Effect of OH-Group Introduction on Gas and Liquid Separation Properties of Polydecylmethylsiloxane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
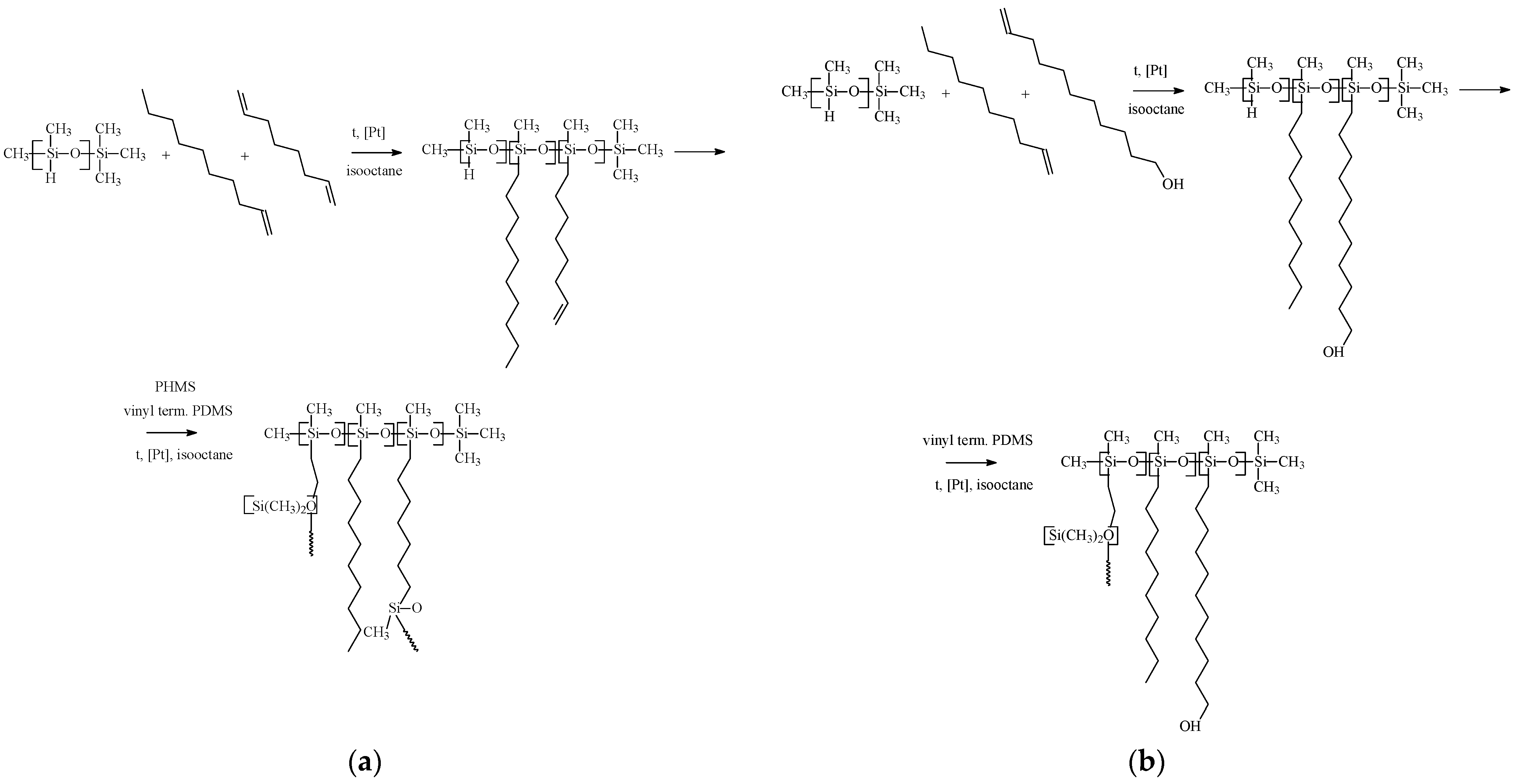
2.2. Membrane Materials Synthesis
2.3. Development Flat Sheet Composite Membrane
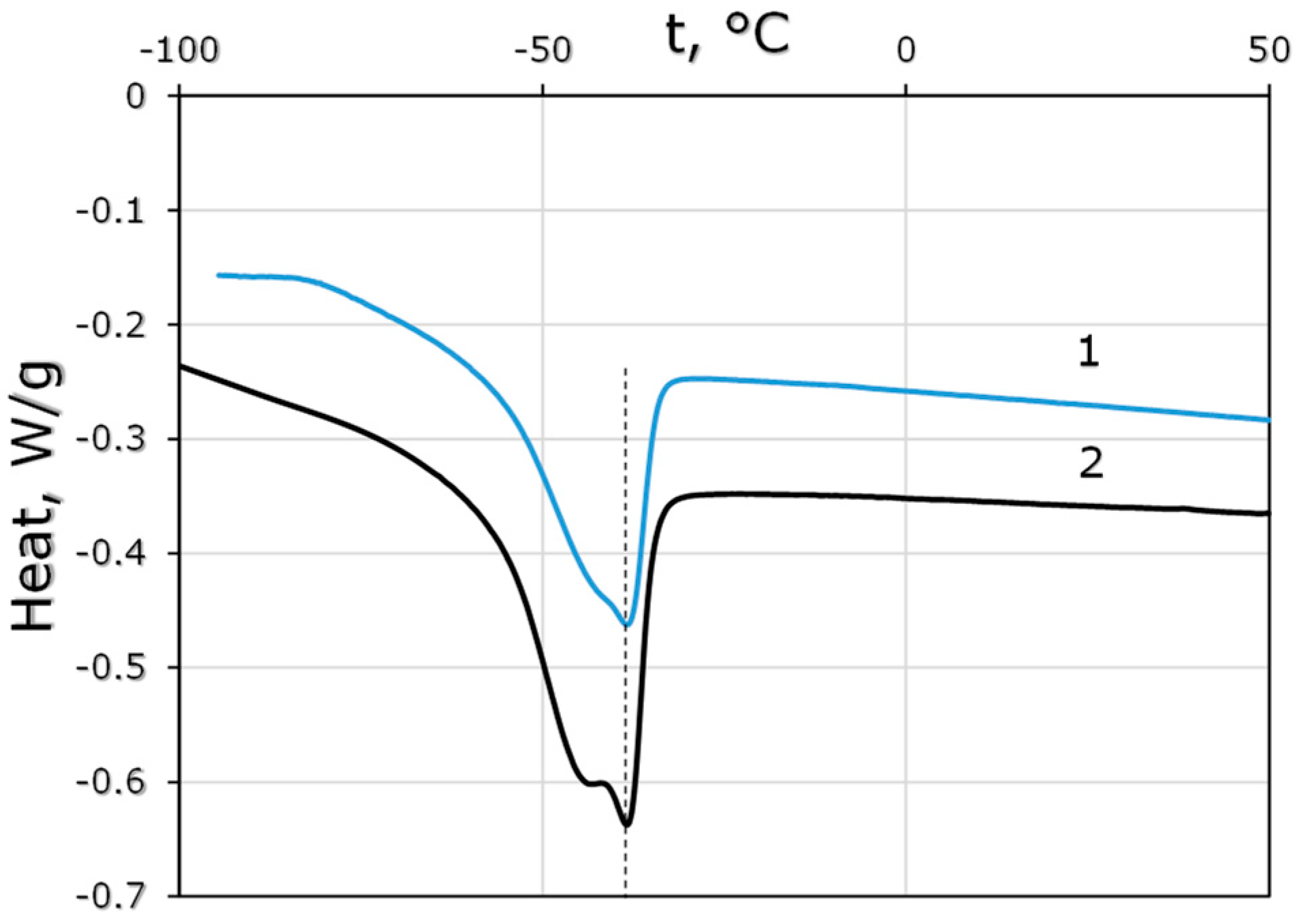
2.4. Differential-Scanning Calorimetry (DSC)
2.5. Nuclear Magnetic Resonance (NMR)
2.6. Scanning Electron Microscopy (SEM)
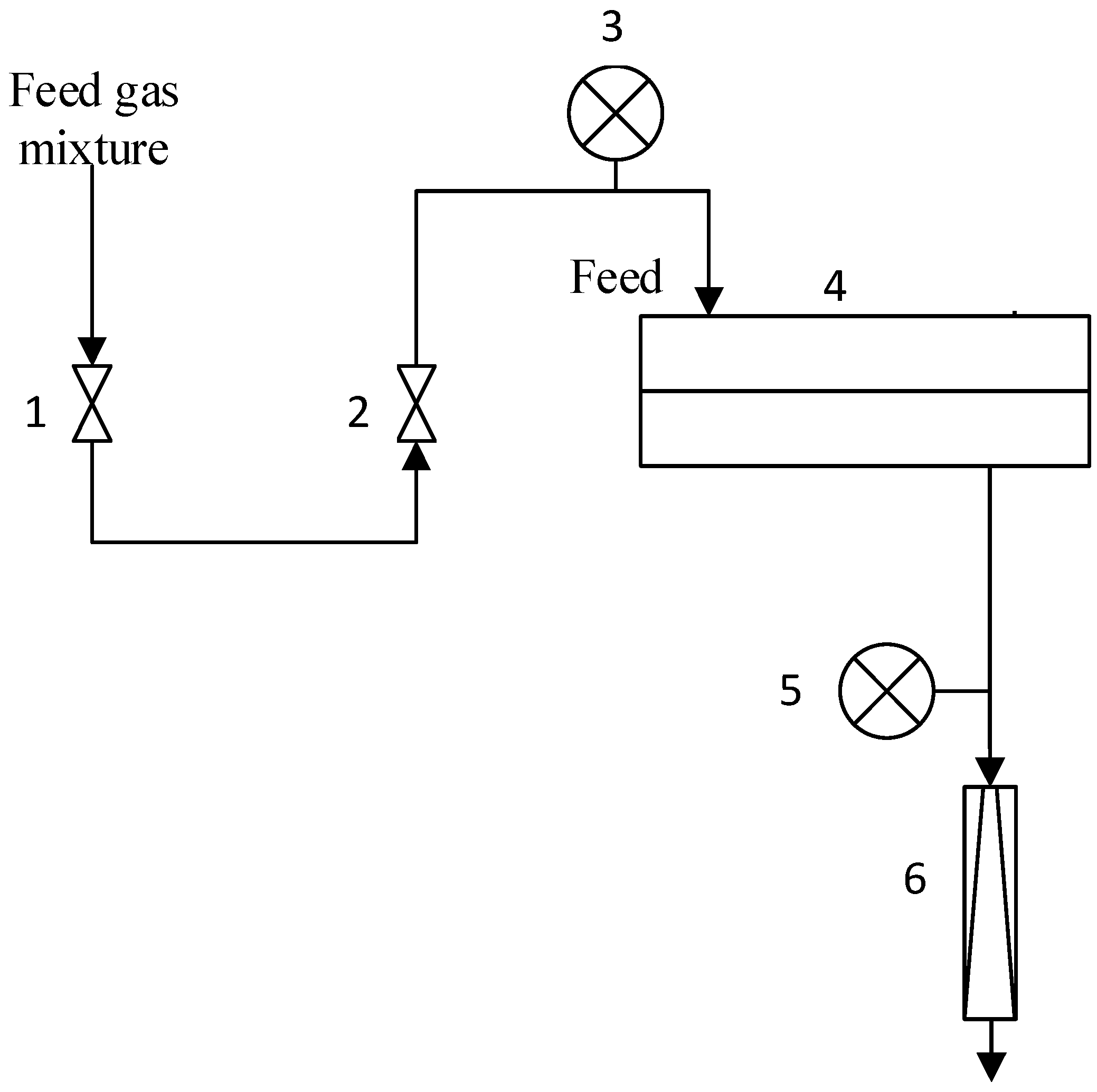
2.7. Gas Transport Properties Measurments
2.8. Sorption Measurments
2.9. Study of Aldehyde and Olefin Transport through PDecMS-Based Membranes in the Vacuum Pervaporation Mode
3. Results
3.1. Membrane Material Characterization
3.1.1. Polymer Properties
3.1.2. Gas Transport Properties of the Membrane Materials
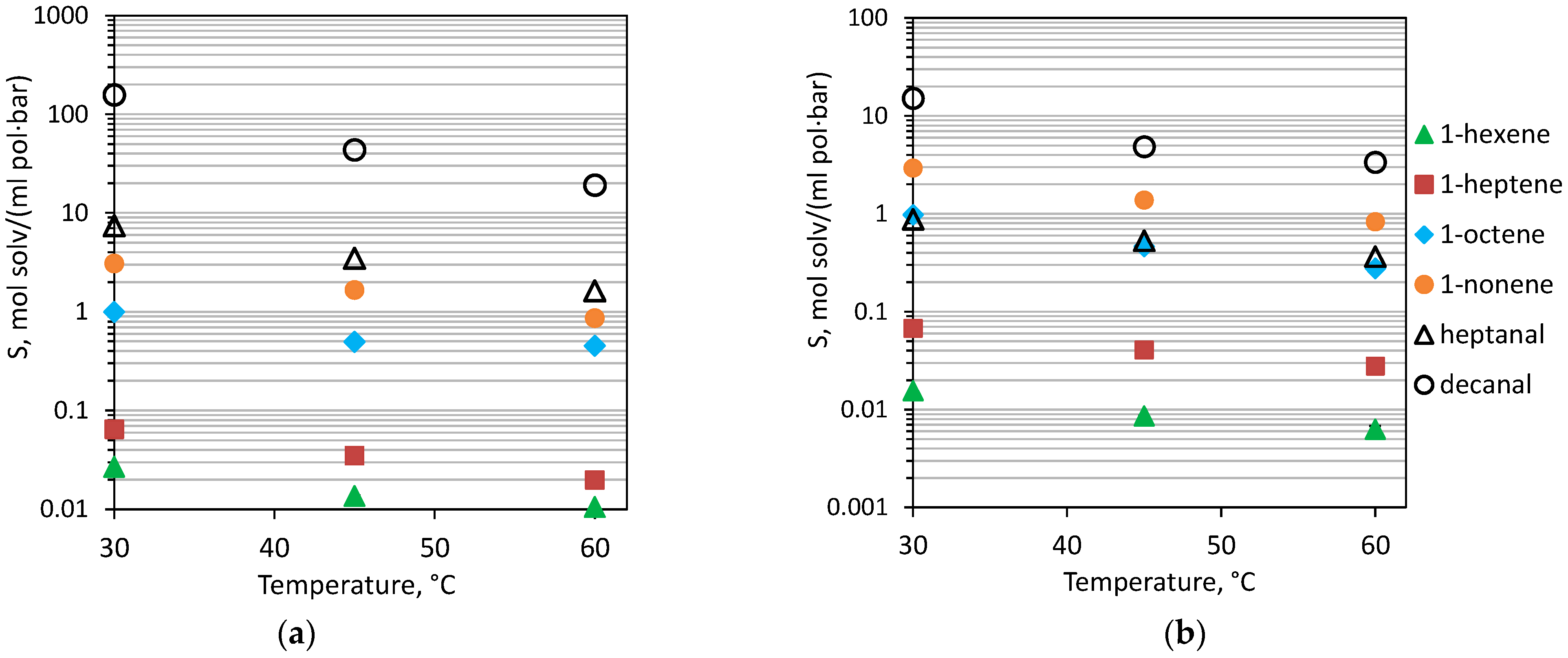
3.1.3. Sorption Hydrophobic and Hydrophilic Liquids in PDecMS and OH-PDecMS
3.2. Composite Membrane Characterisation
3.2.1. Gas Permeation Properties of Composite Membranes
3.2.2. Mass Transport of Olefines and Aldehydes through Composite Membranes in Vacuum Pervaporation Mode
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jue, M.L.; Lively, R.P. Targeted Gas Separations through Polymer Membrane Functionalization. React. Funct. Polym. 2015, 86, 88–110. [Google Scholar] [CrossRef]
- Russo, F.; Galiano, F.; Iulianelli, A.; Basile, A.; Figoli, A. Biopolymers for Sustainable Membranes in CO2 Separation: A Review. Fuel Process. Technol. 2021, 213, 106643. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Leo, C.P.; Ahmad, A.L.; Ramli, W.K.W. Membranes with Great Hydrophobicity: A Review on Preparation and Characterization. Sep. Purif. Rev. 2015, 44, 109–134. [Google Scholar] [CrossRef]
- Grushevenko, E.A.; Borisov, I.L.; Volkov, A.V. High-Selectivity Polysiloxane Membranes for Gases and Liquids Separation (A Review). Pet. Chem. 2021, 61, 959–976. [Google Scholar] [CrossRef]
- Alent’ev, A.Y.; Volkov, A.V.; Vorotyntsev, I.V.; Maksimov, A.L.; Yaroslavtsev, A.B. Membrane Technologies for Decarbonization. Membr. Membr. Technol. 2021, 3, 255–273. [Google Scholar] [CrossRef]
- Kwon, Y.; Lee, S.Y.; Hong, S.; Jang, J.H.; Henkensmeier, D.; Yoo, S.J.; Kim, H.-J.; Kim, S.-H. Novel Sulfonated Poly(Arylene Ether Sulfone) Containing Hydroxyl Groups for Enhanced Proton Exchange Membrane Properties. Polym. Chem. 2015, 6, 233–239. [Google Scholar] [CrossRef]
