Nitrogen-Rich Polyaniline-Based Activated Carbon for Water Treatment: Adsorption Kinetics of Anionic Dye Methyl Orange
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Adsorbents
2.3. Adsorbate
2.4. Characterization
2.5. Modeling of Adsorption Kinetics Processes
2.5.1. PFO Model
2.5.2. PSO Model
2.5.3. Elovich Model
2.5.4. IPD Model
3. Results and Discussion
3.1. Adsorbent Characterization
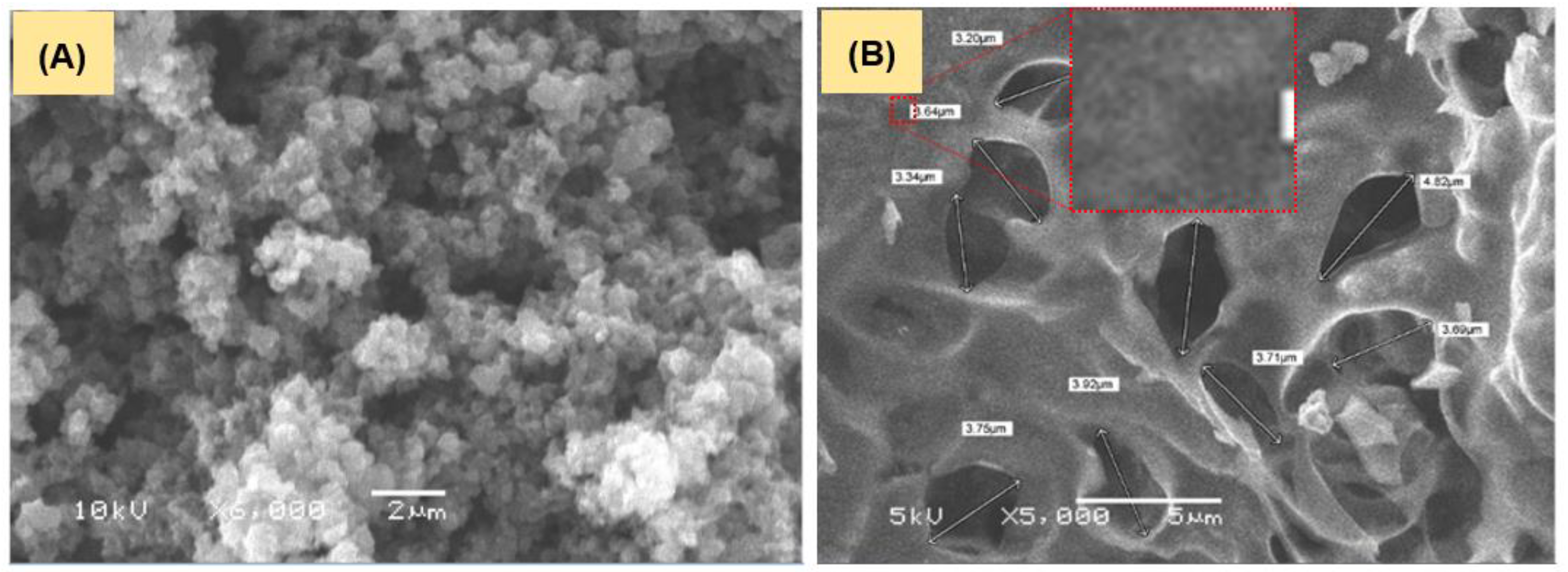
3.1.1. Surface Morphology and Charge
3.1.2. FTIR Analysis
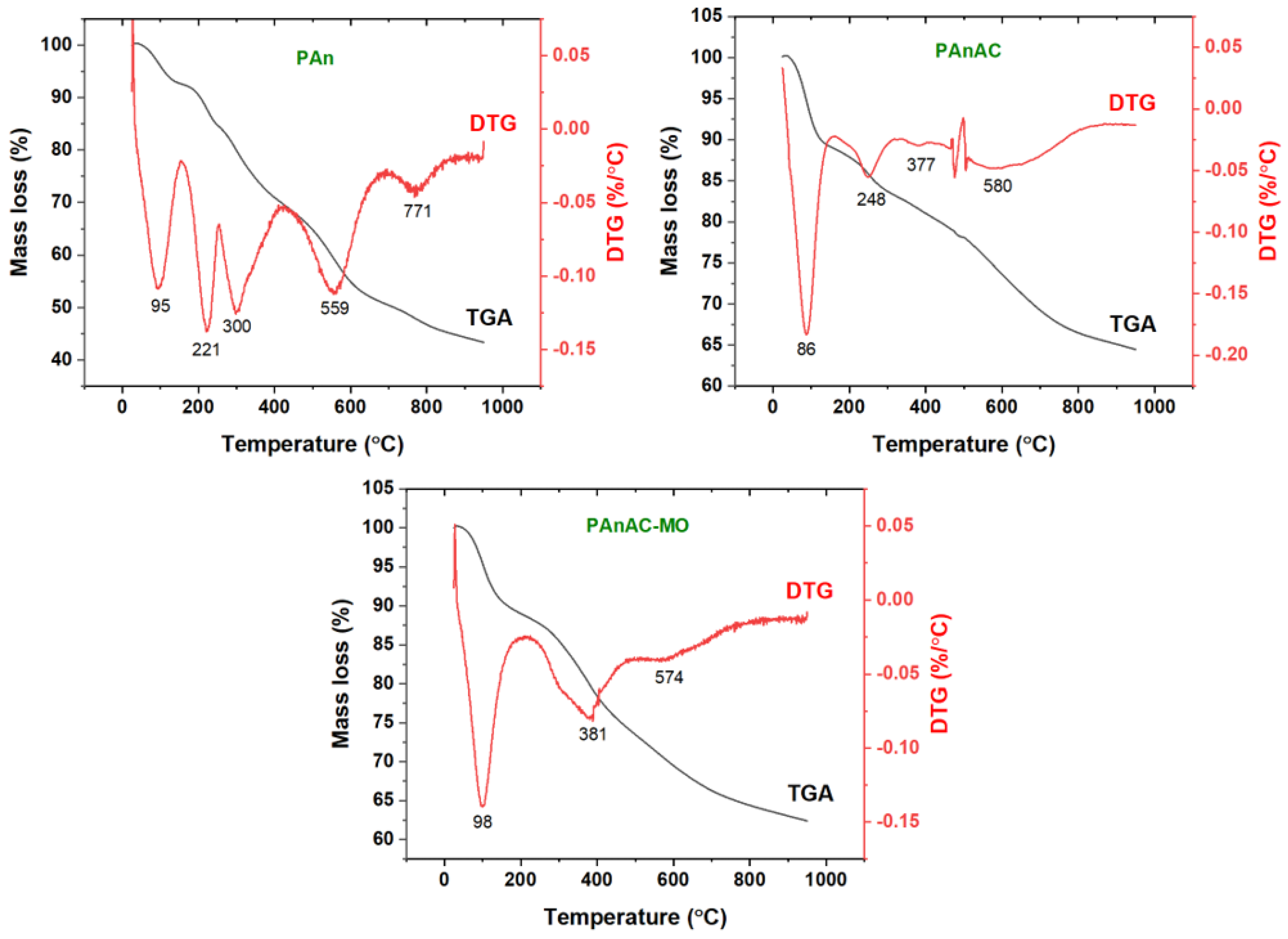
3.1.3. Thermal Analysis
3.2. Adsorption Studies
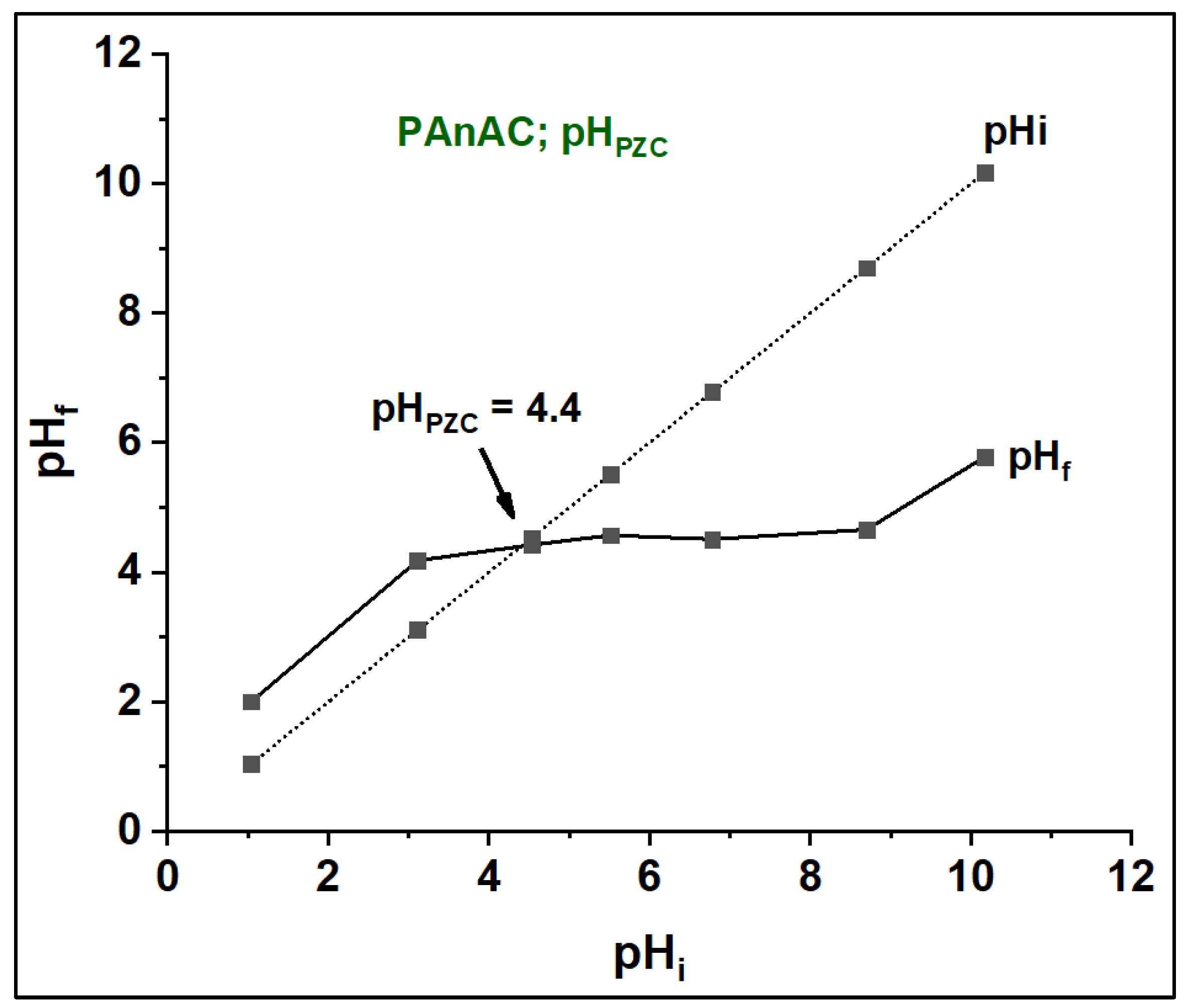
3.2.1. Effect of pH
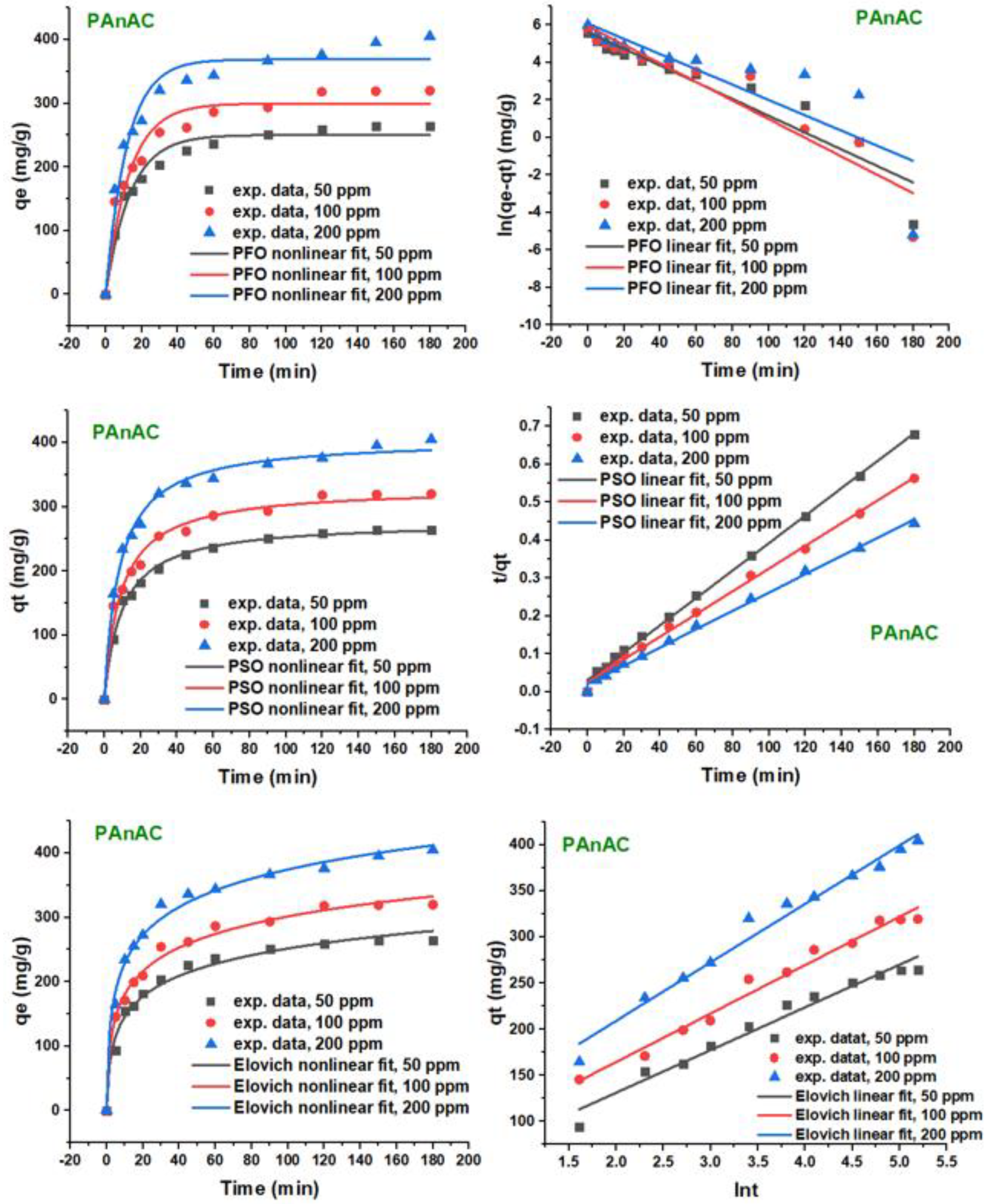
3.2.2. Kinetic Studies: Effect of Adsorbate Concentration
3.2.3. Kinetic Studies: Effect of Adsorbent Dose
3.3. Adsorption-Based Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayaswal, K.; Sahu, V.; Gurjar, B. Water pollution, human health and remediation. In Water Remediation; Springer: Berlin, Germany, 2018; pp. 11–27. [Google Scholar]
- Hairom, N.H.H.; Soon, C.F.; Mohamed, R.M.S.R.; Morsin, M.; Zainal, N.; Nayan, N.; Zulkifli, C.Z.; Harun, N.H. A review of nanotechnological applications to detect and control surface water pollution. Environ. Technol. Innov. 2021, 24, 102032. [Google Scholar] [CrossRef]
- Iyinbor, A.; Adebesin, B.; Abimbola, O.; Adelani-Akande, A. Water pollution: Effects, prevention and climatic impact. Water Chall. Urban. World 2018, 33, 33–47. [Google Scholar]
- Alghamdi, A.A.; Al-Odayni, A.-B.; Saeed, W.S.; Almutairi, M.S.; Alharthi, F.A.; Aouak, T.; Al-Kahtani, A. Adsorption of azo dye methyl orange from aqueous solutions using alkali-activated polypyrrole-based graphene oxide. Molecules 2019, 24, 3685. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Mahanpoor, K.; Moradi, O. Removal of dye molecules from aqueous solution by carbon nanotubes and carbon nanotube functional groups: Critical review. Rsc Adv. 2017, 7, 47083–47090. [Google Scholar] [CrossRef]
