [Google Scholar] [CrossRef]
- de Oliveira, C.P.; Soares, N.D.F.F.; Fontes, E.A.F.; de Oliveira, T.V.; Filho, A.M.M. Behaviour of polydiacetylene vesicles under different conditions of temperature, pH and chemical components of milk. Food Chem. 2012, 135, 1052–1056. [Google Scholar] [CrossRef]
- Kolusheva, S.; Shahal, T.; Jelinek, R. Cation-Selective Color Sensors Composed of Ionophore−Phospholipid−Polydiacetylene Mixed Vesicles. J. Am. Chem. Soc. 2000, 122, 776–780. [Google Scholar] [CrossRef]
- Friedman, S.; Kolusheva, S.; Volinsky, R.; Zeiri, L.; Schrader, T.; Jelinek, R. Lipid/Polydiacetylene Films for Colorimetric Protein Surface-Charge Analysis. Anal. Chem. 2008, 80, 7804–7811. [Google Scholar] [CrossRef] [PubMed]
- Kolusheva, S.; Zadmard, R.; Schrader, T.; Jelinek, R. Color Fingerprinting of Proteins by Calixarenes Embedded in Lipid/Polydiacetylene Vesicles. J. Am. Chem. Soc. 2006, 128, 13592–13598. [Google Scholar] [CrossRef]
- Hartmann, H. Ein einfacher weg zu neuartigen borhaltigen spiroverbindungen. J. Für Prakt. Chemie 1986, 328, 755–762. [Google Scholar] [CrossRef]
- Klein, C.; Baranoff, E.; Nazeeruddin, K.; Grätzel, M. Convenient synthesis of functionalized 4,4′-disubstituted-2,2′-bipyridine with extended π-system for dye-sensitized solar cell applications. Tetrahedron Lett. 2010, 51, 6161–6165. [Google Scholar] [CrossRef]
- Akhmadeev, B.; Podyachev, S.; Katsyuba, S.; Spicher, S.; Sudakova, S.; Gimazetdinova, G.S.; Syakaev, V.; Sinyashin, O.; Mustafina, A. The incorporation of upper vs lower rim substituted thia- and calix[4]arene ligands into polydiacethylene polymeric bilayers for rational design of sensors to heavy metal ions. Polymer 2022, 245, 124728. [Google Scholar] [CrossRef]
- Sheldrick, G. SADABS; Program for Empirical X-ray Absorption Correction; Bruker-Nonius, University of Gottingen: Gottingen, Germany, 2004. [Google Scholar]
- Sheldrick, G. SHELXTL, v.6.12; Structure Determination Software Suite: Bruker AXS: Madison, WI, USA, 2000.
- APEX3, Version 2018.7-2; BrukerAXS Inc.: Madison, WI, USA, 2016.
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 389–397. [Google Scholar] [CrossRef]
- Pracht, P.; Bohle, F.; Grimme, S. Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys. Chem. Chem. Phys. 2020, 22, 7169–7192. [Google Scholar] [CrossRef]
- Grimme, S.; Bohle, F.; Hansen, A.; Pracht, P.; Spicher, S.; Stahn, M. Efficient Quantum Chemical Calculation of Structure Ensembles and Free Energies for Nonrigid Molecules. J. Phys. Chem. A 2021, 125, 4039–4054. [Google Scholar] [CrossRef]
- Grimme, S.; Hansen, A.; Ehlert, S.; Mewes, J.-M. r2SCAN-3c: A “Swiss army knife” composite electronic-structure method. J. Chem. Phys. 2021, 154, 064103. [Google Scholar] [CrossRef] [PubMed]
- Klamt, A.; Diedenhofen, M. Calculation of Solvation Free Energies with DCOSMO-RS. J. Phys. Chem. A 2015, 119, 5439–5445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckert, F.; Klamt, A. Fast solvent screening via quantum chemistry: COSMO-RS approach. AIChE J. 2002, 48, 369–385. [Google Scholar] [CrossRef] [Green Version]
- Eckert, F.; Klamt, A. COSMO Therm, Release 19; COSMOlogic GmbH & Co. KG: Leverkusen, Germany, 2012. [Google Scholar]
- Spicher, S.; Grimme, S. Single-point Hessian calculations for improved vibrational frequencies and rigid-rotor-harmonic-oscillator thermodynamics. J. Chem. Theory Comput. 2021, 17, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Supramolecular Binding Thermodynamics by Dispersion-Corrected Density Functional Theory. Chem.—A Eur. J. 2012, 18, 9955–9964. [Google Scholar] [CrossRef]
- Spicher, S.; Grimme, S. Efficient Computation of Free Energy Contributions for Association Reactions of Large Molecules. J. Phys. Chem. Lett. 2020, 11, 6606–6611. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef] [Green Version]