- Alentiev, D.A.; Nikiforov, R.Y.; Rudakova, M.A.; Zarezin, D.P.; Topchiy, M.A.; Asachenko, A.F.; Alentiev, A.Y.; Bolshchikov, B.D.; Belov, N.A.; Finkelshtein, E.S.; et al. Polynorbornenes Bearing Ether Fragments in Substituents: Promising Membrane Materials with Enhanced CO2 Permeability. J. Membr. Sci. 2022, 648, 120340. [Google Scholar] [CrossRef]
- Deng, J.; Bai, L.; Zeng, S.; Zhang, X.; Nie, Y.; Deng, L.; Zhang, S. Ether-Functionalized Ionic Liquid Based Composite Membranes for Carbon Dioxide Separation. RSC Adv. 2016, 6, 45184–45192. [Google Scholar] [CrossRef]
- Favre, E. Carbon dioxide recovery from post-combustion processes: Can gas permeation membranes compete with absorption? J. Membr. Sci. 2007, 294, 50–59. [Google Scholar] [CrossRef]
- Xu, J.; Wu, H.; Wang, Z.; Qiao, Z.; Zhao, S.; Wang, J. Recent Advances on the Membrane Processes for CO2 Separation. Chin. J. Chem. Eng. 2018, 26, 2280–2291. [Google Scholar] [CrossRef]
- Sarfraz, M.; Ba-Shammakh, M. Synergistic Effect of Adding Graphene Oxide and ZIF-301 to Polysulfone to Develop High Performance Mixed Matrix Membranes for Selective Carbon Dioxide Separation from Post Combustion Flue Gas. J. Membr. Sci. 2016, 514, 35–43. [Google Scholar] [CrossRef]
- Kiadehi, A.D.; Rahimpour, A.; Jahanshahi, M.; Ghoreyshi, A.A. Novel Carbon Nano-Fibers (CNF)/Polysulfone (PSf) Mixed Matrix Membranes for Gas Separation. J. Ind. Eng. Chem. 2015, 22, 199–207. [Google Scholar] [CrossRef]
- Yave, W.; Car, A.; Funari, S.S.; Nunes, S.P.; Peinemann, K.-V. CO2-Philic Polymer Membrane with Extremely High Separation Performance. Macromolecules 2010, 43, 326–333. [Google Scholar] [CrossRef]
- Bondar, V.I.; Freeman, B.D.; Pinnau, I. Gas Transport Properties of Poly(Ether-b-Amide) Segmented Block Copolymers. J. Polym. Sci. B Polym. Phys. 2000, 38, 2051–2062. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.; Li, W.; Liu, Y.; Wang, J.; Wang, S. High-Performance Multilayer Composite Membranes with Mussel-Inspired Polydopamine as a Versatile Molecular Bridge for CO2 Separation. ACS Appl. Mater. Interfaces 2015, 7, 15481–15493. [Google Scholar] [CrossRef] [PubMed]
- Robeson, L.M. The Upper Bound Revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Suleman, M.S.; Lau, K.K.; Yeong, Y.F. Enhanced Gas Separation Performance of PSF Membrane after Modification to PSF/PDMS Composite Membrane in CO2/CH4 Separation. J. Appl. Polym. Sci. 2018, 135, 45650. [Google Scholar] [CrossRef]