- Alghamdi, A.A.; Al-Odayni, A.-B.; Saeed, W.S.; Al-Kahtani, A.; Alharthi, F.A.; Aouak, T. Efficient adsorption of lead (II) from aqueous phase solutions using polypyrrole-based activated carbon. Materials 2019, 12, 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Zandi-Mehri, E.; Taghavi, L.; Moeinpour, F.; Khosravi, I.; Ghasemi, S. Facile application of OH-ended triazinic dendrimer/halloysite nanotube for removal of methylene blue with a high capacity. Int. J. Environ. Anal. Chem. 2022, 102, 4768–4783. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Ighalo, J.O.; Emenike, E.C.; Ogunfowora, L.A.; Igwegbe, C.A. Adsorption of methyl orange: A review on adsorbent performance. Curr. Res. Green Sustain. Chem. 2021, 4, 100179. [Google Scholar] [CrossRef]
- Gupta, V. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Tarmahi, M.H.; Moeinpour, F. Phosphate removal from aqueous solutions using polyaniline/Ni0. 5Zn0. 5Fe2O4 magnetic nanocomposite. Environ. Health Eng. Manag. J. 2017, 4, 65–71. [Google Scholar] [CrossRef]
- Nourmoradi, H.; Nikaeen, M.; Khiadani, M. Removal of benzene, toluene, ethylbenzene and xylene (BTEX) from aqueous solutions by montmorillonite modified with nonionic surfactant: Equilibrium, kinetic and thermodynamic study. Chem. Eng. J. 2012, 191, 341–348. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Doke, K.; Yusufi, M.; Joseph, R.; Khan, E. Comparative adsorption of crystal violet and congo red onto ZnCl2 activated carbon. J. Dispers. Sci. Technol. 2016, 37, 1671–1681. [Google Scholar] [CrossRef]
- Dey, A.; Singh, R.; Purkait, M. Cobalt ferrite nanoparticles aggregated schwertmannite: A novel adsorbent for the efficient removal of arsenic. J. Water Process Eng. 2014, 3, 1–9. [Google Scholar] [CrossRef]