- Ehlert, S.; Stahn, M.; Spicher, S.; Grimme, S. Robust and Efficient Implicit Solvation Model for Fast Semiempirical Methods. J. Chem. Theory Comput. 2021, 17, 4250–4261. [Google Scholar] [CrossRef]
- Gross, E.; Dobson, J.; Petersilka, M.; Nalewajski, R.F. (Eds.) Density Functional Theory II; Springer: Berlin/Heidelberg, Germany, 1996; Chapter 181. [Google Scholar]
- Casida, M. Recent Advances in Density Functional Methods; World Scientific: Hackensack, NJ, USA, 1995. [Google Scholar]
- Furche, F. On the density matrix based approach to time-dependent density functional response theory. J. Chem. Phys. 2001, 114, 5982–5992. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Eichkorn, K.; Treutler, O.; Öhm, H.; Häser, M.; Ahlrichs, R. Auxiliary basis sets to approximate Coulomb potentials. Chem. Phys. Lett. 1995, 240, 283–290. [Google Scholar] [CrossRef]
- Eichkorn, K.; Weigend, F.; Treutler, O.; Ahlrichs, R. Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theor. Chem. Accounts 1997, 97, 119–124. [Google Scholar] [CrossRef]
- Weigend, F.; Häser, M.; Patzelt, H.; Ahlrichs, R. RI-MP2: Optimized auxiliary basis sets and demonstration of efficiency. Chem. Phys. Lett. 1998, 294, 143–152. [Google Scholar] [CrossRef]
- Spicher, S.; Plett, C.; Pracht, P.; Hansen, A.; Grimme, S. Automated Molecular Cluster Growing for Explicit Solvation by Efficient Force Field and Tight Binding Methods. J. Chem. Theory Comput. 2022, 18, 3174–3189. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Bannwarth, C.; Caldeweyher, E.; Pisarek, J.; Hansen, A. A general intermolecular force field based on tight-binding quantum chemical calculations. J. Chem. Phys. 2017, 147, 161708. [Google Scholar] [CrossRef]
- Spicher, S.; Grimme, S. Robust atomistic modeling of materials, organometallic, and biochemical systems. Angew. Chem. Int. Ed. 2020, 59, 15665–15673. [Google Scholar] [CrossRef]
- Furche, F.; Ahlrichs, R.; Hättig, C.; Klopper, W.; Sierka, M.; Weigend, F. TURBOMOLE V7.5.1 2021, a Development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH Since 2007. Available online: http://www.turbomole.com (accessed on 1 January 2023).
- Cornago, M.D.P.; Cabildo, P.; Sanz, D.; Claramunt, R.M.; Torralba, M.C.; Torres, M.R.; Elguero, J. Structures of Hemi-Curcuminoids in the Solid State and in Solution. Eur. J. Org. Chem. 2013, 2013, 6043–6054. [Google Scholar] [CrossRef]
- Tsaplev, Y.B.; Lapina, V.A.; Trofimov, A.V. Fluorescence of curcumin in alkaline dimethyl sulfoxide and the effects of alkali metal cations on it. J. Photochem. Photobiol. A Chem. 2021, 405, 112967. [Google Scholar] [CrossRef]
- Gobrogge, C.A.; Kong, V.A.; Walker, R.A. Temperature Dependent Solvation and Partitioning of Coumarin 152 in Phospholipid Membranes. J. Phys. Chem. B 2015, 120, 1805–1812. [Google Scholar] [CrossRef]
- Goto, M.; Wilk, A.; Kazama, A.; Chodankar, S.; Kohlbrecher, J.; Matsuki, H. Chain elongation of diacylphosphatidylcholine induces fully bilayer interdigitation under atmospheric pressure. Colloids Surfaces B Biointerfaces 2011, 84, 44–48. [Google Scholar] [CrossRef]
- Elistratova, J.; Akhmadeev, B.; Zairov, R.; Dovzhenko, A.; Podyachev, S.; Sudakova, S.; Syakaev, V.; Jelinek, R.; Kolusheva, S.; Mustafina, A. Tb(III) complexes with nonyl-substituted calix[4]arenes as building blocks of hydrophilic luminescent mixed polydiacetylene-based aggregates. J. Mol. Liq. 2018, 268, 463–470. [Google Scholar] [CrossRef]
- Gerasimova, T.P.; Burganov, T.I.; Katsyuba, S.A.; Kalinin, A.A.; Islamova, L.N.; Fazleeva, G.M.; Ahmadeev, B.S.; Mustafina, A.R.; Monari, A.; Assfeld, X.; et al. Halochromic luminescent quinoxalinones as a basis for pH-sensing in organic and aqueous solutions. Dye. Pigment. 2021, 186, 108958. [Google Scholar] [CrossRef]