- Suleman, M.S.; Lau, K.K.; Yeong, Y.F. Development and Performance Evaluation of Polydimethyl Siloxane/Polysulfone (PDMS/PSF) Composite Membrane for CO2/CH4 Separation. IOP Conf. Ser. Earth Environ. Sci. 2016, 36, 012014. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Liu, G.; Wei, W.; Xiangli, F.; Jin, W. Ceramic Supported PDMS and PEGDA Composite Membranes for CO2 Separation. Chin. J. Chem. Eng. 2013, 21, 348–356. [Google Scholar] [CrossRef]
- Stern, S.A.; Shah, V.M.; Hardy, B.J. Structure-Permeability Relationships in Silicone Polymers. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 1263–1298. [Google Scholar] [CrossRef]
- Senthilkumar, U.; Reddy, B.S.R. Polysiloxanes with Pendent Bulky Groups Having Amino-Hydroxy Functionality: Structure–Permeability Correlation. J. Membr. Sci. 2007, 292, 72–79. [Google Scholar] [CrossRef]
- Prabhakar, R.S.; Raharjo, R.; Toy, L.G.; Lin, H.; Freeman, B.D. Self-Consistent Model of Concentration and Temperature Dependence of Permeability in Rubbery Polymers. Ind. Eng. Chem. Res. 2005, 44, 1547–1556. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Merkel, T.C.; Gupta, R.P.; Turk, B.S.; Freeman, B.D. Mixed-Gas Permeation of Syngas Components in Poly(Dimethylsiloxane) and Poly(1-Trimethylsilyl-1-Propyne) at Elevated Temperatures. J. Membr. Sci. 2001, 191, 85–94. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, J.H.; Lee, S.B. Sorption and Permeation Behaviors of a Series of Olefins and Nitrogen through PDMS Membranes. J. Membr. Sci. 2007, 299, 54–62. [Google Scholar] [CrossRef]
- Catarino, M.; Ferreira, A.; Mendes, A. Study and Optimization of Aroma Recovery from Beer by Pervaporation. J. Membr. Sci. 2009, 341, 51–59. [Google Scholar] [CrossRef]
- Börjesson, J.; Karlsson, H.O.E.; Trägårdh, G. Pervaporation of a Model Apple Juice Aroma Solution: Comparison of Membrane Performance. J. Membr. Sci. 1996, 119, 229–239. [Google Scholar] [CrossRef]
- Olsson, J.; Trägårdh, G.; Lipnizki, F. The Influence of Permeant and Membrane Properties on Mass Transfer in Pervaporation of Volatile Organic Compounds from Dilute Aqueous Solutions. Sep. Sci. Technol. 2002, 37, 1199–1223. [Google Scholar] [CrossRef]