- López-Luna, J.; Ramírez-Montes, L.E.; Martinez-Vargas, S.; Martínez, A.I.; Mijangos-Ricardez, O.F.; González-Chávez, M.d.C.A.; Carrillo-González, R.; Solís-Domínguez, F.A.; Cuevas-Díaz, M.d.C.; Vázquez-Hipólito, V. Linear and nonlinear kinetic and isotherm adsorption models for arsenic removal by manganese ferrite nanoparticles. SN Appl. Sci. 2019, 1, 950. [Google Scholar] [CrossRef]
- Tejada, C.; Herrera, A.; Ruiz, E. Kinetic and isotherms of biosorption of Hg (II) using citric acid treated residual materials. Ing. Y Compet. 2016, 18, 117–127. [Google Scholar] [CrossRef]
- Shikuku, V.O.; Zanella, R.; Kowenje, C.O.; Donato, F.F.; Bandeira, N.M.; Prestes, O.D. Single and binary adsorption of sulfonamide antibiotics onto iron-modified clay: Linear and nonlinear isotherms, kinetics, thermodynamics, and mechanistic studies. Appl. Water Sci. 2018, 8, 175. [Google Scholar] [CrossRef]
- Belhamdi, B.; Merzougui, Z.; Trari, M.; Addoun, A. A kinetic, equilibrium and thermodynamic study of l-phenylalanine adsorption using activated carbon based on agricultural waste (date stones). J. Appl. Res. Technol. 2016, 14, 354–366. [Google Scholar] [CrossRef]
- Tan, K.; Hameed, B. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Ferreira, A.; Mota, A.; Oliveira, A.; Rodrigues, F.; Pacífico, S.; Da Silva, J.; Abagaro, B.; Saraiva, G.; De Castro, A.; Teixeira, R. Equilibrium and kinetic modelling of adsorption: Evaluating the performance of an adsorbent in softening water for irrigation and animal consumption. Rev. Virtual Química 2019, 11, 1752–1766. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Lagegren, S.; Svenska, B. Zur theorie der sogenannten adsorption geloester stoffe. Vaternskapsakad Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber Jr, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Etorki, A.; Kammashi, M.; Elhabbat, M.; Shaban, I. Application of polyaniline nanoparticles modified screen printed carbon electrode as a sensor for determination of Hg (II) in environmental samples. J. Environ. Anal. Toxicol. 2017, 7, 2161. [Google Scholar]
- Kalyani, D.C.; Telke, A.A.; Govindwar, S.P.; Jadhav, J.P. Biodegradation and detoxification of reactive textile dye by isolated Pseudomonas sp. SUK1. Water Environ. Res. 2009, 81, 298–307. [Google Scholar] [CrossRef]
- Joshi, S.; Pokharel, B.P. Preparation and characterization of activated carbon from lapsi (Choerospondias axillaris) seed stone by chemical activation with potassium hydroxide. J. Inst. Eng. 2013, 9, 79–88. [Google Scholar] [CrossRef]
- Shao, W.; Jamal, R.; Xu, F.; Ubul, A.; Abdiryim, T. The effect of a small amount of water on the structure and electrochemical properties of solid-state synthesized polyaniline. Materials 2012, 5, 1811–1825. [Google Scholar] [CrossRef]
- Turczyn, R.; Krukiewicz, K.; Katunin, A.; Sroka, J.; Sul, P. Fabrication and application of electrically conducting composites for electromagnetic interference shielding of remotely piloted aircraft systems. Compos. Struct. 2020, 232, 111498. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, I.; Husain, A.; Khan, A.; Asiri, A.M. Electrical conductivity based ammonia sensing properties of polypyrrole/MoS2 nanocomposite. Polymers 2020, 12, 3047. [Google Scholar] [CrossRef]
- Tchuifon, D.; Anagho, S.; Njanja, E.; Ghogomu, J.; Ndifor-Angwafor, N.; Kamgaing, T. Equilibrium and kinetic modelling of methyl orange adsorption from aqueous solution using rice husk and egussi peeling. Int. J. Chem. Sci. 2014, 12, 741–761. [Google Scholar]
