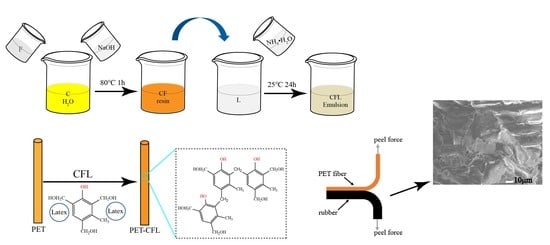
Highly Strong Interface Adhesion of Polyester Fiber Rubber Composite via Fiber Surface Modification by Meta-Cresol/Formaldehyde Latex Dipping Emulsion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of M-Cresol/Formaldehyde Resin and Preparation of Different CFL Emulsions
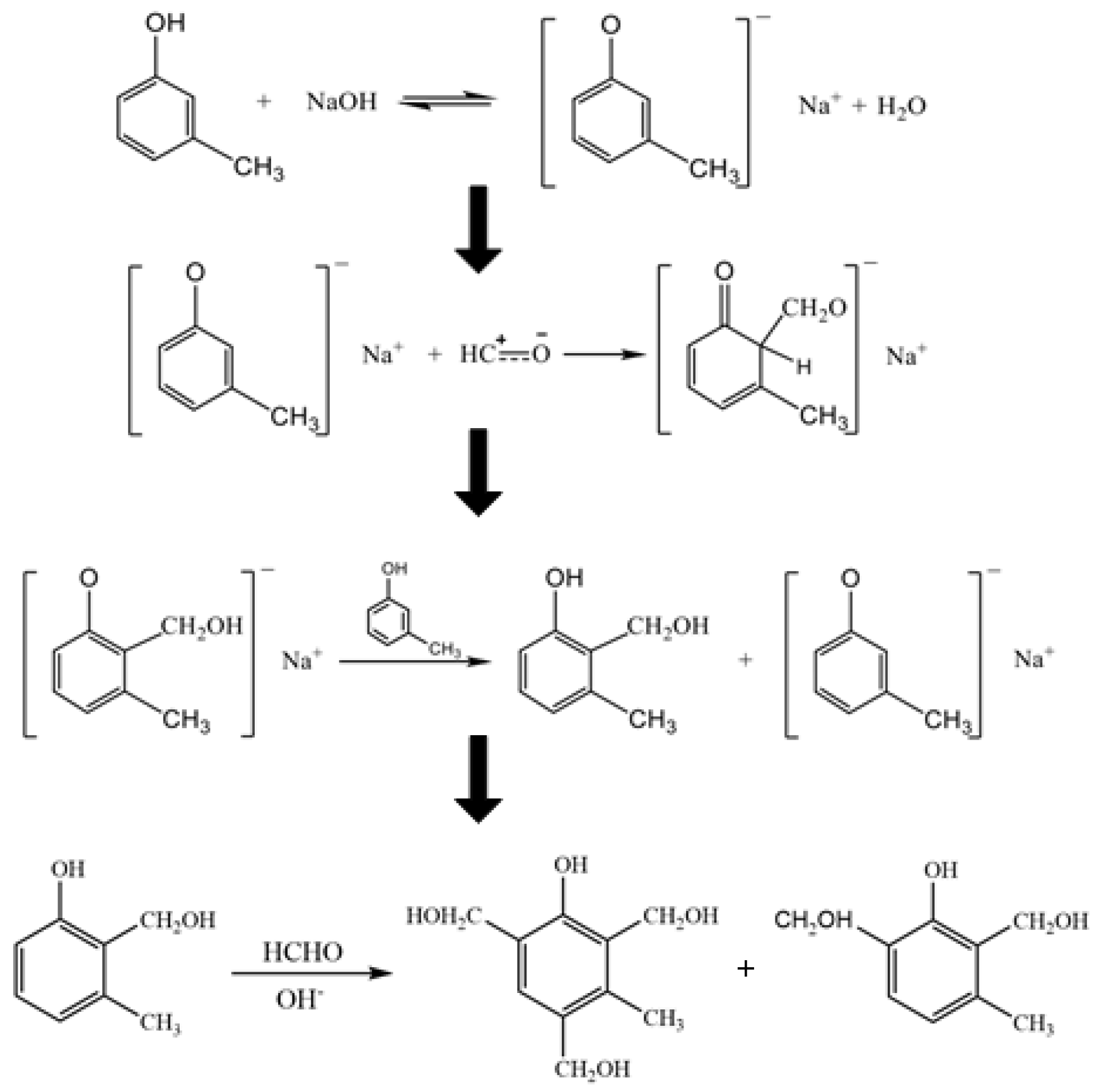
2.2.1. Synthesis of M-Cresol Resin and Preparation of CFL Emulsions with Different M-Cresol/Formaldehyde Molar Ratios
2.2.2. Preparation of CFL Emulsions with Different Resin/Latex Wet Weight Ratios
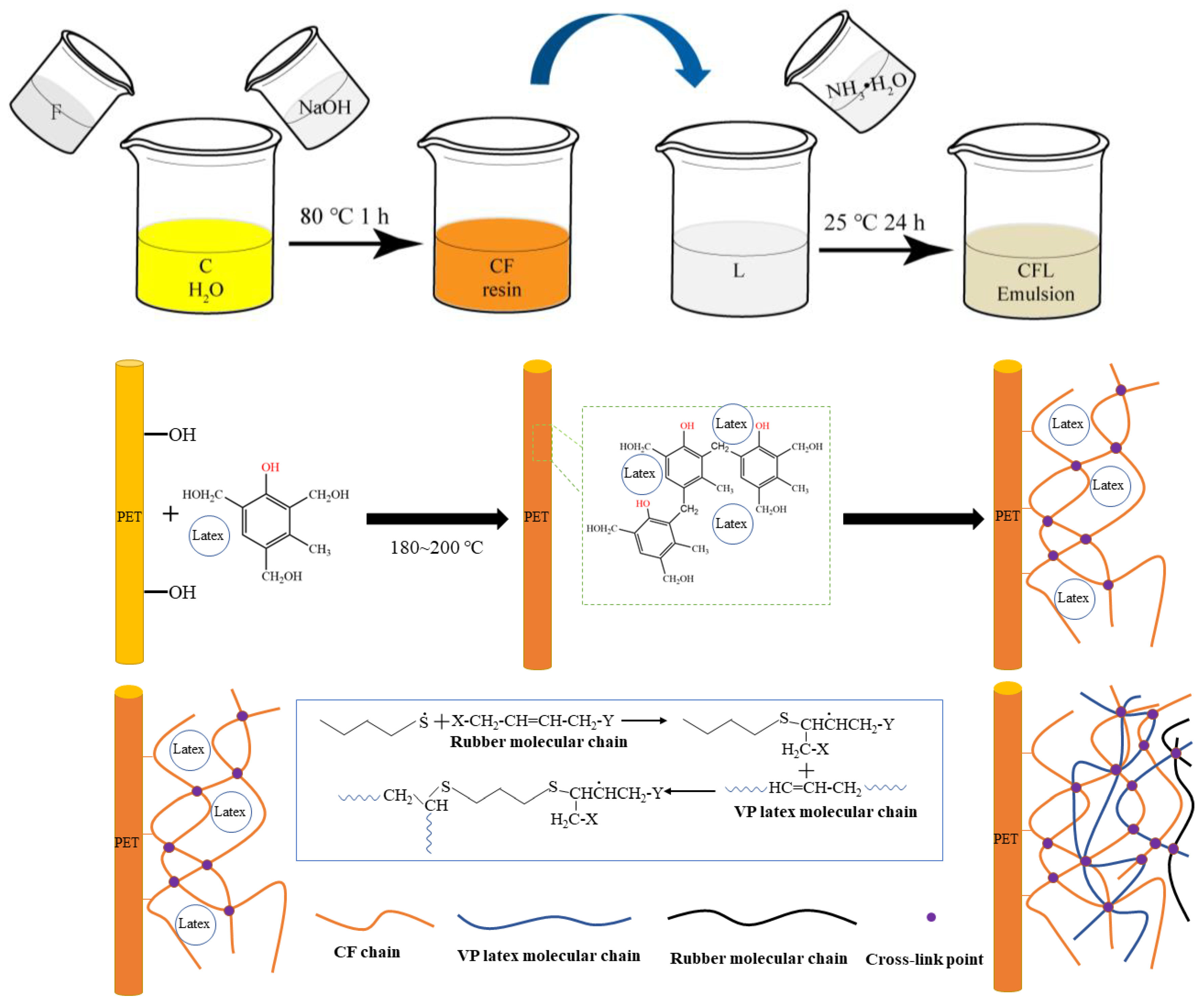
2.3. Preparation of PET/CFL Composites