- Borisov, I.; Podtynnikov, I.; Grushevenko, E.; Scharova, O.; Anokhina, T.; Makaev, S.; Volkov, A.; Volkov, V. High Selective Composite Polyalkylmethylsiloxane Membranes for Pervaporative Removal of MTBE from Water: Effect of Polymer Side-Chain. Polymers 2020, 12, 1213. [Google Scholar] [CrossRef]
- Bennett, M.; Brisdon, B.J.; England, R.; Field, R.W. Performance of PDMS and Organofunctionalised PDMS Membranes for the Pervaporative Recovery of Organics from Aqueous Streams. J. Membr. Sci. 1997, 137, 63–88. [Google Scholar] [CrossRef]
- León, J.A.; Fontalvo, J. PDMS Modified Membranes by 1-Dodecanol and Its Effect on Ethanol Removal by Pervaporation. Sep. Purif. Technol. 2019, 210, 364–370. [Google Scholar] [CrossRef]
- Logemann, M.; Alders, M.; Wist, M.; Pyankova, V.; Krakau, D.; Gottschalk, D.; Wessling, M. Can PDMS membranes separate aldehydes and alkenes at high temperatures? J. Membr. Sci. 2020, 615, 118334. [Google Scholar] [CrossRef]
- Kossov, A.A.; Yushkin, A.A.; Khotimskiy, V.S.; Volkov, A.V. Study of accessible free volume and transport properties of TFPS-co-TMSP copolymer. Pet. Chem. 2015, 55, 783–790. [Google Scholar] [CrossRef]
- Lejeune, A.; Rabiller-Baudry, M.; Renouard, T. Design of Membrane Cascades According to the Method of McCabe-Thiele: An Organic Solvent Nanofiltration Case Study for Olefin Hydroformylation in Toluene. Sep. Purif. Technol. 2018, 195, 339–357. [Google Scholar] [CrossRef]
- Kim, J.S.; Datta, R. Supported Liquid-Phase Catalytic Membrane Reactor–Separator for Homogeneous Catalysis. AIChE J. 1991, 37, 1657–1667. [Google Scholar] [CrossRef]
- Ramezani, M.; Kashfipour, M.A.; Abolhasani, M. Minireview: Flow Chemistry Studies of High-Pressure Gas-Liquid Reactions with Carbon Monoxide and Hydrogen. J. Flow Chem. 2020, 10, 93–101. [Google Scholar] [CrossRef]
- Gorbunov, D.N.; Volkov, A.; Kardasheva, Y.S.; Maksimov, A.L.; Karakhanov, E.A. Hydroformylation in Petroleum Chemistry and Organic Synthesis: Implementation of the Process and Solving the Problem of Recycling Homogeneous Catalysts (Review). Pet. Chem. 2015, 55, 587–603. [Google Scholar] [CrossRef]
- Priske, M.; Wiese, K.D.; Drews, A.; Kraume, M.; Baumgarten, G. Reaction Integrated Separation of Homogenous Catalysts in the Hydroformylation of Higher Olefins by Means of Organophilic Nanofiltration. J. Membr. Sci. 2010, 360, 77–83. [Google Scholar] [CrossRef]