- Kajjumba, G.W.; Emik, S.; Öngen, A.; Özcan, H.K.; Aydın, S. Modelling of adsorption kinetic processes—Errors, theory and application. Adv. Sorpt. Process Appl. 2018, 1–19. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, X.; Han, M.; Ma, W. Adsorption kinetic character of copper ions onto a modified chitosan transparent thin membrane from aqueous solution. J. Hazard. Mater. 2010, 182, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Mashkoor, F.; Nasar, A. Magnetized Tectona grandis sawdust as a novel adsorbent: Preparation, characterization, and utilization for the removal of methylene blue from aqueous solution. Cellulose 2020, 27, 2613–2635. [Google Scholar] [CrossRef]
- Ribas, M.C.; Adebayo, M.A.; Prola, L.D.; Lima, E.C.; Cataluña, R.; Feris, L.A.; Puchana-Rosero, M.; Machado, F.M.; Pavan, F.A.; Calvete, T. Comparison of a homemade cocoa shell activated carbon with commercial activated carbon for the removal of reactive violet 5 dye from aqueous solutions. Chem. Eng. J. 2014, 248, 315–326. [Google Scholar] [CrossRef]
- Cardoso, N.F.; Lima, E.C.; Royer, B.; Bach, M.V.; Dotto, G.L.; Pinto, L.A.; Calvete, T. Comparison of Spirulina platensis microalgae and commercial activated carbon as adsorbents for the removal of Reactive Red 120 dye from aqueous effluents. J. Hazard. Mater. 2012, 241, 146–153. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, Á.; Suárez-García, F.; Martínez-Alonso, A.; Tascón, J. Synthesis, characterization and dye removal capacities of N-doped mesoporous carbons. J. Colloid Interface Sci. 2015, 450, 91–100. [Google Scholar] [CrossRef]
- Khattabi, E.H.E.; Rachdi, Y.; Bassam, R.; Mourid, E.; Naimi, Y.; Alouani, M.E.; Belaaouad, S. Enhanced elimination of methyl orange and recycling of an eco-friendly adsorbent activated carbon from aqueous solution. Russ. J. Phys. Chem. B 2021, 15, S149–S159. [Google Scholar] [CrossRef]
- Smita, J.; Dipika, J.; Shraddha, K. Polyaniline for removal of methyl orange dye from waste water. Int. J. Sci. Eng. Manag. 2016, 3, 1–6. [Google Scholar]