2.3.1. Impregnation of PET Fibers in CFL Emulsions
2.3.2. Preparation of PET/Rubber Composites
2.4. Test Instruments and Methods
3. Results and Discussion
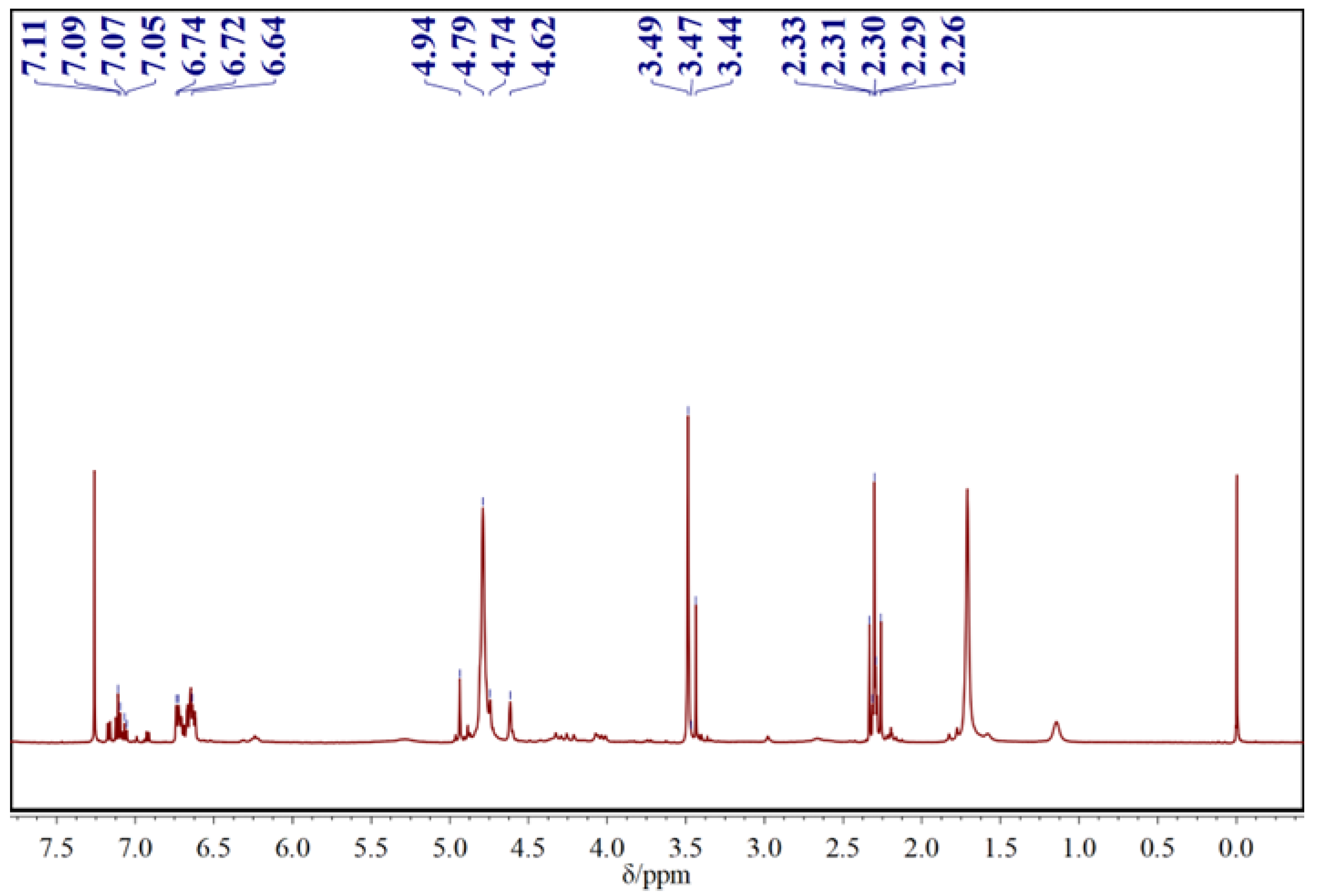
3.1. Chemical Composition of the CF Resin
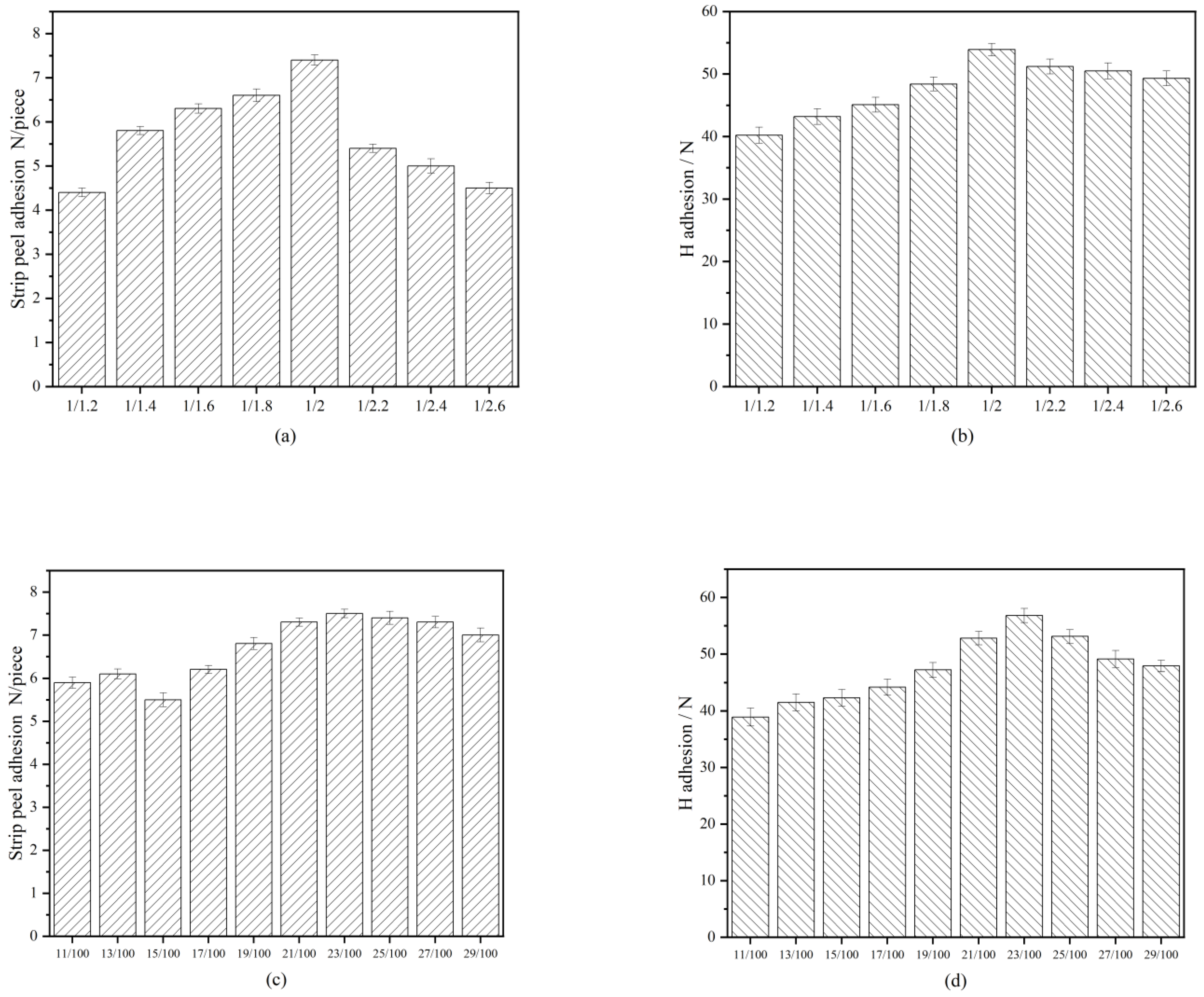
3.2. Adhesion and Strength Results
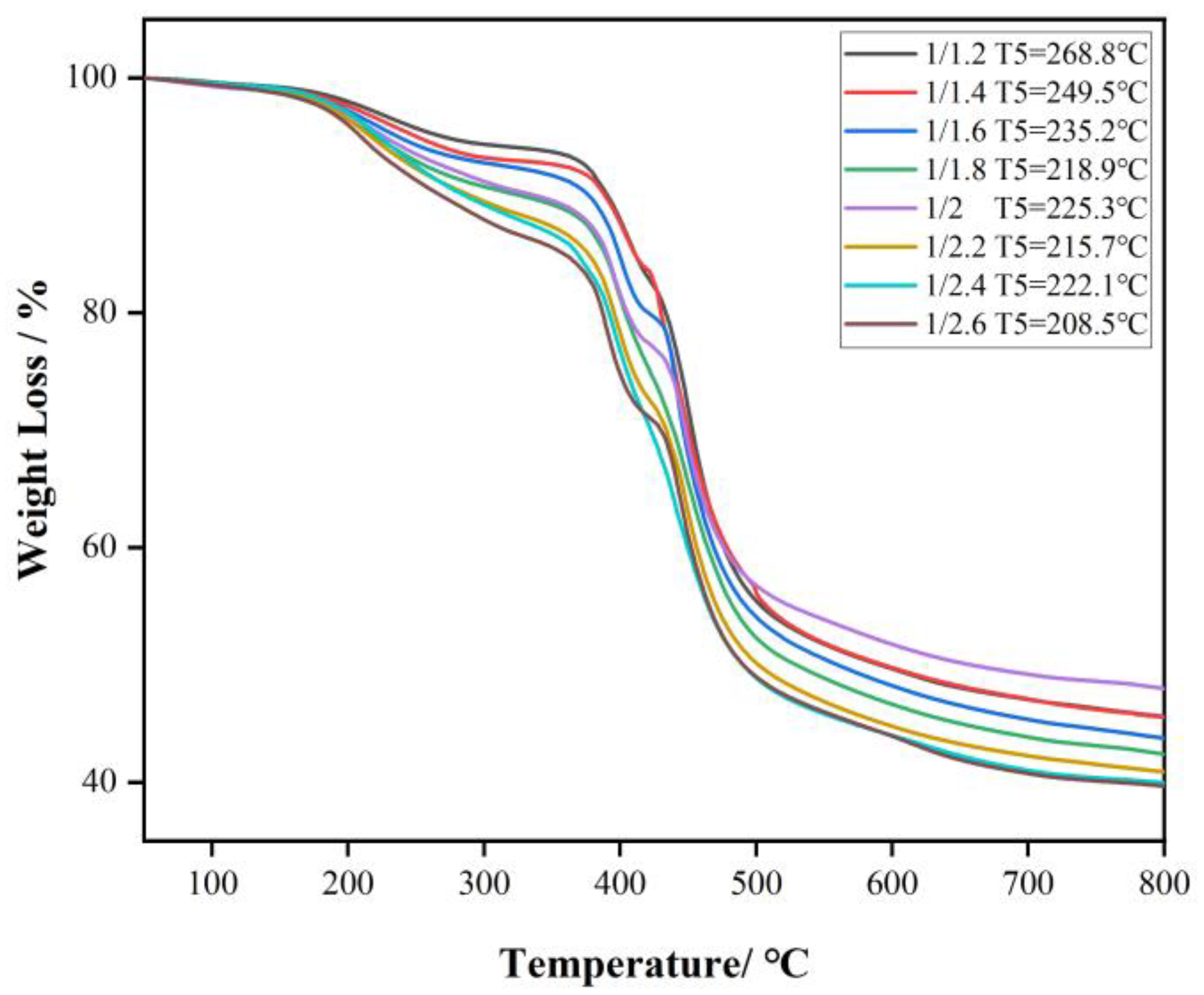
