Study on Degradation of Natural Rubber Latex Using Hydrogen Peroxide and Sodium Nitrite in the Presence of Formic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Degradation of Natural Rubber Latex
2.3. Characterization
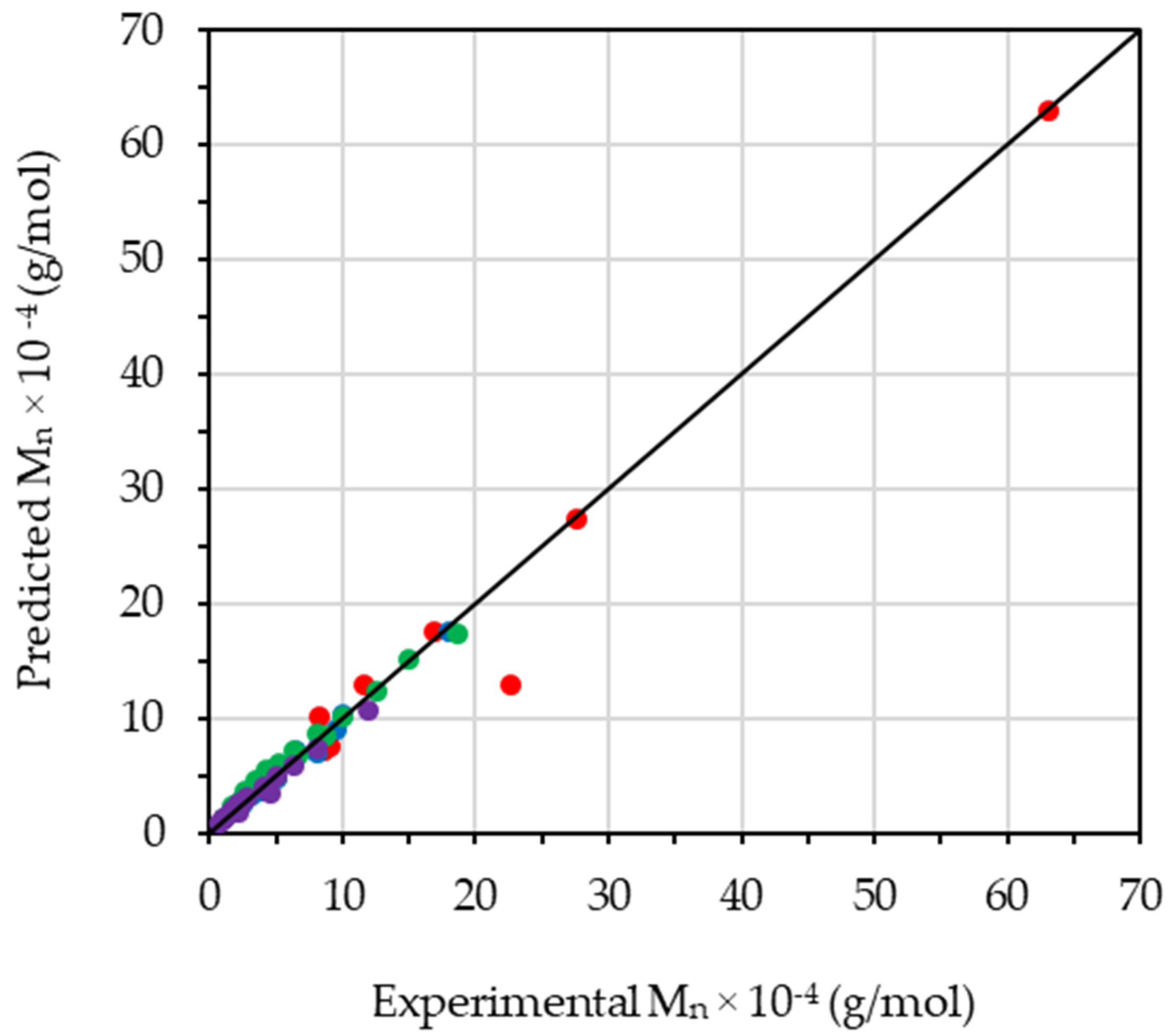
2.3.1. Number-Average Molecular Weights
2.3.2. FTIR Spectroscopy
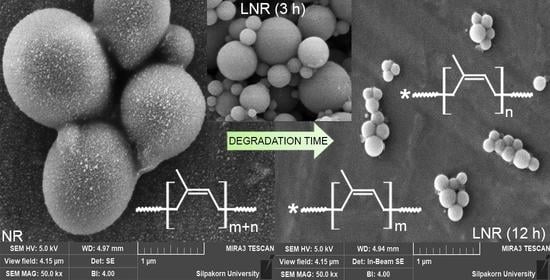
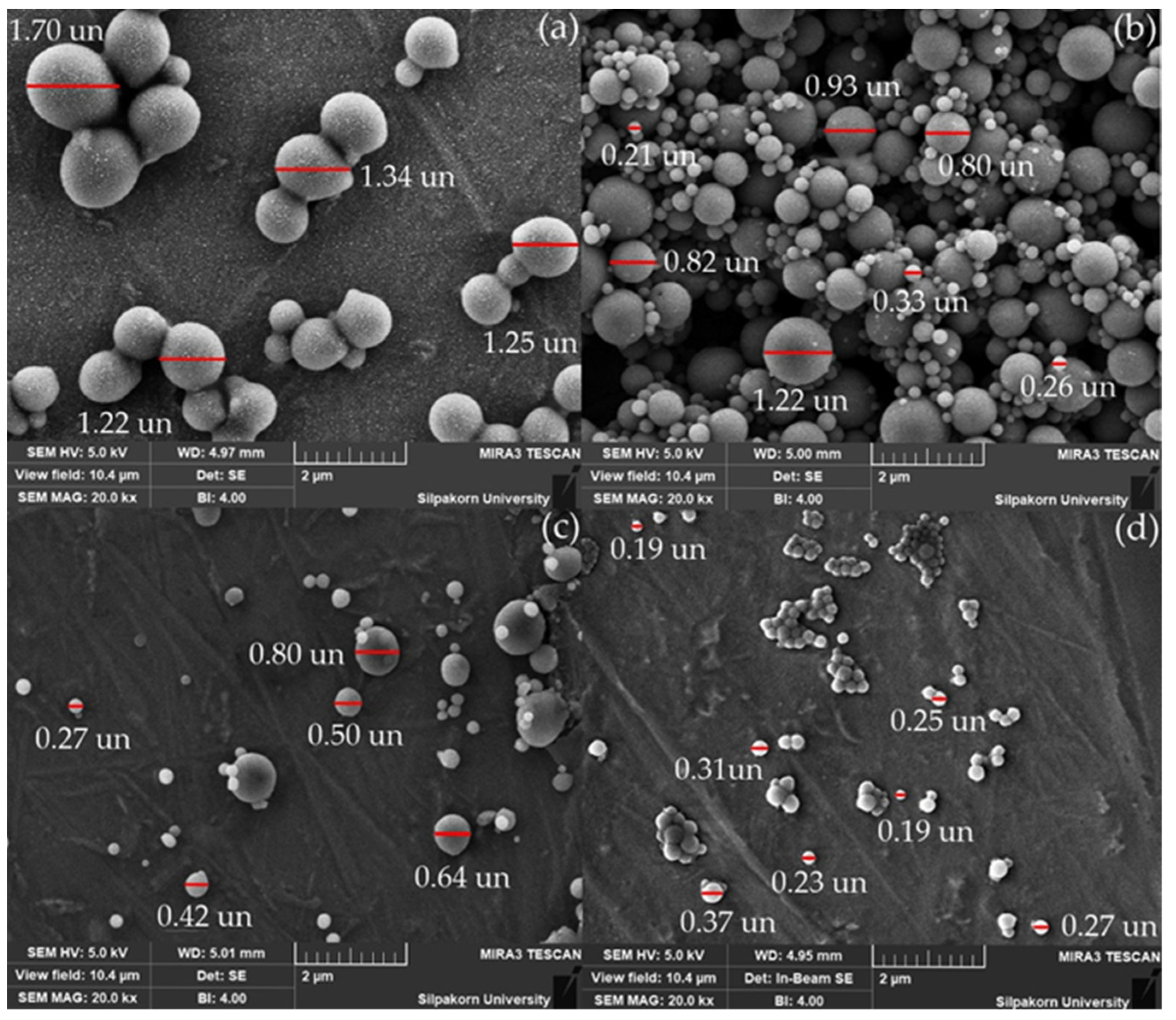
2.3.3. Morphology
2.3.4. Thermal Analysis
3. Results and Discussion
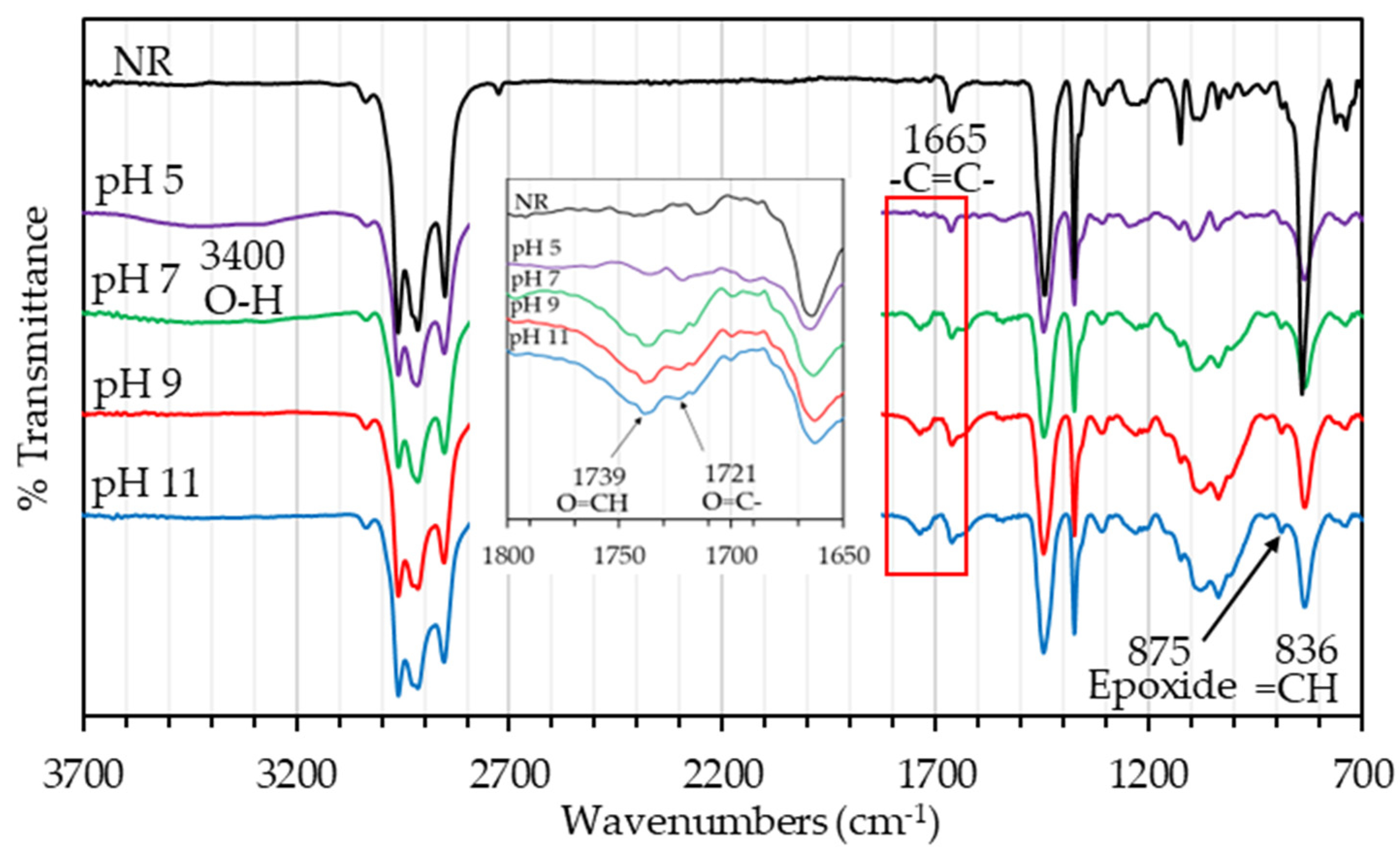
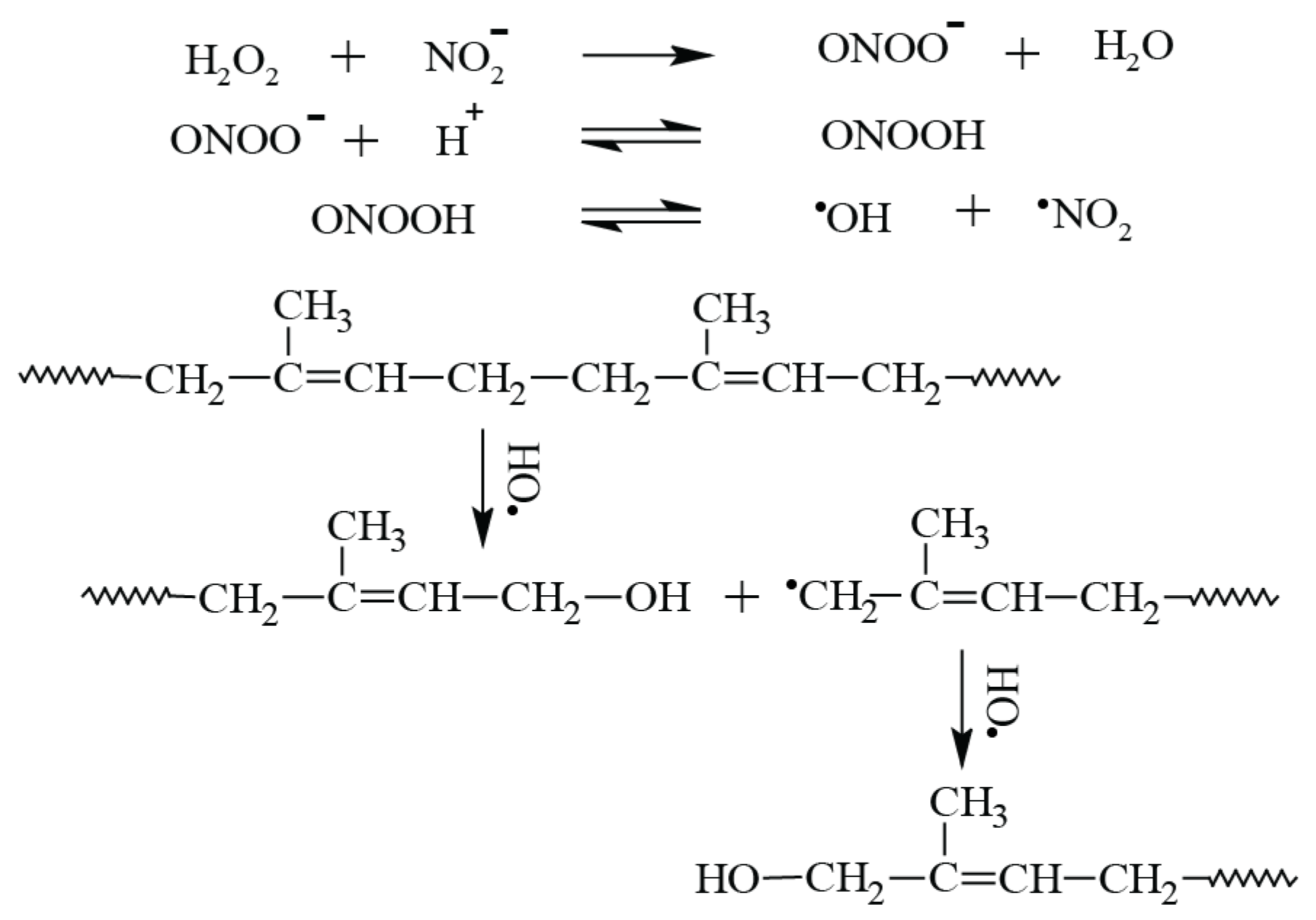
3.1. FTIR Spectroscopy
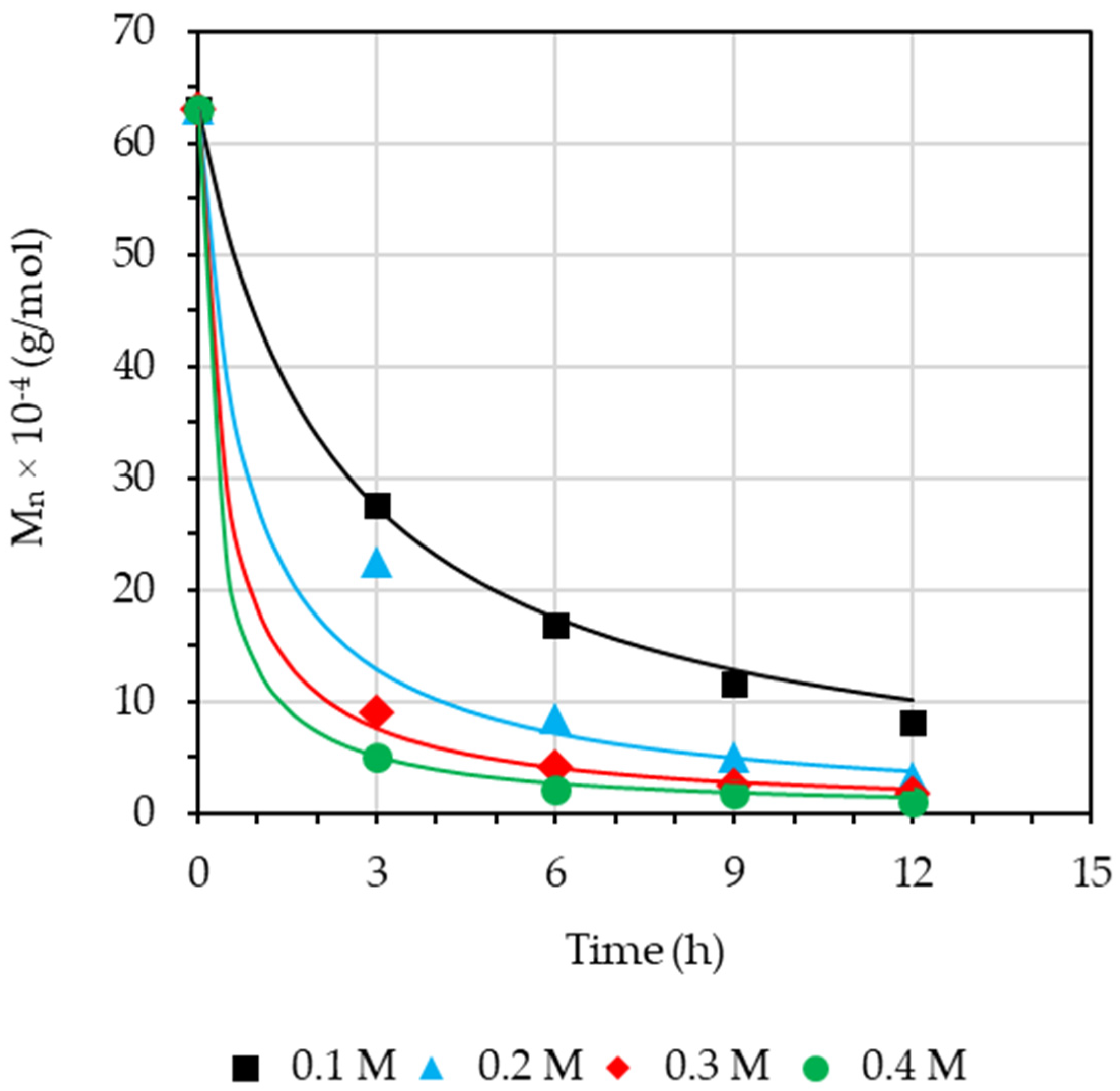
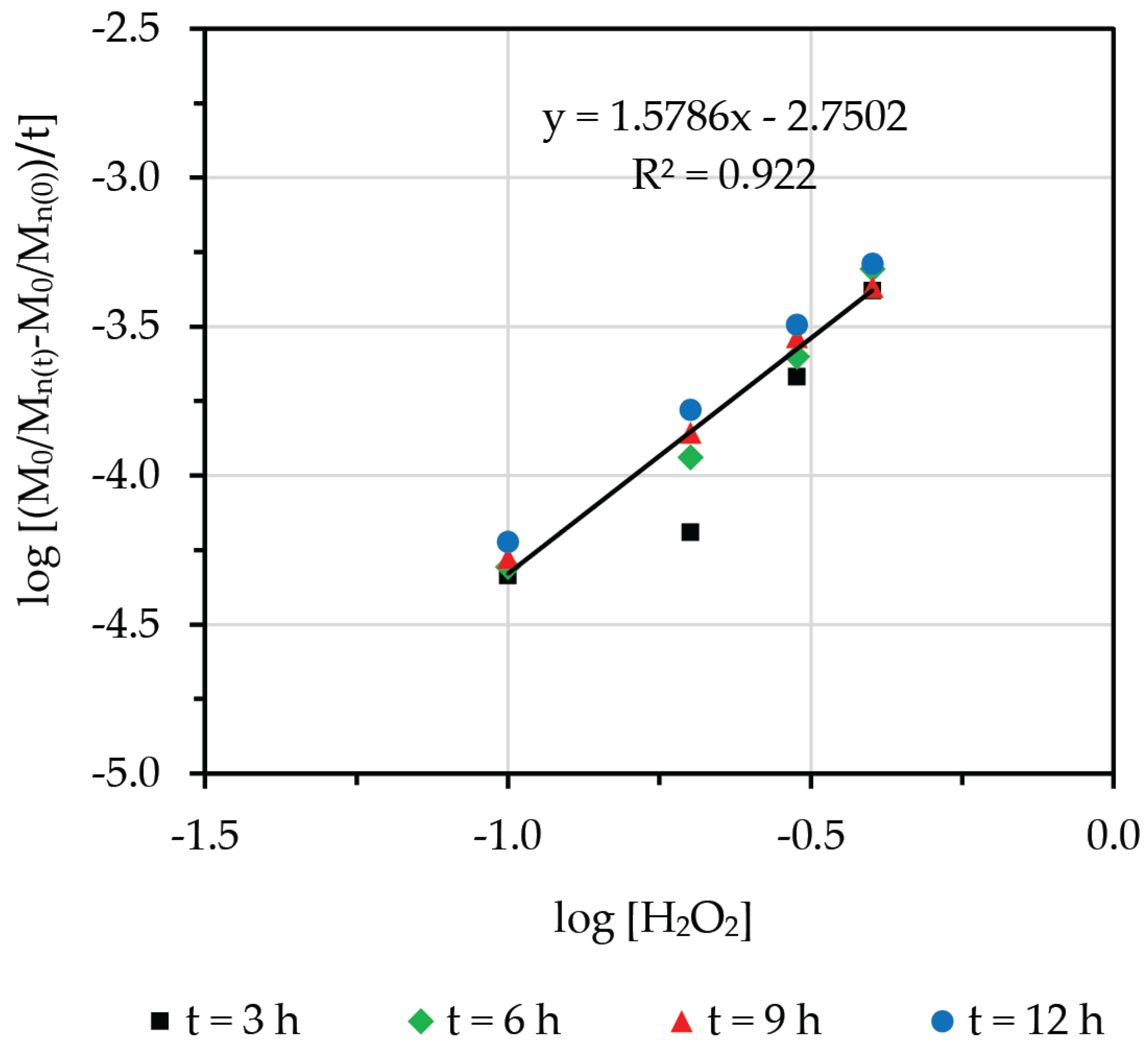
3.2. Effect of Concentration of Hydrogen Peroxide
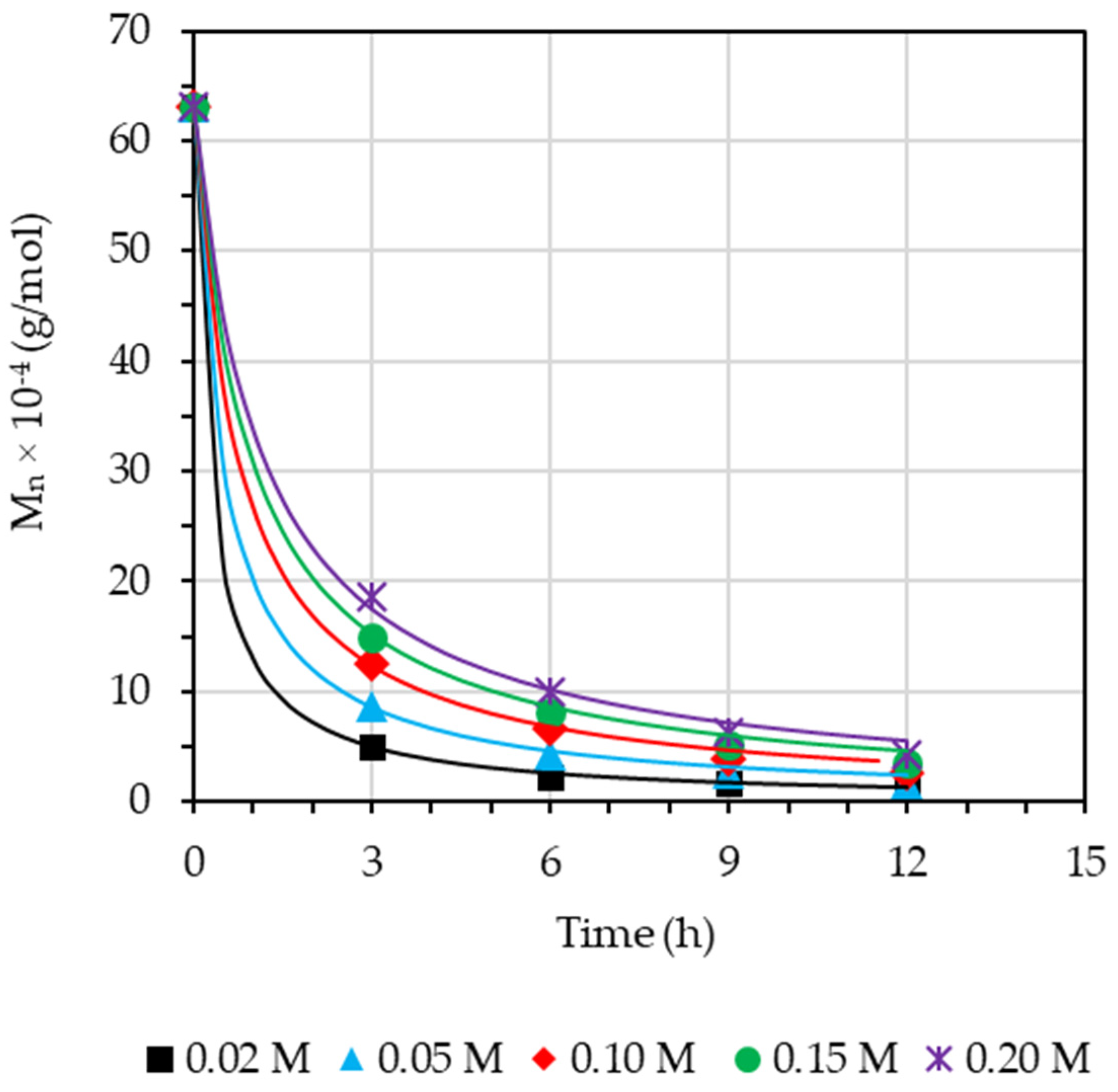
3.3. Effect of Concentration of Formic Acid
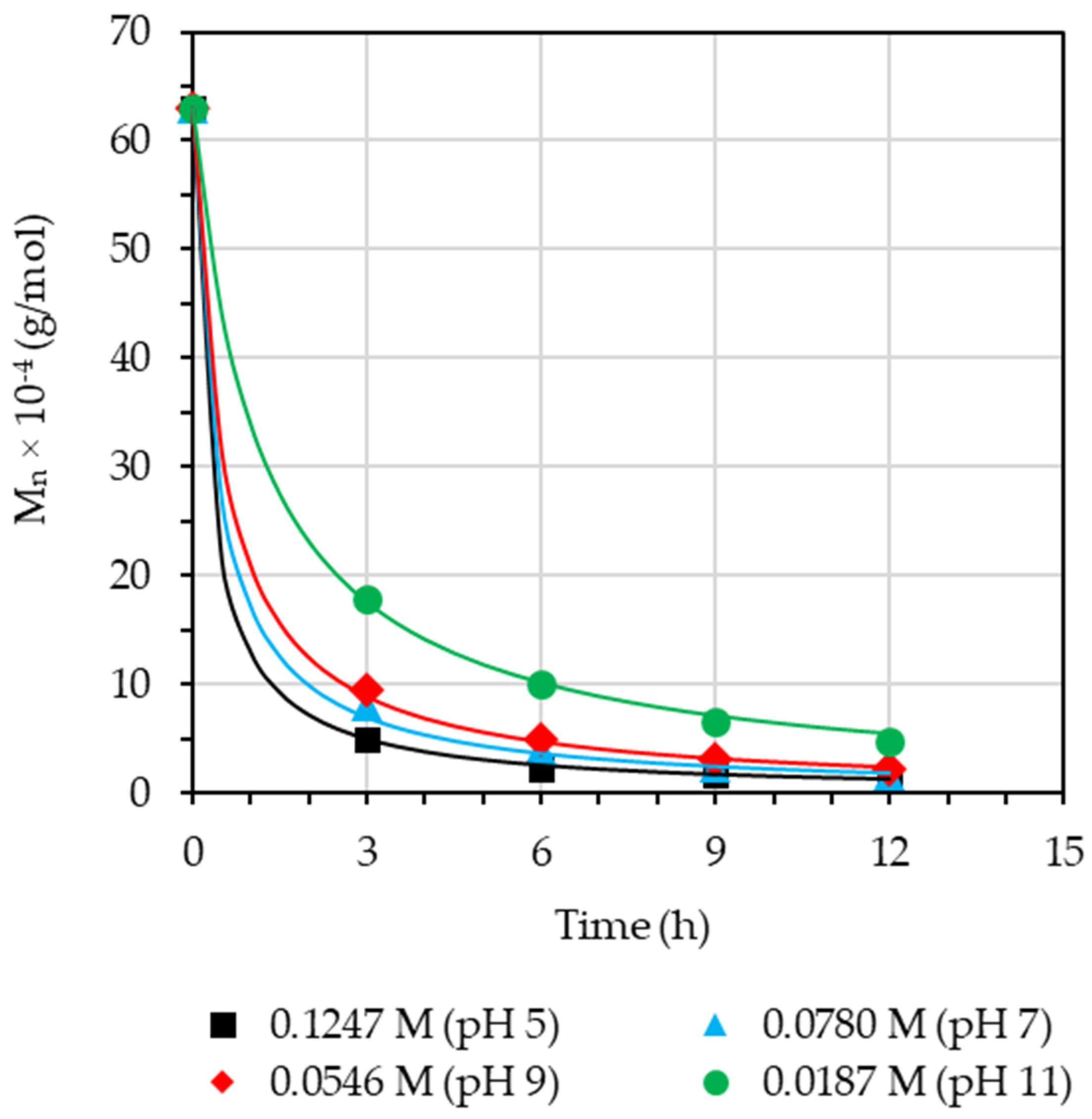
3.4. Effect of Concentration of Sodium Nitrile
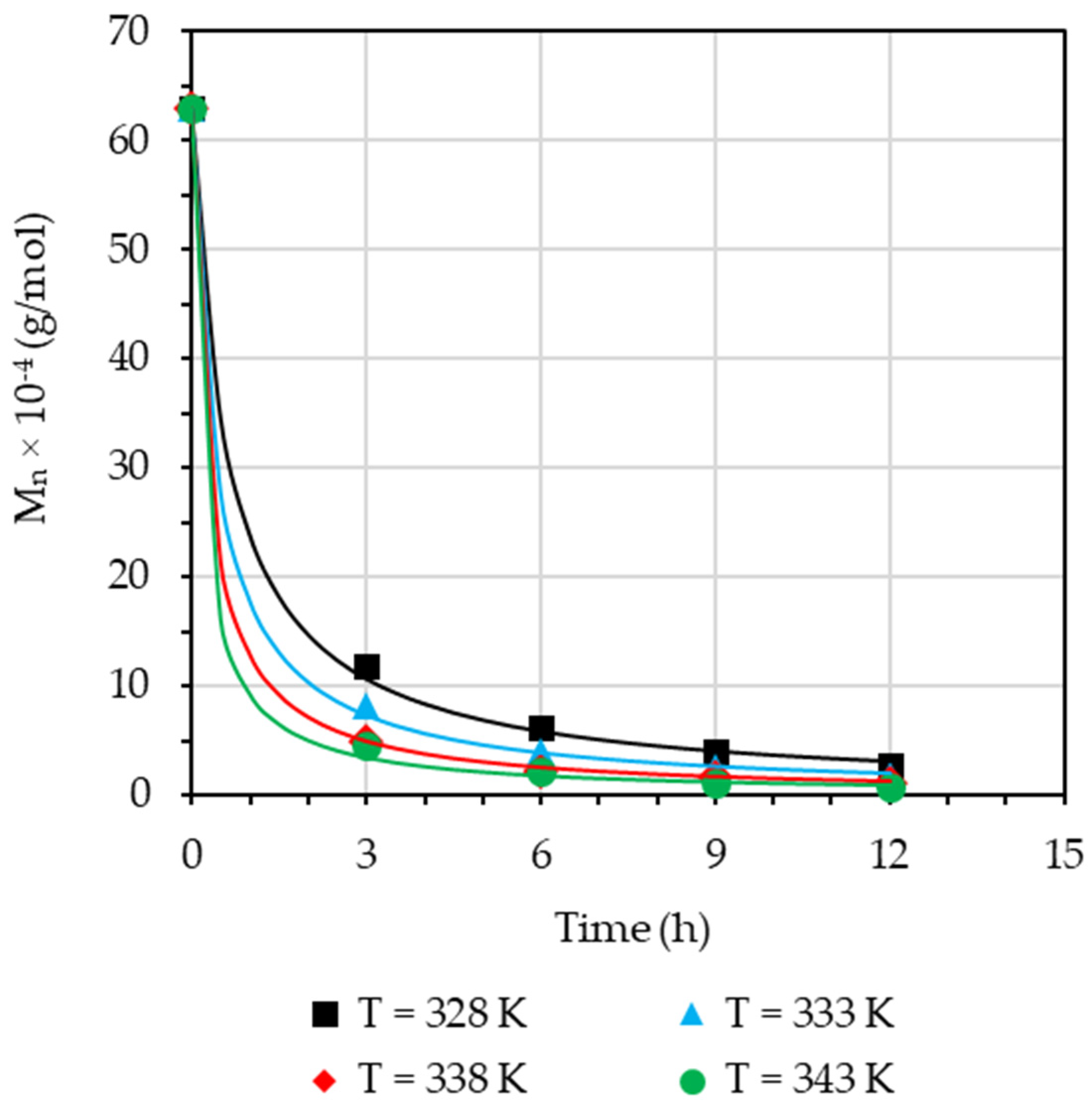
3.5. Effect of Reaction Temperature
3.6. Effect of Reaction Time