- Duhan, M.; Kaur, R. Adsorptive removal of methyl orange with polyaniline nanofibers: An unconventional adsorbent for water treatment. Environ. Technol. 2020, 41, 2977–2990. [Google Scholar] [CrossRef] [PubMed]
- Khairy, M.; Kamar, E.M.; Yehia, M.; Masoud, E.M. High removal efficiency of methyl orange dye by pure and (Cu, N) doped TiO2/polyaniline nanocomposites. Biointerface Res. Appl. Chem 2021, 12, 893–909. [Google Scholar]






| Kinetic Models | Nonlinear | Linear | Constants | Ref. | ||
|---|---|---|---|---|---|---|
| Equation | Plot | Equation | Plot | |||
| PFO | qt vs. t | ln(qe − qt) vs. t | k1 (min−1) | [24] | ||
| PSO | qt vs. t | t/qt vs. t | k2 (g/mg·min); h = k2qe2 (mg/g·min) | [25] | ||
| Elovich | qt vs. t | qt vs. lnt | 𝛼 (mg/g·min); 𝛽 (g/mg) | [16] | ||
| IPD | - | - | qt vs. t0.5 | kid (mg/g·min0.5); C (mg/g) | [26] | |
| Material | BET Surface Area (m2/g) | Average Pore Width (nm) | CO2 Adsorption Capacity (mg/g) | Elemental Analysis (wt%) | |||
|---|---|---|---|---|---|---|---|
| C | H | N | O * | ||||
| PAnAC | 1893 | 163.5 | 56.14 | 58.80 | 3.27 | 18.01 | 19.92 |
| Steps | Property | PAn | PAnAC | PAnAC-MO |
|---|---|---|---|---|
| Step 1 | Temperature (°C) | 25–153 | 25–153 | 25–208 |
| Mass Loss (%) | 7.4 | 10.9 | 11.2 | |
| DTG (°C) | 95 | 86 | 98 | |
| Assig. | Adsorbed volatiles | |||
| Step 2 | Temperature (°C) | 154–255 | 154–326 | 209–474 |
| Mass Loss (%) | 8.2 | 6.0 | 14.3 | |
| DTG (°C) | 221 | 248 | 381 | |
| Assig. | Weak parts and sensitive functional groups | |||
| Step 3 | Temperature (°C) | 256–419 | 327–417 | - |
| Mass Loss (%) | 14.7 | 2.5 | - | |
| DTG (°C) | 300 | 377 | - | |
| Assig. | Oxidative process | - | ||
| Step 4 | Temperature (°C) | 420–686 | 418–785 | - |
| Mass Loss (%) | 18.9 | 13.8 | - | |
| DTG (°C) | 559 | 580 | - | |