- Logemann, M.; Marinkovic, J.M.; Schörner, M.; García-Suárez, E.J.; Hecht, C.; Franke, R.; Wessling, M.; Riisager, A.; Fehrmann, R.; Haumann, M. Continuous Gas-Phase Hydroformylation of but-1-Ene in a Membrane Reactor by Supported Liquid-Phase (SLP) Catalysis. Green Chem. 2020, 22, 5691–5700. [Google Scholar] [CrossRef]
- Udayakumar, V.; Alexander, S.; Gayathri, V.; Shivakumaraiah; Patil, K.R.; Viswanathan, B. Polymer-Supported Palladium-Imidazole Complex Catalyst for Hydrogenation of Substituted Benzylideneanilines. J. Mol. Catal. A Chem. 2010, 317, 111–117. [Google Scholar] [CrossRef]
- Zhuchkov, D.P.; Nenasheva, M.V.; Terenina, M.V.; Kardasheva, Y.S.; Gorbunov, D.N.; Karakhanov, E.A. Polymeric Heterogeneous Catalysts in the Hydroformylation of Unsaturated Compounds. Pet. Chem. 2021, 61, 1–14. [Google Scholar] [CrossRef]
- Brunetti, A.; Zito, P.F.; Borisov, I.; Grushevenko, E.; Volkov, V.; Volkov, A.; Barbieri, G. CO2 Separation from Humidified Ternary Gas Mixtures Using a Polydecylmethylsiloxane Composite Membrane. Fuel Process. Technol. 2020, 210, 106550. [Google Scholar] [CrossRef]
- Grushevenko, E.A.; Rohmanka, T.N.; Dibrov, G.A.; Volkov, V.V.; Volkov, A.V. Evaluation of the Efficiency of Polydecylmethylsiloxane in the Separation of a 1-Hexene–Heptanal Mixture. Membr. Membr. Technol. 2022, 4, 357–366. [Google Scholar] [CrossRef]
- Wei, J.-Z.; Lang, J.-W.; Fu, H.-Y.; Li, R.-X.; Zheng, X.-L.; Yuan, M.-L.; Chen, H. Aqueous Biphasic Hydroformylation of Higher Alkenes and Highly Efficient Catalyst Recycling in the Presence of a Polar Low Boiling Solvent. Transit. Met. Chem. 2016, 41, 599–603. [Google Scholar] [CrossRef]
- Diebolt, O.; Müller, C.; Vogt, D. “On-Water” Rhodium-Catalysed Hydroformylation for the Production of Linear Alcohols. Catal. Sci. Technol. 2012, 2, 773–777. [Google Scholar] [CrossRef]
- Macdougall, J.K.; Simpson, M.C.; Cole-Hamilton, D.J. Metal Hydroxycarbene-like Intermediates in the Hydrocarbonylation of Alkenes to Alcohols Catalysed by Rhodium Complexes. Polyhedron 1993, 12, 2877–2881. [Google Scholar] [CrossRef]
- Kang, N.; Du, Z.; Li, H.; Zhang, C. Synthesis of Polysiloxane-Type Multifunctional Flame Retardant and Its Application in Epoxy Systems. J. Appl. Polym. Sci. 2011, 124, 4915–4919. [Google Scholar] [CrossRef]
- Morgan, A.M.; Pollack, S.K.; Beshah, K. Synthesis and Multidimensional NMR Characterization of PDMS- b -PS Prepared by Combined Anionic Ring-Opening and Nitroxide-Mediated Radical Polymerization. Macromolecules 2002, 35, 4238–4246. [Google Scholar] [CrossRef]