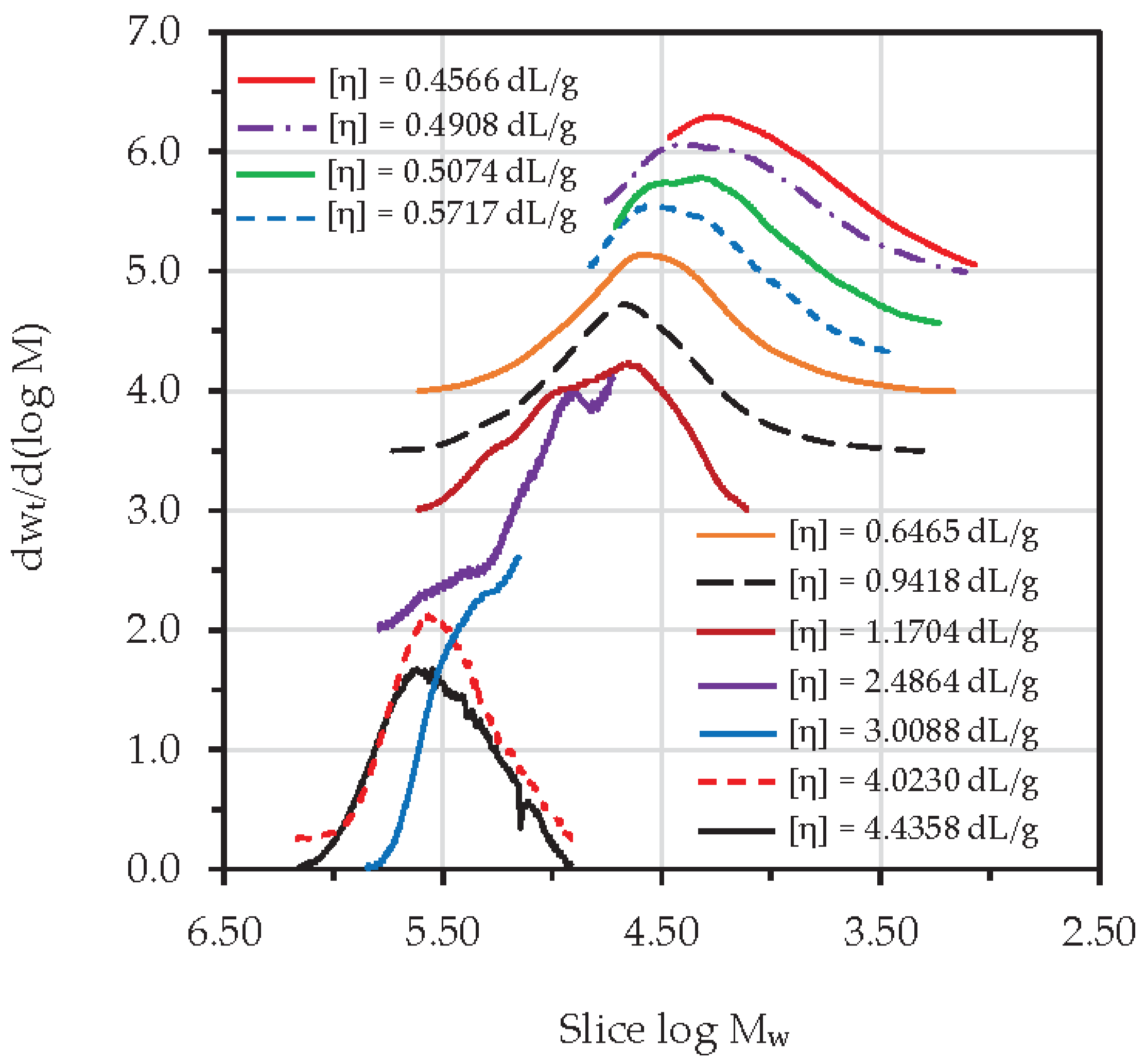
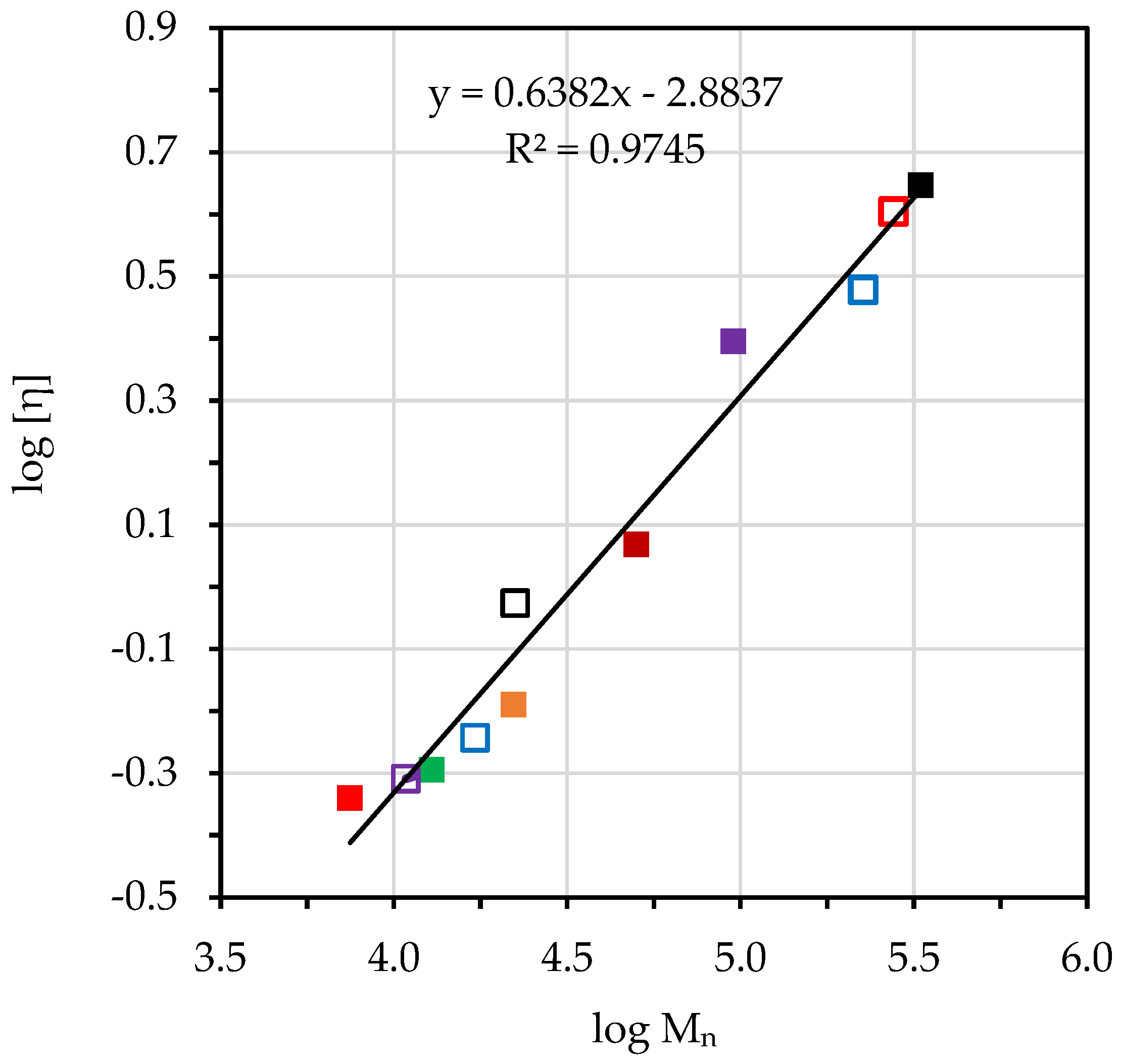
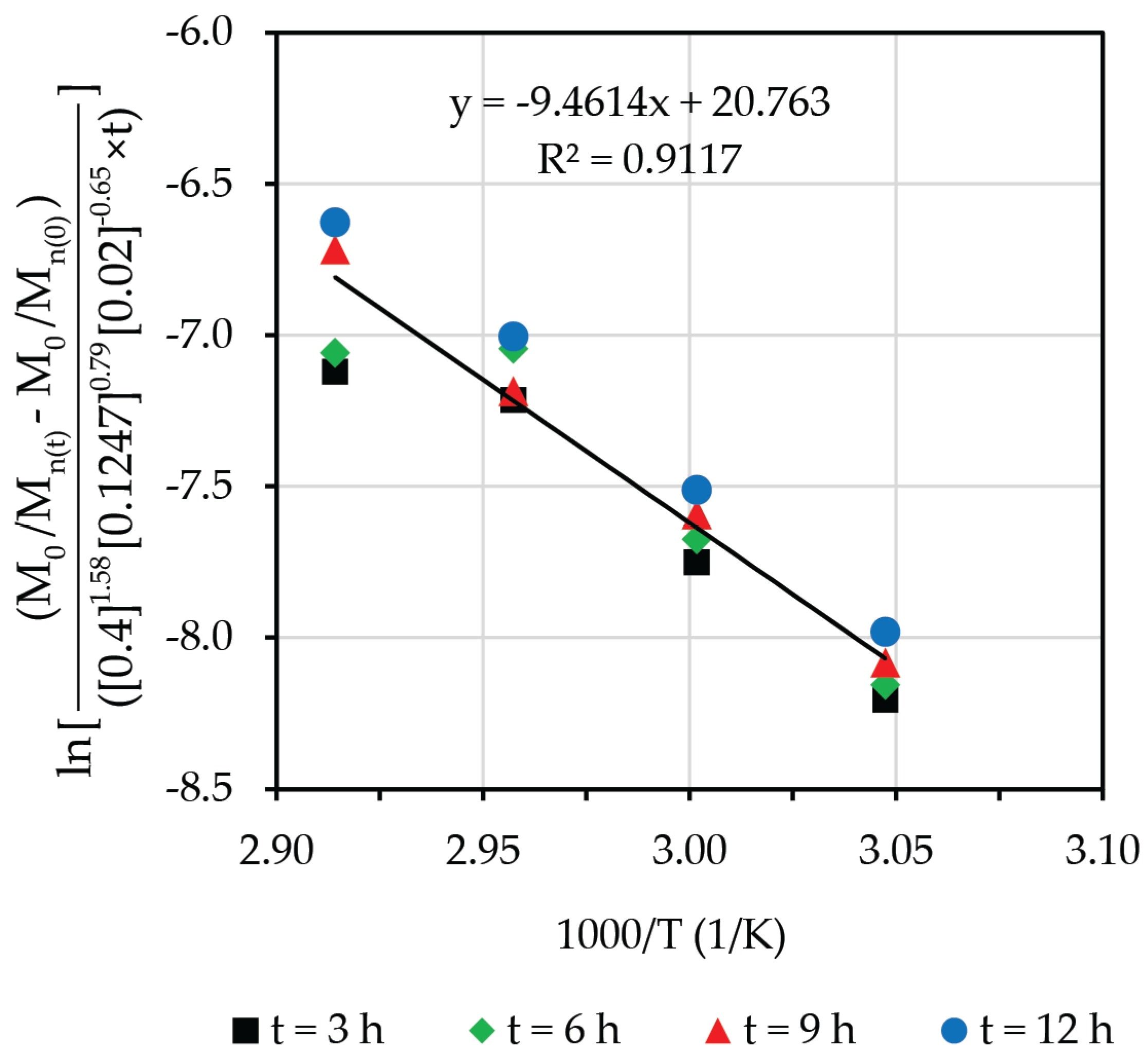
3.7. Degradative Kinetics
3.8. TGA Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahim, S.; Othman, N.; Ismail, H. Degradation of natural rubber latex. In Natural Rubber: Properties, Behavior and Application; Hamilton, J.L., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2016; pp. 105–136. [Google Scholar]
- Azhar, N.H.A.; Jamaluddin, N.; Md Rasid, H.; Mohd Yusof, M.J.; Yusoff, S.F.M. Studies on Hydrogenation of Liquid Natural Rubber Using Diimide. Int. J. Polym. Sci. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Abdullah, I. Liquid natural rubber: Preparation and application. Prog. Polym. Sci. 1994, 3, 351–365. [Google Scholar]
- Ibrahim, S.; Othman, N.; Sreekantan, S.; Tan, K.; Mohd Nor, Z.; Ismail, H. Preparation and Characterization of Low-Molecular-Weight Natural Rubber Latex via Photodegradation Catalyzed by Nano TiO2. Polymers 2018, 10, 1216–1233. [Google Scholar] [CrossRef]
