Effect of Annealing Process and Molecular Weight on the Polymorphic Transformation from Form II to Form I of Poly(1-butene)
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Differential Scanning Calorimetry (DSC)
3. Results and Discussion
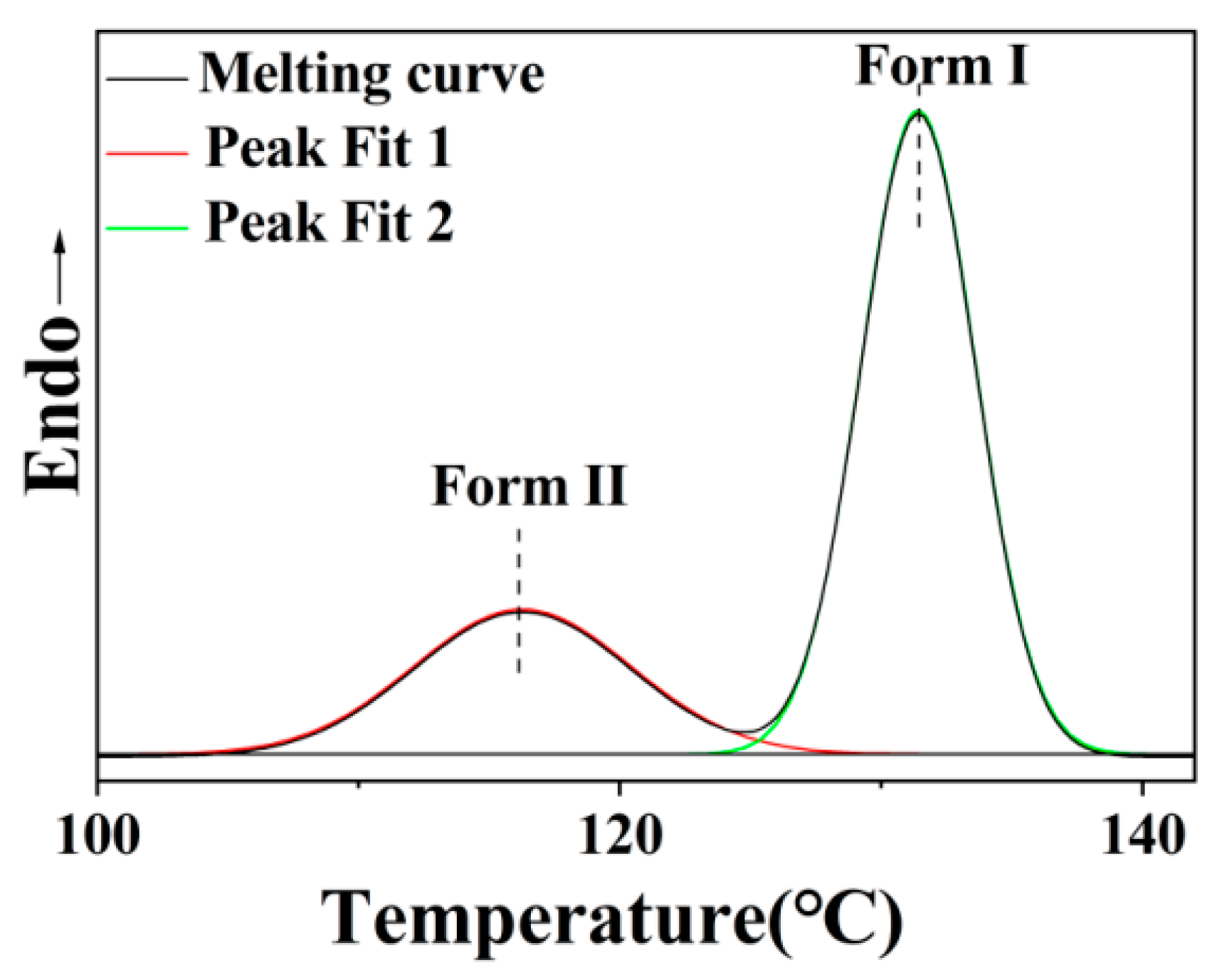
3.1. Characterization of Samples
3.2. Influence of Stepwise Annealing and Molecular Weight on Crystal Transformation
3.3. Influence of Low Annealing Temperature Tl and Molecular Weight on Crystal Transformation
3.4. Influence of High Annealing Temperature Th and Molecular Weight on Crystal Transformation
3.5. Influence of Low-Temperature Annealing Time tl and Molecular Weight on Crystal Transformation
3.6. Influence of High-Temperature Annealing Time th and Molecular Weight on Crystal Transformation
4. Conclusions
- (1)
- When annealed at one temperature, XI obtained by annealing at −10 °C was more than that obtained by annealing at 40 °C. Compared with the single annealing temperature, the step-by-step annealing process can significantly speed up the transformation rate from crystal form II to form I.