- Merkel, T.C.; Bondar, V.I.; Nagai, K.; Freeman, B.D.; Pinnau, I. Gas Sorption, Diffusion, and Permeation in Poly(Dimethylsiloxane). J. Polym. Sci. Part B Polym. Phys. 2000, 38, 415–434. [Google Scholar] [CrossRef]
- Teplyakov, V.; Meares, P. Correlation Aspects of the Selective Gas Permeabilities of Polymeric Materials and Membranes. Gas Sep. Purif. 1990, 4, 66–74. [Google Scholar] [CrossRef]
- Borisov, I.L.; Grushevenko, E.A.; Anokhina, T.S.; Bakhtin, D.S.; Levin, I.S.; Bondarenko, G.N.; Volkov, V.V.; Volkov, A.V. Influence of side chains assembly on the structure and transport properties of comb-like polysiloxanes in hydrocarbon separation. Mater. Today Chem. 2021, 22, 100598. [Google Scholar] [CrossRef]
- Raharjo, R.D.; Freeman, B.D.; Sanders, E.S. Pure and Mixed Gas CH4 and N-C4H10 Sorption and Dilation in Poly(Dimethylsiloxane). J. Membr. Sci. 2007, 292, 45–61. [Google Scholar] [CrossRef]
- van Krevelen, D.W.; te Nijenhuis, K. Properties of Polymers Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions, 4th ed.; Elsevier: Oxford, UK, 2009; ISBN 978-0-08-054819-7. [Google Scholar]
- Hansen, C. (Ed.) Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-0-8493-7248-3. [Google Scholar]
- Dibrov, G.A.; Novitsky, E.G.; Vasilevsky, V.P.; Volkov, V.V. Cold Rolling for Controllable Narrowing of Pore Size and Pore Size Distribution of Commercial Fluoroplastic Microfiltration Membrane. Pet. Chem. 2014, 54, 568–572. [Google Scholar] [CrossRef]
- Huang, L.; McCutcheon, J.R. Impact of Support Layer Pore Size on Performance of Thin Film Composite Membranes for Forward Osmosis. J. Membr. Sci. 2015, 483, 25–33. [Google Scholar] [CrossRef]
- Banihashemi, F.; Pakizeh, M.; Ahmadpour, A. CO2 Separation Using PDMS/ZSM-5 Zeolite Composite Membrane. Sep. Purif. Technol. 2011, 79, 293–302. [Google Scholar] [CrossRef]
- Li, P.; Chen, H.Z.; Chung, T.-S. The Effects of Substrate Characteristics and Pre-Wetting Agents on PAN–PDMS Composite Hollow Fiber Membranes for CO2/N2 and O2/N2 Separation. J. Membr. Sci. 2013, 434, 18–25. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K.-V. Pebax®/Polyethylene Glycol Blend Thin Film Composite Membranes for CO2 Separation: Performance with Mixed Gases. Sep. Purif. Technol. 2008, 62, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Schultz, J.; Peinemann, K.-V. Membranes for Separation of Higher Hydrocarbons from Methane. J. Membr. Sci. 1996, 110, 37–45. [Google Scholar] [CrossRef]