- Sillapasuwan, A.; Saekhow, P.; Rojruthai, P.; Sakdapipanich, J. The Preparation of Hydroxyl-Terminated Deproteinized Natural Rubber Latex by Photochemical Reaction Utilizing Nanometric TiO2 Depositing on Quartz Substrate as a Photocatalyst. Polymers 2022, 14, 2877–2889. [Google Scholar] [CrossRef]
- Dahlan, H.M.; Khairul Zaman, M.D.; Ibrahim, A. Liquid natural rubber (LNR) as a compatibilizer in NR/LLDPE blends. J. Appl. Polym. Sci. 2000, 78, 1776–1782. [Google Scholar] [CrossRef]
- Abdullah, I.; Ahmad, S.; Sulaiman, C.S. Blending of natural rubber with linear low-density polyethylene. J. Appl. Polym. Sci. 1995, 58, 1125–1133. [Google Scholar] [CrossRef]
- Ibrahim, A. Thermoplastic natural rubber blends. Prog. Polym. Sci. 1998, 23, 665–706. [Google Scholar] [CrossRef]
- Panwiriyarat, W.; Tanrattanakul, V.; Pilard, J.-F.; Pasetto, P.; Khaokong, C. Effect of the diisocyanate structure and the molecular weight of diols on bio-based polyurethanes. J. Appl. Polym. Sci. 2013, 130, 453–462. [Google Scholar] [CrossRef]