| Assig. | Carbonaceous step (C. step) | - | ||
| Step 5 | Temperature (°C) | 687–950 | - | - |
| Mass Loss (%) | 7.4 | - | - | |
| DTG (°C) | 771 | - | - | |
| Assig. | C. step | - | - | |
| Residue at 950 °C | 43.4 | 64.5 | 62.3 | |
| Conc. (mg/L) | qe,exp. (mg/g) | PFO | PSO | Elovich | Best Fit | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10−3k1 (min−1) | qe,calc. (mg/g) | R2 | 10−3 k2 (min−1) | qe,calc. (mg/g) | h (mg/(g·min)) | R2 | 𝛼 | 𝛽 | R2 | |||
| Linear models | ||||||||||||
| 50 | 264.7 | 44.6 | 276.5 | 0.894 | 0.43 | 276.2 | 32.8 | 0.998 | 106.7 | 0.0216 | 0.968 | PSO |
| 100 | 319.7 | 49.5 | 376.5 | 0.896 | 0.35 | 333.3 | 38.9 | 0.997 | 163.2 | 0.0190 | 0.980 | PSO |
| 200 | 405.0 | 40.7 | 433.7 | 0.706 | 0.29 | 413.2 | 49.5 | 0.997 | 232.5 | 0.0158 | 0.978 | PSO |
| Nonlinear models | ||||||||||||
| 50 | 264.7 | 73.6 | 250.3 | 0.969 | 0.37 | 277.4 | 28.5 | 0.995 | 101.2 | 0.0214 | 0.985 | PSO |
| 100 | 319.7 | 76.1 | 299.7 | 0.942 | 0.33 | 330.1 | 36.0 | 0.984 | 152.8 | 0.0178 | 0.992 | Elovich |
| 200 | 405.0 | 84.4 | 368.9 | 0.957 | 0.30 | 405.9 | 49.4 | 0.992 | 223.1 | 0.0157 | 0.991 | PSO |
| Conc. (mg/L) | qe,exp. (mg/g) | IPD Model | |||||
|---|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | ||||||
| Kid1 (mg/(g·min0.5)) | C1 | R2 | Kid2 (mg/(g·min0.5)) | C2 | R2 | ||
| 50 | 264.7 | 21.16 | 85.35 | 0.990 | 5.91 | 190.40 | 0.936 |
| 100 | 319.7 | 25.74 | 94.39 | 0.966 | 6.69 | 235.29 | 0.854 |
| 200 | 405.0 | 37.46 | 101.94 | 0.994 | 10.49 | 264.75 | 0.992 |
| Adsorbent | Dose (mg), MO = 100 ppm | ||
|---|---|---|---|
| 20 mg | 40 mg | 80 mg | |
| PAnAC; qe,exp. (mg/g) | 320.09 | 319.73 | 287.73 |
| Adsorbent | Azo Dye Used | Dye Conc. (mg/L) | Capacity (qe; mg/g) | Mixing Time (min) | Adsorbent Dosage (g/L) | pH | Temp. (°C) | Kinetic Model | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Commercial activated carbon (CAC; Merck) | Reactive violet 5 | 1000 | 246 | 150 | 2.5 | 2 | 25 | Pseudo-second-order | [37] |