- Pinnau, I.; He, Z. Pure- and Mixed-Gas Permeation Properties of Polydimethylsiloxane for Hydrocarbon/Methane and Hydrocarbon/Hydrogen Separation. J. Membr. Sci. 2004, 244, 227–233. [Google Scholar] [CrossRef]
- Zhmakin, V.V.; Teplyakov, V.V. The Evaluation of the C1–C4 Hydrocarbon Permeability Parameters in the Thin Film Composite Membranes. Sep. Purif. Technol. 2017, 186, 145–155. [Google Scholar] [CrossRef]
- Raharjo, R.D.; Freeman, B.D.; Paul, D.R.; Sarti, G.C.; Sanders, E.S. Pure and Mixed Gas CH4 and N-C4H10 Permeability and Diffusivity in Poly(Dimethylsiloxane). J. Membr. Sci. 2007, 306, 75–92. [Google Scholar] [CrossRef]





| Liquid | Molecular Mass, g/mol | Boiling Temperature, °C | Saturated Vapor Pressure at 30 °C, kPa | Saturated Vapor Pressure at 100 °C, kPa |
|---|---|---|---|---|
| Olefine | ||||
| 1-hexene | 84 | 63 | 29.7 | 290 |
| 1-heptene | 98 | 94 | 9.54 | 122 |
| 1-octene | 112 | 121 | 3.0 | 53.7 |
| 1-nonene | 126 | 151 | 1.01 | 24 |
| Aldehydes | ||||
| heptanal | 114 | 153 | 0.817 | 19.8 |
| decanal | 156 | 208 | 0.027 | 2.21 |
| Membrane Material | P, Barrer | D·108, cm2/s | S·102, cm3/(cm3·cm Hg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N2 | CH4 | CO2 | N2 | CH4 | CO2 | N2 | CH4 | CO2 | |
| PdecMS | 120 | 400 | 1300 | 1010 | 420 | 500 | 0.12 | 0.95 | 2.60 |
| OH-PdecMS | 230 | 770 | 2700 | 1050 | 420 | 570 | 0.22 | 1.83 | 4.74 |
| PDMS [50] | 400 | 1200 | 3800 | 3400 | 2200 | 2200 | 0.12 | 0.55 | 1.73 |
| Membrane Material | αP | αD | αS | |||
|---|---|---|---|---|---|---|
| CO2/N2 | CO2/CH4 | CO2/N2 | CO2/CH4 | CO2/N2 | CO2/CH4 | |
| PdecMS | 10.8 | 3.3 | 0.50 | 1.19 | 21.9 | 2.7 |
| OH-PdecMS | 11.7 | 3.5 | 0.54 | 1.36 | 21.6 | 2.6 |
| PDMS [50] | 9.5 | 3.2 | 0.65 | 1.00 | 14.7 | 3.2 |
| Liquid Pair | Polymer | Temperature, °C | ||
|---|---|---|---|---|
| 30 | 45 | 60 | ||
| heptanal/1-hexene | OH-PDecMS | 440 | 250 | 150 |
| PDecMS | 56 | 60 | 58 | |
| decanal/1-nonene | OH-PDecMS | 50 | 26 | 22 |
| PDecMS | 4.4 | 3.5 | 3.5 | |
| Polymer | Permeability, GPU | Permselectivity | |||
|---|---|---|---|---|---|
| CO2 | O2 | N2 | CO2/O2 | CO2/N2 | |
| OH-PDecMS | 160 | 30 | 15 | 5.3 | 10.6 |
| PDecMS | 320 | 65 | 35 | 4.9 | 9.2 |