- (2)
- PB-1 samples with different molecular weights have the same dependence on annealing temperature, and the optimal low annealing temperature Tl and high annealing temperature Th were −10 °C and 40 °C, respectively. The crystal form I obtained by step-by-step annealing at these two temperatures had the highest content and the fastest transformation rate.
- (3)
- Under the same annealing temperature Tl or Th, with the increasing of molecular weight, XI obtained by the step-by-step annealing process firstly showed an increasing trend and then decreased. The molecular weight of sample F4 (360 K) was neither lowest nor highest in five samples, but its form I content was the highest one in all, which was because that molecular weight had double influence on the polymorphic transition from form II to form I.
- (4)
- Under the same annealing temperature Tl and Th, XI firstly increased with annealing time tl, then the rate slowed down and gradually reached a plateau, but the time to reach the plateau was different due to the different molecular weights of the five samples. XI of the samples with a relatively lower molecular weight monotonously increased with annealing time th. While the molecular weight was higher, XI increased rapidly with th at the beginning, and then transition speed slowed down.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Natta, G.; Pino, P.; Corradini, P.; Danusso, F.; Mantica, E.; Mazzanti, G.; Moraglio, G. Crystalline high polymers of alfa-olefins. J. Am. Chem. Soc. 1955, 77, 1708–1710. [Google Scholar] [CrossRef]
- Luciani, L.; Seppala, J.; Lofgren, B. Poly-1-butene: Its preparation, properties and challenges. Prog. Polym. Sci. 1988, 13, 37–62. [Google Scholar] [CrossRef]
- Miller, R.L.; Holland, V.F. On transformations in isotactic polybutene-1. J. Polym. Sci. Part B Polym. Lett. 1964, 2, 519–521. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Nie, M. Structure and performance of polybutene-1 pipes produced via mandrel rotation extrusion. J. Polym. Eng. 2014, 34, 15–22. [Google Scholar] [CrossRef]
- Wang, Y.T.; Liu, P.R.; Lu, Y.; Men, Y.F. Mechanism of polymorph selection during crystallization of random butene-1/ethylene copolymer. Chin. J. Polym. Sci. 2016, 34, 1014–1020. [Google Scholar] [CrossRef]
- Qin, Y.N.; Litvinov, V.; Chassé, W.; Zhang, B.; Men, Y.F. Change of lamellar morphology upon polymorphic transition of form II to form I crystals in isotactic polybutene-1 and its copolymer. Polymer 2021, 215, 123355–123365. [Google Scholar] [CrossRef]
- Miyoshi, T.; Mamun, A.; Reichert, D. Fast dynamics and conformations of polymer in a conformational disordered crystal characterized by 1H-13C WISE NMR. Macromolecules 2010, 43, 3986–3989. [Google Scholar] [CrossRef]
- Miyoshi, T.; Mamun, A. Critical roles of molecular dynamics in the superior mechanical properties of isotactic-poly(1-butene) elucidated by solid-state NMR. Polym. J. 2012, 44, 65–71. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Zhang, C.; Liu, C.T.; Wang, Z.; Liu, Y.P. Polymorphic transition of pre-oriented polybutene-1 under tensile deformation: In situ FTIR study. Chin. J. Polym. Sci. 2020, 38, 888–897. [Google Scholar] [CrossRef]
- Su, F.M.; Li, X.Y.; Zhou, W.M.; Chen, W.; Li, H.L.; Cong, Y.H.; Hong, Z.H.; Qi, Z.M.; Li, L.B. Accelerating crystal-crystal transition in poly(1-butene) with two-step crystallization: An in-situ microscopic infrared imaging and microbeam X-ray diffraction study. Polymer 2013, 54, 3408–3416. [Google Scholar] [CrossRef]
- Cheng, J.G.; Zhong, Z.X.; Lin, Y.; Su, Z.H.; Zhang, C.Y.; Zhang, X.Q. Miscibility of isotactic poly(1-butene)/isotactic polypropylene blends studied by atomic force microscopy-infrared. Polymer 2022, 239, 124445–124450. [Google Scholar] [CrossRef]