- Panwiriyarat, W.; Tanrattanakul, V.; Pilard, J.-F.; Pasetto, P.; Khaokong, C. Preparation and Properties of Bio-based Polyurethane Containing Polycaprolactone and Natural Rubber. J Polym. Environ. 2013, 21, 807–815. [Google Scholar] [CrossRef]
- Saetung, A.; Rungvichaniwat, A.; Campistron, I.; Klinpituksa, P.; Laguerre, A.; Phinyocheep, P.; Doutres, O.; Pilard, J.-F. Preparation and physico-mechanical, thermal and acoustic properties of flexible polyurethane foams based on hydroxytelechelic natural rubber. J. Appl. Polym. Sci. 2010, 117, 828–837. [Google Scholar] [CrossRef]
- Dileep, U.; Avirah, S.A. Studies on carboxy-terminated liquid natural rubber in NBR. J. Appl. Polym. Sci. 2002, 84, 261–267. [Google Scholar] [CrossRef]
- Nor, H.M.; Ebdon, J.R. Ozonolysis of natural rubber in chloroform solution Part 1. A study by GPC and FTIR spectroscopy. Polymer. 2000, 41, 2359–2365. [Google Scholar] [CrossRef]
- Bac, N.V.; Terlemezyan, L.; Mihailov, M. Epoxidation of natural rubber in latex in the presence of a reducing agent. J. Appl. Polym. Sci. 1993, 50, 845–849. [Google Scholar] [CrossRef]
- Isa, S.Z.; Yahya, R.; Hassan, A.; Tahir, M. The influence of temperature and reaction time in the degradation of natural rubber latex. Malays. J. Anal. Sci. 2007, 11, 42–47. [Google Scholar]
- Ibrahim, S.; Daik, R.; Abdullah, I. Functionalization of Liquid Natural Rubber via Oxidative Degradation of Natural Rubber. Polymers 2014, 6, 2928–2941. [Google Scholar] [CrossRef]
- Thuong, N.T.; Manh, N.D.; Tue, N.N. Characterization of liquid deproteinized natural rubber prepared via oxidative degradation. Vietnam J. Chem. 2020, 58, 826–831. [Google Scholar]
- Fadhillah, I.; Wiranata, A.; Zahrina, I. Molecular Weight of Liquid Natural Rubber (LNR) Product from the Chemical Depolymerization Process of High Molecular Weight Natural Rubber Latex. J. Phys. Conf. Ser. 2020, 1655, 012091. [Google Scholar]