| PDecMS (wet gas) [43] | 116 | 30 | 15 | 3.9 | 7.6 |
| PDMS-ZSM-5 [58] | 18 | - | - | - | 21 |
| PDMS/PAN [59] | 3700 | 860 | 370 | 4.3 | 10 |
| Penetrant | OH-PDecMS | PDecMS | |
|---|---|---|---|
| Total flux, kg·m−2·h−1 | 1-hexene | 2.16 | 7.53 |
| 1-heptene | 1.59 | 6.19 | |
| 1-octene | 1.36 | 5.76 | |
| 1-nonone | 1.02 | 3.02 | |
| Heptanal | 1.42 | 1.48 | |
| Decanal | 0.62 | 0.49 | |
| Component flux | 1-hexene | 0.75 | 6.28 |
| 1-heptene | 0.68 | 5.61 | |
| 1-octene | 0.32 | 4.44 | |
| 1-nonone | 0.21 | 1.23 | |
| Heptanal | 0.45 | 0.42 | |
| Decanal | 0.07 | 0.04 | |
| Separation factor (penetrant/ethanol) | 1-hexene | 1.7 | 13.8 |
| 1-heptene | 1.7 | 13.4 | |
| 1-octene | 1.6 | 10.0 | |
| 1-nonone | 1.3 | 7.2 | |
| Heptanal | 0.5 | 0.5 | |
| Decanal | 0.2 | 0.1 |
| Selectivity | Temperature, °C | OH-PDecMS | PDecMS |
|---|---|---|---|
| heptanal/1-hexene | 30 | 55.9 | 5.8 |
| 45 | 39.3 | 7.7 | |
| 60 | 29.0 | 9.9 | |
| decanal/1-nonene | 30 | 12.3 | 0.5 |
| 45 | 7.7 | 0.6 | |
| 60 | 5.1 | 0.7 |
| Penetrant | OH-PDecMS | PDecMS | |
|---|---|---|---|
| Apparent permeation activation energy, kJ/mol | 1-hexene | −29.8 | −25.2 |
| 1-heptene | −44.8 | −19.6 | |
| 1-octene | −44.0 | −29.5 | |
| 1-nonone | −47.5 | −27.7 | |
| Heptanal | −47.6 | −10.3 | |
| Decanal | −72.3 | −21.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grushevenko, E.A.; Rokhmanka, T.N.; Borisov, I.L.; Volkov, A.V.; Bazhenov, S.D. Effect of OH-Group Introduction on Gas and Liquid Separation Properties of Polydecylmethylsiloxane. Polymers 2023, 15, 723. https://doi.org/10.3390/polym15030723
Grushevenko EA, Rokhmanka TN, Borisov IL, Volkov AV, Bazhenov SD. Effect of OH-Group Introduction on Gas and Liquid Separation Properties of Polydecylmethylsiloxane. Polymers. 2023; 15(3):723. https://doi.org/10.3390/polym15030723
Chicago/Turabian StyleGrushevenko, Evgenia A., Tatiana N. Rokhmanka, Ilya L. Borisov, Alexey V. Volkov, and Stepan D. Bazhenov. 2023. "Effect of OH-Group Introduction on Gas and Liquid Separation Properties of Polydecylmethylsiloxane" Polymers 15, no. 3: 723. https://doi.org/10.3390/polym15030723
APA StyleGrushevenko, E. A., Rokhmanka, T. N., Borisov, I. L., Volkov, A. V., & Bazhenov, S. D. (2023). Effect of OH-Group Introduction on Gas and Liquid Separation Properties of Polydecylmethylsiloxane. Polymers, 15(3), 723. https://doi.org/10.3390/polym15030723