- Li, Y.K.; Zhang, X.X.; Sun, Z.Y. Influence of the coexistence of thin and thick lamellae on the transformation from crystalline form II to form I in isotactic polybutylene-1. Polymer 2020, 188, 122137–122144. [Google Scholar] [CrossRef]
- Zhang, X.X.; Sun, Z.Y. Competitive growth of crystalline form II and form I in isotactic polybutene-1. Polymer 2019, 171, 133–139. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Z.J.; Huang, S.Y.; Chen, Q. Form II to I transformation of polybutene-1 and copolymer of butane-1 and ethylene: A role of amorphous phase. Polymer 2018, 149, 146–153. [Google Scholar] [CrossRef]
- Ma, Y.P.; Zheng, W.P.; Liu, C.G.; Shao, H.F.; Nie, H.R.; He, A.H. Differential polymorphic transformation behavior of polybutene-1 with multiple isotactic sequences. Chin. J. Polym. Sci. 2020, 38, 164–173. [Google Scholar] [CrossRef]
- Wang, Z.F.; Dong, X.; Cavallo, D.; Müller, A.J.; Wang, D.J. Promotion of self-nucleation with latent form I nuclei in polybutene-1 and its copolymer. Macromolecules 2018, 51, 6037–6046. [Google Scholar] [CrossRef]
- Zheng, L.R.; Liu, L.; Shao, C.G.; Wang, W.; Wang, B.; Pan, L.; Li, Y.S.; Ma, Z. Phase transition from tetragonal form II to hexagonal form I of butene-1/4-methyl-1-pentene random copolymers: Molecular factor versus stretching stimuli. Macromolecules 2019, 52, 1188–1199. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, T.; Li, L.; Li, D.C.; Yuan, W.K.; Zhao, L. Controlling crystal phase transition from form II to I in isotactic poly-1-butene using CO2. Polymer 2012, 53, 6102–6111. [Google Scholar] [CrossRef]
- Qiao, Y.N.; Liu, J.; Zhao, J.C.; Xu, M.L.; Qi, Q.; Chen, Z.L.; Men, Y.F.; Park, C.B.; Lee, P.C. Promotion of form I’ in the polymorph selection of polybutene-1 during crystallization under high gas/supercritical fluid pressure via enhancing chain mobility. Macromolecules 2020, 53, 10069–10077. [Google Scholar] [CrossRef]
- Shao, H.F.; Ma, Y.P.; Nie, H.R.; He, A.H. Solvent vapor annealing induced polymorphic transformation of polybutene-1. Chin. J. Polym. Sci. 2016, 34, 1141–1149. [Google Scholar] [CrossRef]
- Qiu, X.; Azhar, U.; Li, J.Q.; Huang, D.H.; Jiang, S.C. Ultrafast form II to I transition of isotactic polybutene-1. Chin. J. Polym. Sci. 2019, 37, 633–636. [Google Scholar] [CrossRef]
- Qiao, Y.N.; Wang, Q.; Men, Y.F. Kinetics of nucleation and growth of form II to I polymorphic transition in polybutene-1 as revealed by stepwise annealing. Macromolecules 2016, 49, 5126–5136. [Google Scholar] [CrossRef]
- Liu, P.R.; Men, Y.F. Glass-transition-temperature-independent form II to I phase transition of low-molar-mass isotactic polybutene-1. Macromolecules 2021, 54, 858–865. [Google Scholar] [CrossRef]
- Qiao, Y.N.; Men, Y.F. Intercrystalline links determined kinetics of form II to I polymorphic transition in polybutene-1. Macromolecules 2017, 50, 5490–5497. [Google Scholar] [CrossRef]
- Xue, Y.H.; Liu, W.; Li, P.; Men, Y.F.; Bo, S.Q.; Ji, X.L. Preparative temperature rising elution fractionation of one poly(1-butene) copolymer and its chain microstructure characterization. Ind. Eng. Chem. Res. 2019, 58, 16869–16876. [Google Scholar] [CrossRef]
- Xue, Y.H.; Shi, L.; Liu, W.; Bo, S.; Cui, Y.Y.; Ji, X.L. Solvent gradient fractionation of polybutene-1 resin and its molecular weight dependency of form II to I transformation. Polymer 2020, 198, 122536–122545. [Google Scholar] [CrossRef]
- Shi, L.; Liu, W.; Xue, Y.H.; Hong, M.; Ji, X.L. Influence of isothermal crystallization temperature on the temperature rising elution fractionation for a poly(1-butene-co-ethylene) resin. Polymer 2021, 221, 123584–123594. [Google Scholar] [CrossRef]