| Acidified cocoa shell activated carbon (ACC-1.0) | 400 | ||||||||
| Commercial activated carbon (AC; Merck) | Reactive red 120 | 50 | 267 | 180 | 2.5 | 2 | 25 | General-order | [38] |
| S. platensis microalgae (SP) | 482 | ||||||||
| Nitrogen-doped mesoporous carbons (N-OMCs) | Methyl orange | 200 | 135 | 90 | 1.0 | - | 25 | - | [39] |
| Nondoped mesoporous carbons (N-OMCs) | 120 | ||||||||
| Commercial activated carbon (LOBA Chemie) | Methyl orange | 80 | 96 | 250 | 0.75 | 2 | 25 | Pseudo-second-order | [40] |
| Polyaniline | Methyl orange | 131 | 63 | 360 | 1.2 | 3 | 30 | - | [41] |
| Polyaniline nanofibers | Methyl orange | 7 | 22 | 80 | 0.5 | 7 | 25 | Pseudo-second-order | [42] |
| Polyaniline | Methyl orange | 120 | 111 | 20 | 1.0 | 7 | 25 | Pseudo-second-order | [43] |
| TiO2/Polyaniline composite | 119 | ||||||||
| PAnAC | Methyl orange | 200 | 406 | 60 | 0.16 | 6.4 | 23 | Pseudo-second-order | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Odayni, A.-B.; Alsubaie, F.S.; Saeed, W.S. Nitrogen-Rich Polyaniline-Based Activated Carbon for Water Treatment: Adsorption Kinetics of Anionic Dye Methyl Orange. Polymers 2023, 15, 806. https://doi.org/10.3390/polym15040806
Al-Odayni A-B, Alsubaie FS, Saeed WS. Nitrogen-Rich Polyaniline-Based Activated Carbon for Water Treatment: Adsorption Kinetics of Anionic Dye Methyl Orange. Polymers. 2023; 15(4):806. https://doi.org/10.3390/polym15040806
Chicago/Turabian StyleAl-Odayni, Abdel-Basit, Faisal S. Alsubaie, and Waseem Sharaf Saeed. 2023. "Nitrogen-Rich Polyaniline-Based Activated Carbon for Water Treatment: Adsorption Kinetics of Anionic Dye Methyl Orange" Polymers 15, no. 4: 806. https://doi.org/10.3390/polym15040806