- Tanaka, H.; Gomez, M.A.; Tonelli, A.E.; Lovinger, A.J.; Davis, D.D.; Thakur, M. Structural and morphological study of a melt-crystallized polydiacetylene. Macromolecules 1989, 22, 2427–2432. [Google Scholar] [CrossRef]
- Jiang, H.; Jelinek, R. Mixed Diacetylene/Octadecyl Melamine Nanowires Formed at the Air/Water Interface Exhibit Unique Structural and Colorimetric Properties. Langmuir 2015, 31, 5843–5850. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Song, J.E.; Yoon, B.; Kim, J.M.; Cho, E.C. Role of Gel to Fluid Transition Temperatures of Polydiacetylene Vesicles with 10,12-Pentacosadiynoic Acid and Cholesterol in Their Thermochromisms. Bull. Korean Chem. Soc. 2014, 35, 1809–1816. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Wang, Y.; Jiang, H.; Zou, G.; Zhang, Q. Benzo-15-crown-5 functionalized polydiacetylene-based colorimetric self-assembled vesicular receptors for lead ion recognition. J. Mater. Chem. 2011, 21, 3604–3610. [Google Scholar] [CrossRef]
- Jose, D.A.; König, B. Polydiacetylenevesicles functionalized with N-heterocyclic ligands for metal cation binding. Org. Biomol. Chem. 2010, 8, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, F.; Wang, Y.; Zhang, W.; Chen, X. Polydiacetylene-based sensor for highly sensitive and selective Pb2+ detection. Dye. Pigment. 2015, 120, 307–313. [Google Scholar] [CrossRef]
- Guo, J.; Yang, L.; Zhu, L.; Chen, D. Selective detection of metal ions based on nanocrystalline ionochromic polydiacetylene. Polymer 2013, 54, 743–749. [Google Scholar] [CrossRef]
- Batys, P.; Morga, M.; Bonarek, P.; Sammalkorpi, M. pH-Induced Changes in Polypeptide Conformation: Force-Field Comparison with Experimental Validation. J. Phys. Chem. B 2020, 124, 2961–2972. [Google Scholar] [CrossRef]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 2017, 27, 112–128. [Google Scholar] [CrossRef] [PubMed]



| PdI | Size by Intensity (dI, nm) | Size by Volume (dI, nm) | |
|---|---|---|---|
| HCur-PC | 0.430 | 47 ± 8 265 ± 88 | 43 ± 9 274 ± 99 |
| HCur-PS | 0.594 | 125 ± 63 | 66 ± 49 |
| HCur-PDA | 0.450 | 271 ± 100 | 283 ± 115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhmadeev, B.S.; Retyunskaya, O.O.; Podyachev, S.N.; Katsyuba, S.A.; Gubaidullin, A.T.; Sudakova, S.N.; Syakaev, V.V.; Babaev, V.M.; Sinyashin, O.G.; Mustafina, A.R. Supramolecular Optimization of Sensory Function of a Hemicurcuminoid through Its Incorporation into Phospholipid and Polymeric Polydiacetylenic Vesicles: Experimental and Computational Insight. Polymers 2023, 15, 714. https://doi.org/10.3390/polym15030714
Akhmadeev BS, Retyunskaya OO, Podyachev SN, Katsyuba SA, Gubaidullin AT, Sudakova SN, Syakaev VV, Babaev VM, Sinyashin OG, Mustafina AR. Supramolecular Optimization of Sensory Function of a Hemicurcuminoid through Its Incorporation into Phospholipid and Polymeric Polydiacetylenic Vesicles: Experimental and Computational Insight. Polymers. 2023; 15(3):714. https://doi.org/10.3390/polym15030714
Chicago/Turabian StyleAkhmadeev, Bulat S., Olga O. Retyunskaya, Sergey N. Podyachev, Sergey A. Katsyuba, Aidar T. Gubaidullin, Svetlana N. Sudakova, Victor V. Syakaev, Vasily M. Babaev, Oleg G. Sinyashin, and Asiya R. Mustafina. 2023. "Supramolecular Optimization of Sensory Function of a Hemicurcuminoid through Its Incorporation into Phospholipid and Polymeric Polydiacetylenic Vesicles: Experimental and Computational Insight" Polymers 15, no. 3: 714. https://doi.org/10.3390/polym15030714
APA StyleAkhmadeev, B. S., Retyunskaya, O. O., Podyachev, S. N., Katsyuba, S. A., Gubaidullin, A. T., Sudakova, S. N., Syakaev, V. V., Babaev, V. M., Sinyashin, O. G., & Mustafina, A. R. (2023). Supramolecular Optimization of Sensory Function of a Hemicurcuminoid through Its Incorporation into Phospholipid and Polymeric Polydiacetylenic Vesicles: Experimental and Computational Insight. Polymers, 15(3), 714. https://doi.org/10.3390/polym15030714