- Acierno, S.; Grizzuti, N.; Winter, H.H. Effects of molecular weight on the isothermal crystallization of poly(1-butene). Macromolecules 2002, 35, 5043–5048. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R.; Righetti, M.C. The irreversible form II to form I transformation in random butene-1/ethylene copolymers. Eur. Polym. J. 2015, 67, 264–273. [Google Scholar] [CrossRef]
- Powers, J.; Hoffman, J.D.; Weeks, J.J.; Quinn, F.A., Jr. Crystallization kinetics and polymorphic transformations in polybutene-1. J. Res. Natl. Bur. Stand. Sect. A 1965, 69A, 335–345. [Google Scholar] [CrossRef]
- Keith, H.D.; Padden, F.J., Jr.; Vadimsky, R.G. Intercrystalline links in bulk polyethylene. Science 1965, 150, 1026–1027. [Google Scholar] [CrossRef] [PubMed]












| Sample | Sample Type | Mw (kg/mol) | PDI | Annealing Method | Manufacturer | References |
|---|---|---|---|---|---|---|
| DP8510M | Copolymer | 153 | 2.2 | Stepwise Annealing | Lyondell Basell Industry | Men et al., 2021 [6] |
| PB0400M | Homopolymer | 188 | 2.7 | Single temperature annealing | Lyondell Basell Industry | Sun et al., 2020 [12] |
| PB0400M | Homopolymer | 188 | 2.7 | Stepwise Annealing | Lyondell Basell Industry | Men et al., 2021 [6] Sun et al., 2019 [13] Men et al., 2017 [24] |
| PB0110 PB8640 PB8220 PB8510 | Homopolymer Copolymer Copolymer Copolymer | 742 552 414 203 | 3.3 3.5 2.7 2.6 | Single temperature annealing/ Stepwise Annealing | Lyondell Basell Industry | Chen et al., 2018 [14] |
| iPB | Homopolymer | 548 | 3.9 | Single temperature annealing | Shandong Dongfang Hongye Chemical Co., Ltd. China | He et al., 2020 [15] |
| PB8340M | Copolymer | 28.1 | 4.3 | Stepwise Annealing | Lyondell Basell Industry | Wang et al., 2018 [16] |
| PB-L | Homopolymer | 8.0 | 2.7 | Stepwise Annealing | Tianjin University | Men et al., 2021 [23] |
| PB0800 | Homopolymer | 77 | 3.0 | Stepwise Annealing | Lyondell Basell Industry | Men et al., 2017 [24] |
| F2 F4 F6 F8 F10 F12 | Homopolymer | 31.9 85.3 135.1 239.7 710.0 1029.8 | 1.53 1.10 1.09 1.14 1.44 1.40 | Single temperature annealing | Changchun Institute of Applied Chemistry | Xue et al., 2020 [26] |
| iPB398 iPB295 iPB177 iPB116 | Homopolymer | 398 295 177 116 | 3.8 4.6 3.3 3.1 | Single temperature annealing | Shell | Winter et al., 2002 [28] |
| Sample | Mw (103 g/mol) | PDI |
|---|---|---|
| F1 | 23 | 1.09 |
| F2 | 109 | 1.10 |
| F3 | 201 | 1.10 |
| F4 | 360 | 1.20 |
| F5 | 710 | 1.44 |
| Sample | Tm (°C) | ∆Hm (J/g) | Tc (°C) | ∆Hc (J/g) |
|---|---|---|---|---|
| F1 | 113.8 | 44.2 | 82.4 | 42.4 |
| F2 | 116.9 | 43.6 | 88.4 | 43.1 |
| F3 | 118.8/132.6 | 36.8/0.2 | 87.4 | 36.6 |
| F4 | 117.9/132.2 | 35.4/0.2 | 88.1 | 34.1 |
| F5 | 117.9/132.5 | 33.3/0.1 | 84.7 | 32.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Xue, Y.; Li, R.; Liu, W.; Liu, P.; Ji, X. Effect of Annealing Process and Molecular Weight on the Polymorphic Transformation from Form II to Form I of Poly(1-butene). Polymers 2023, 15, 800. https://doi.org/10.3390/polym15040800
Zhang Z, Xue Y, Li R, Liu W, Liu P, Ji X. Effect of Annealing Process and Molecular Weight on the Polymorphic Transformation from Form II to Form I of Poly(1-butene). Polymers. 2023; 15(4):800. https://doi.org/10.3390/polym15040800
Chicago/Turabian StyleZhang, Zhenkang, Yanhu Xue, Rui Li, Wei Liu, Peng Liu, and Xiangling Ji. 2023. "Effect of Annealing Process and Molecular Weight on the Polymorphic Transformation from Form II to Form I of Poly(1-butene)" Polymers 15, no. 4: 800. https://doi.org/10.3390/polym15040800
APA StyleZhang, Z., Xue, Y., Li, R., Liu, W., Liu, P., & Ji, X. (2023). Effect of Annealing Process and Molecular Weight on the Polymorphic Transformation from Form II to Form I of Poly(1-butene). Polymers, 15(4), 800. https://doi.org/10.3390/polym15040800