- Phetphaisit, C.W.; Phinyocheep, P. Kinetics and parameters affecting degradation of purified natural rubber. J. Appl. Polym. Sci. 2003, 90, 3546–3555. [Google Scholar] [CrossRef]
- Chen, L.; Xue, W.; Zeng, Z. Synthesis and Intrinsic Viscosity-molecular Weight Relationship of Poly(ethylene adipate). Chem. Sci. Int. J. 2017, 20, 1–9. [Google Scholar] [CrossRef]
- Yoksan, R. Epoxidized Natural Rubber for Adhesive Applications. Kasetsart J. 2008, 42, 325–332. [Google Scholar]
- Marussi, G.; Vione, D. Secondary Formation of Aromatic Nitroderivatives of Environmental Concern: Photonitration Processes Triggered by the Photolysis of Nitrate and Nitrite Ions in Aqueous Solution. Molecules 2021, 26, 2550. [Google Scholar] [CrossRef] [PubMed]
- Elliot, A.J.; Simsons, A.S. Reactions of NO2 and nitrite ion with organic radicals. Can. J. Chem. 1984, 62, 1831–1834. [Google Scholar] [CrossRef]
- Ibrahim, S.; Mustafa, A. Effect of reagents concentration and ratio on degradation of natural rubber latex in acidic medium. Malays. J. Anal. Sci. 2014, 18, 405–414. [Google Scholar]
- Wang, Z.; Luo, W.; Li, S.; Fang, L.; Liao, S.; Li, L.; Lin, H.; He, C. Rheological Behavior of Raw Natural Rubber Coagulated by Microorganisms. Polímeros. 2014, 24, 143–148. [Google Scholar] [CrossRef]
- Elias, H.-G. An Introduction to Polymer Science; VCH: Weinheim, Germany, 1997; p. 166. [Google Scholar]
- Tasakorn, P.; Amatyakul, W. Photochemical reduction of molecular weight and number of double bonds in natural rubber film. Korean J. Chem. Eng. 2008, 25, 1532–1538. [Google Scholar] [CrossRef]



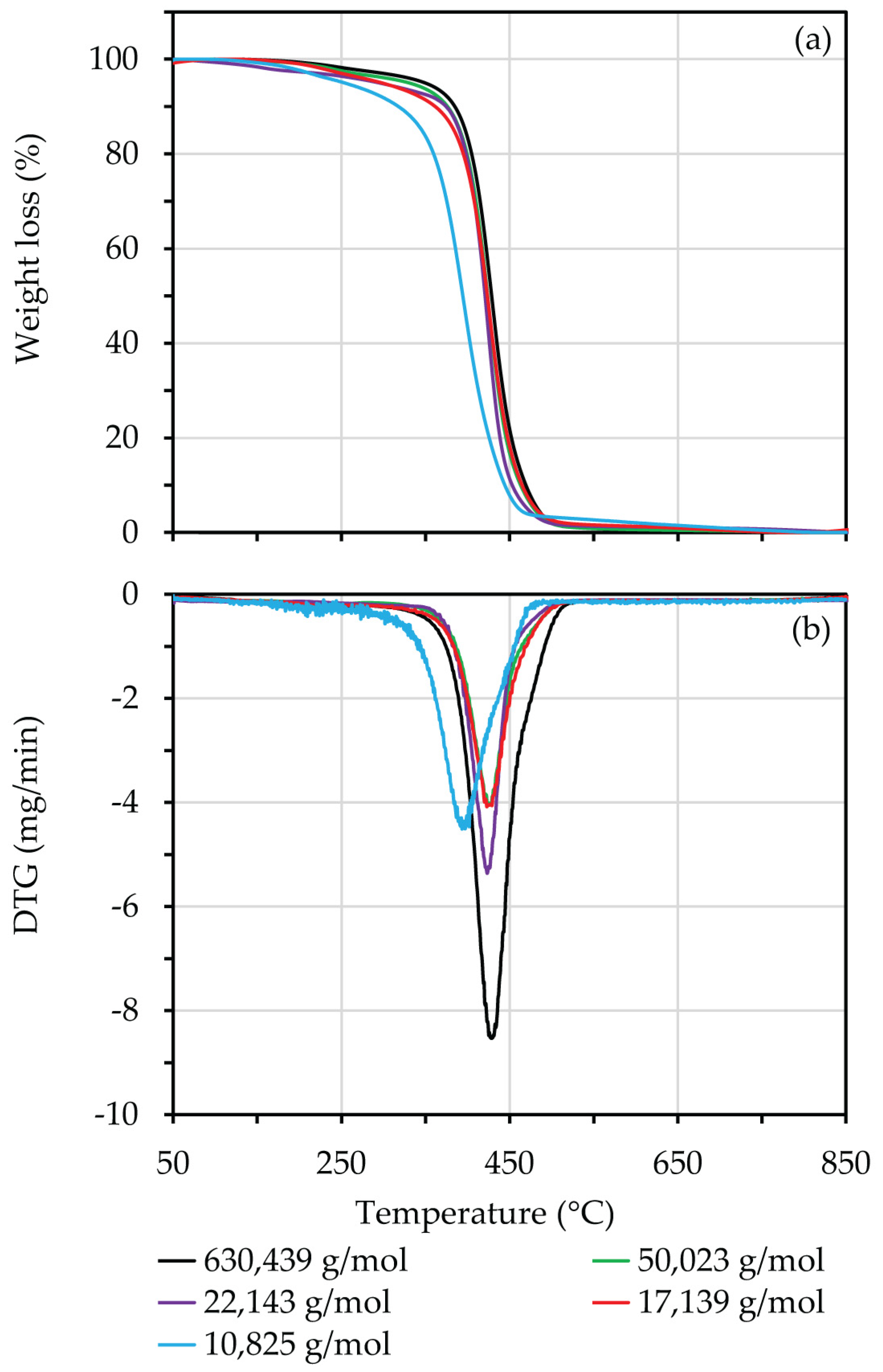
| Mn (g/mol) | Heating Rate = 40 °C/min | ||
|---|---|---|---|
| Ti (°C) | Tm (°C) | Tf (°C) | |
| 630,439 | 392 | 429 | 469 |
| 50,023 | 388 | 428 | 468 |
| 22,143 | 380 | 424 | 466 |
| 17,139 | 375 | 419 | 461 |