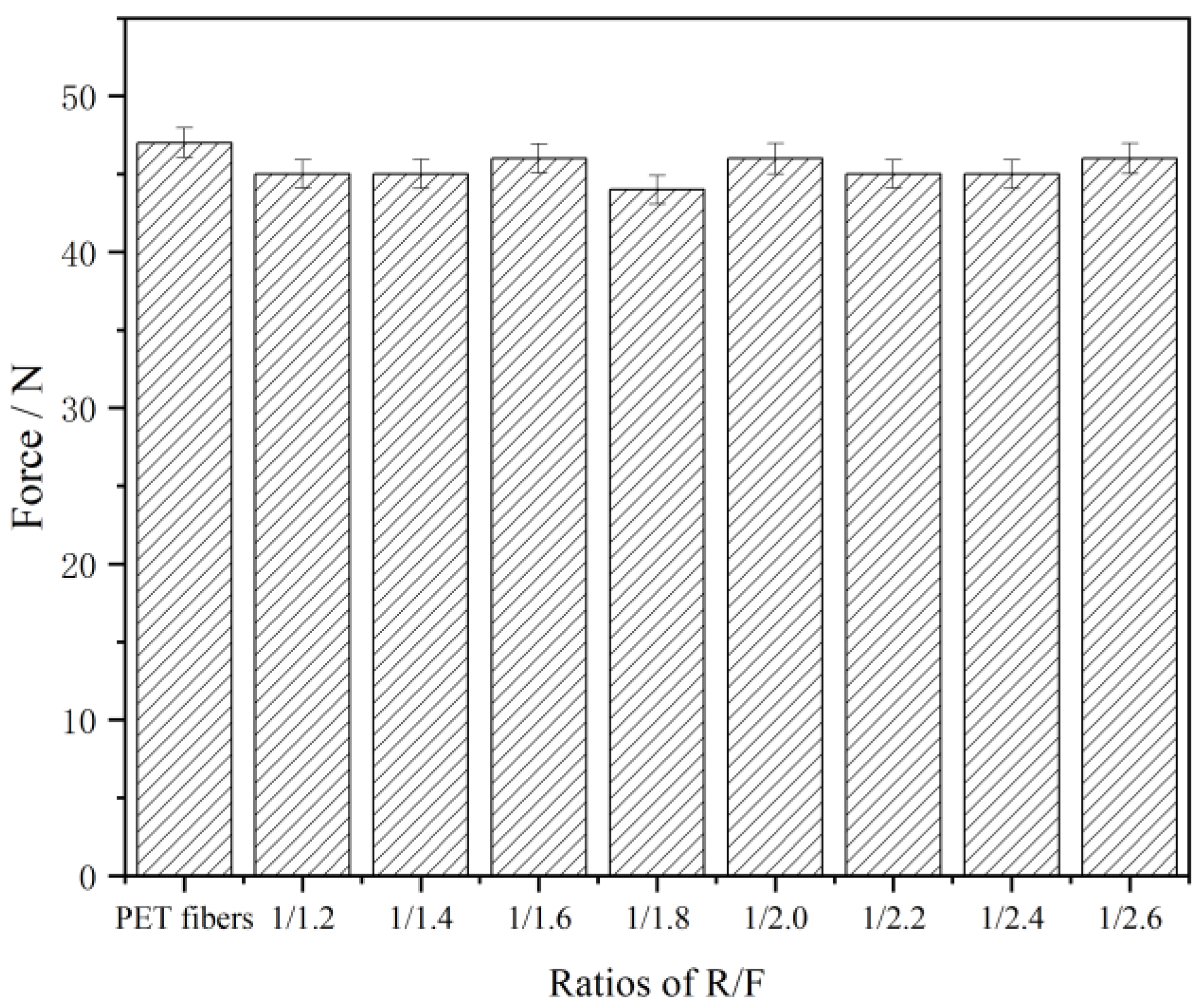
3.3. The Breaking Strength of PET
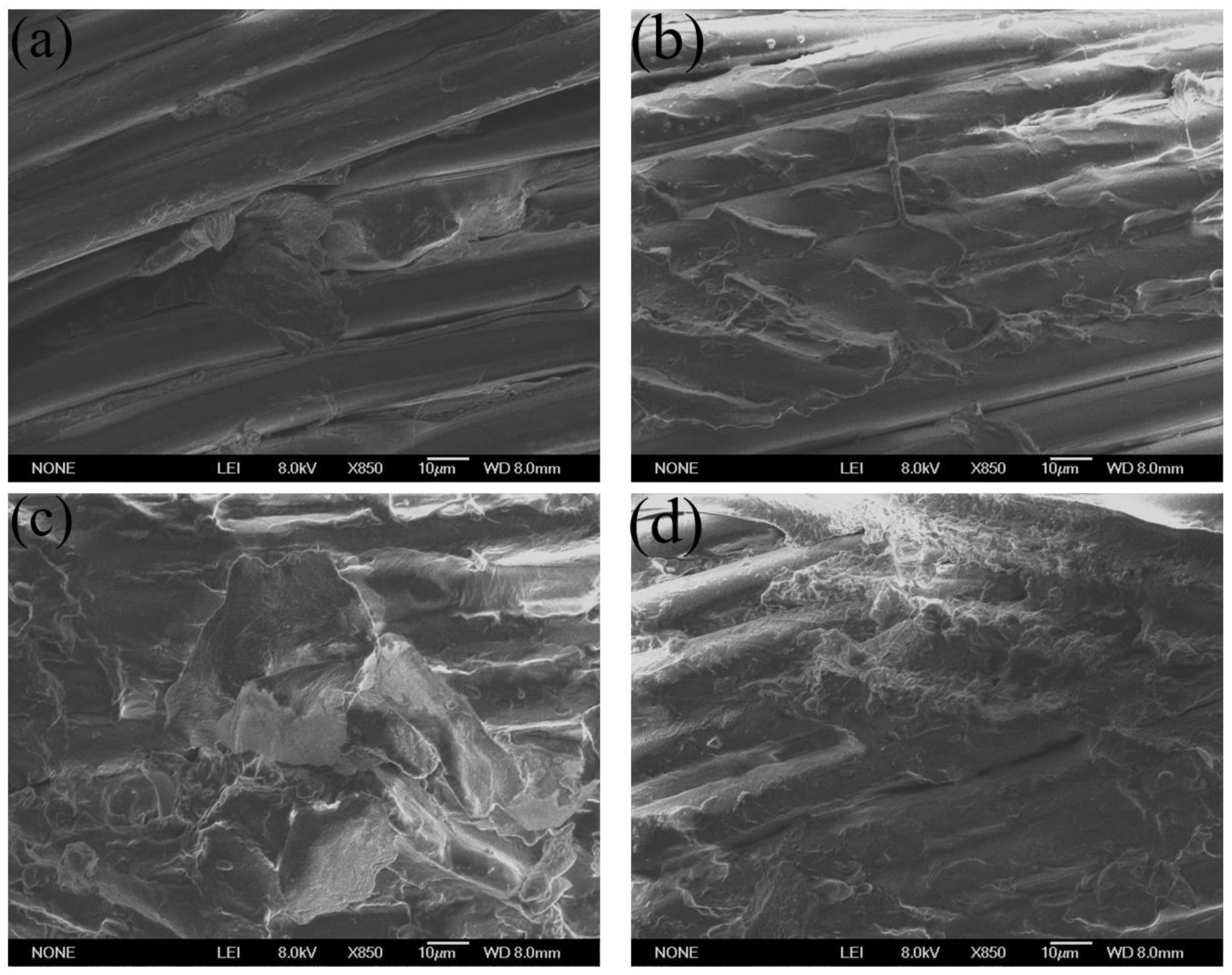
3.4. Morphology of Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Doganci, E. Improving adhesion between polyester cord and rubber by using glycidyl-POSS. J. Appl. Polym. Sci. 2020, 138, 49681. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, M.; Sun, G.; Huang, W.; Niu, H.; Wu, D. Preparation and properties of high-strength-high-modulus polyimide cords/natural rubber composites. J. Appl. Polym. Sci. 2020, 138, 49733. [Google Scholar] [CrossRef]
- Research and Markets Global Polyester Fibers Industry 2020 to 2027-Market Trajectory Analytics-ResearchAndMarkets.com. Medical Letter on the CDC & FDA 2020. Available online: https://www.businesswire.com/news/home/20201022005652/en/Global-Polyester-Fibers-Industry-2020-to-2027---Market-Trajectory-Analytics---ResearchAndMarkets.com,22,10,2020 (accessed on 22 October 2020).
- Li, Z.; Xiao, T.; Kong, L.; Wan, J.; Zhao, S. Structure and adhesive properties of the RFL-coated continuous basalt fiber cord/rubber interface. J. Appl. Polym. Sci. 2017, 134, 44353. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, S.; Wang, W.; Tian, M.; Ning, N.; Zhang, L. Polyester (PET) fabrics coated with environmentally friendly adhesive and its interface structure and adhesive properties with rubber. Compos. Sci. Technol. 2020, 195, 108171. [Google Scholar] [CrossRef]
- Chen, X.; Hu, X.; Lu, Q.; Yang, Y.; Linghu, S.; Zhang, X. Study on the differences in sludge toxicity and microbial community structure caused by catechol, resorcinol and hydroquinone with metagenomic analysis. J. Environ. Manag. 2022, 302, 114027. [Google Scholar] [CrossRef]
- Kondo, Y.; Miyazaki, K.; Takayanagi, K.; Sakurai, K. Surface treatment of PET fiber by EB-irradiation-induced graft polymerization and its effect on adhesion in natural rubber matrix. Eur. Polym. J. 2008, 44, 1567–1576. [Google Scholar] [CrossRef]
- Vecchiato, S.; Ahrens, J.; Pellis, A.; Scaini, D.; Mueller, B.; Herrero Acero, E.; Guebitz, G.M. Enzymatic Functionalization of HMLS-Polyethylene Terephthalate Fabrics Improves the Adhesion to Rubber. ACS Sustain. Chem. Eng. 2017, 5, 6456–6465. [Google Scholar] [CrossRef]
- Dierkes, W.; Louis, A.; Noordermeer, J.; Blume, A. A Novel Approach of Promoting Adhesion of Reinforcing Cord to Elastomers by Plasma Polymerization. Polymers 2019, 11, 577. [Google Scholar] [CrossRef] [Green Version]
- Razavizadeh, M.; Jamshidi, M. Adhesion of nitrile rubber to UV-assisted surface chemical modified PET fabric, part II: Interfacial characterization of MDI grafted PET. Appl. Surf. Sci. 2016, 379, 114–123. [Google Scholar] [CrossRef]
- Palola, S.; Sarlin, E.; Kolahgar Azari, S.; Koutsos, V.; Vuorinen, J. Microwave induced hierarchical nanostructures on aramid fibers and their influence on adhesion properties in a rubber matrix. Appl. Surf. Sci. 2017, 410, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Han, Z.; Wang, G.; Niu, K. In situ grafting coupling agent on polyester fabric to significantly improve the interfacial adhesion to silicone rubber. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127384. [Google Scholar] [CrossRef]
- Yang, X.; Tu, Q.; Shen, X.; Jiang, C.; Pan, M.; Zhu, P.; Li, Y.; Hu, C.; Zhang, Q. Study on interfacial adhesion of the aramid fibers/rubber matrix by grafting mercapto hyperbranched polysiloxane. Polym. Test. 2020, 81, 106259. [Google Scholar] [CrossRef]
- Yazıcı, N.; Dursun, S.; Yarıcı, T.; Kılıç, B.; Arıcan, M.O.; Mert, O.; Karaağaç, B.; Özkoç, G.; Kodal, M. The outstanding interfacial adhesion between acrylo-POSS/natural rubber composites and polyamide-based cords: ‘An environmentally friendly alternative to resorcinol-formaldehyde latex coating’. Polymer 2021, 228, 123880. [Google Scholar] [CrossRef]
- Zhong, J.; Luo, Z.; Hao, Z.; Guo, Y.; Zhou, Z.; Li, P.; Xue, B. Enhancing fatigue properties of styrene butadiene rubber composites by improving interface adhesion between coated aramid fibers and matrix. Compos. Part B Eng. 2019, 172, 485–495. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, B.; Tian, M.; Ning, N.; Zhang, L.; Wang, W. Surface construction of ANF/CNT onto aramid fibers to enhance interfacial adhesion and provide real-time monitoring of deformation. Compos. Sci. Technol. 2022, 223, 109336. [Google Scholar] [CrossRef]