| 10,825 | 352 | 398 | 439 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wisetkhamsai, K.; Patthaveekongka, W.; Arayapranee, W. Study on Degradation of Natural Rubber Latex Using Hydrogen Peroxide and Sodium Nitrite in the Presence of Formic Acid. Polymers 2023, 15, 1031. https://doi.org/10.3390/polym15041031
Wisetkhamsai K, Patthaveekongka W, Arayapranee W. Study on Degradation of Natural Rubber Latex Using Hydrogen Peroxide and Sodium Nitrite in the Presence of Formic Acid. Polymers. 2023; 15(4):1031. https://doi.org/10.3390/polym15041031
Chicago/Turabian StyleWisetkhamsai, Kraiwut, Weerawat Patthaveekongka, and Wanvimon Arayapranee. 2023. "Study on Degradation of Natural Rubber Latex Using Hydrogen Peroxide and Sodium Nitrite in the Presence of Formic Acid" Polymers 15, no. 4: 1031. https://doi.org/10.3390/polym15041031
APA StyleWisetkhamsai, K., Patthaveekongka, W., & Arayapranee, W. (2023). Study on Degradation of Natural Rubber Latex Using Hydrogen Peroxide and Sodium Nitrite in the Presence of Formic Acid. Polymers, 15(4), 1031. https://doi.org/10.3390/polym15041031