- Li, W.; Meng, L.; Ma, R. Effect of surface treatment with potassium permanganate on ultra-high molecular weight polyethylene fiber reinforced natural rubber composites. Polym. Test. 2016, 55, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Niroomand, M.; Hejazi, S.M.; Sheikhzadeh, M.; Alirezazadeh, A. Pull-out analysis of laser modified polyamide tire cords through rubber matrix. Eng. Fail. Anal. 2017, 80, 431–443. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Y.; Sa, R.; Ning, N.; Wang, W.; Tian, M.; Zhang, L. Surface Modification of Aramid Fibers by Catechol/Polyamine Codeposition Followed by Silane Grafting for Enhanced Interfacial Adhesion to Rubber Matrix. Ind. Eng. Chem. Res. 2016, 55, 12547–12556. [Google Scholar] [CrossRef]
- Sa, R.; Yan, Y.; Wei, Z.; Zhang, L.; Wang, W.; Tian, M. Surface modification of aramid fibers by bio-inspired poly(dopamine) and epoxy functionalized silane grafting. ACS Appl. Mater. Interfaces 2014, 6, 21730–21738. [Google Scholar] [CrossRef]
- Du, Y.; Zheng, X.; Shao, X.; Hou, Z.; Sun, P.; Jiang, X.; Yue, Z.; Xu, Q.; Wang, Y.; Cheng, F.S.; et al. Improved adhesion properties of natural rubber to polyamide cord through mussel-inspired adhesive. J. Polym. Res. 2021, 28, 357. [Google Scholar] [CrossRef]
- Zhang, B.; Shao, X.; Tian, M.; Ning, N.; Zhang, L.; Wang, W. Mussel-inspired environmentally friendly dipping system for aramid fiber and its interfacial adhesive mechanism with rubber. Polymer 2022, 238, 124414. [Google Scholar] [CrossRef]
- Yang, X.; Tu, Q.; Shen, X.; Pan, M.; Jiang, C.; Zhu, P.; Li, Y.; Li, P.; Hu, C. Surface modification of Poly(p-phenylene terephthalamide) fibers by polydopamine-polyethyleneimine/graphene oxide multilayer films to enhance interfacial adhesion with rubber matrix. Polym. Test. 2019, 78, 105985. [Google Scholar] [CrossRef]
- Zhang, B.; Liang, T.; Shao, X.; Tian, M.; Ning, N.; Zhang, L.; Wang, W. Nondestructive Grafting of ZnO on the Surface of Aramid Fibers Followed by Silane Grafting to Improve its Interfacial Adhesion Property with Rubber. ACS Appl. Polym. Mater. 2021, 3, 4587–4594. [Google Scholar] [CrossRef]
- Liu, J.; Chao, L.; Ding, J.; Yu, Q. Stabilization Modification and Thermal Degradation Behaviors of Hydroxyl-terminated Hyperbranched Polyesters. J. Chem. Eng. Chin. Univ. 2014, 28, 346–351. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Wu, S. The pyrolysis mechanism of phenol formaldehyde resin. Polym. Degrad. Stab. 2012, 97, 1527–1533. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Kang, L.; Guo, X.; Tang, X.; Liu, L.; Shen, M. Highly Strong Interface Adhesion of Polyester Fiber Rubber Composite via Fiber Surface Modification by Meta-Cresol/Formaldehyde Latex Dipping Emulsion. Polymers 2023, 15, 1009. https://doi.org/10.3390/polym15041009
Meng X, Kang L, Guo X, Tang X, Liu L, Shen M. Highly Strong Interface Adhesion of Polyester Fiber Rubber Composite via Fiber Surface Modification by Meta-Cresol/Formaldehyde Latex Dipping Emulsion. Polymers. 2023; 15(4):1009. https://doi.org/10.3390/polym15041009
Chicago/Turabian StyleMeng, Xiangze, Le Kang, Xin Guo, Xiaohao Tang, Li Liu, and Mei Shen. 2023. "Highly Strong Interface Adhesion of Polyester Fiber Rubber Composite via Fiber Surface Modification by Meta-Cresol/Formaldehyde Latex Dipping Emulsion" Polymers 15, no. 4: 1009. https://doi.org/10.3390/polym15041009
APA StyleMeng, X., Kang, L., Guo, X., Tang, X., Liu, L., & Shen, M. (2023). Highly Strong Interface Adhesion of Polyester Fiber Rubber Composite via Fiber Surface Modification by Meta-Cresol/Formaldehyde Latex Dipping Emulsion. Polymers, 15(4), 1009. https://doi.org/10.3390/polym15041009