Selective Sorption of Cerium Ions from Uranium-Containing Solutions by Remotely Activated Ion Exchangers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Equipment
2.2. Determination of the Polymer Chain Binding Degree
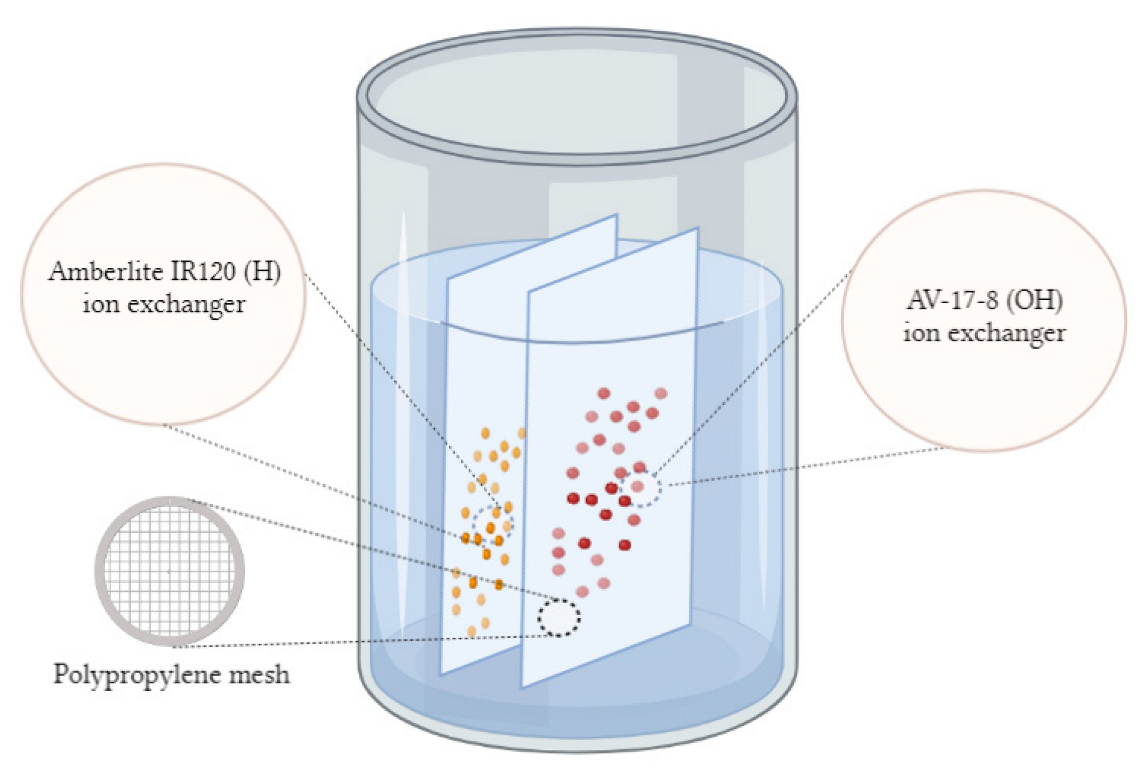
2.3. Preparation of the Interpolymer System
2.4. Activation of the Interpolymer System
2.5. Determination of the Cerium Ion Concentration and Desorption Studies
3. Results
3.1. Determination of the Polymer Chain Binding Degree
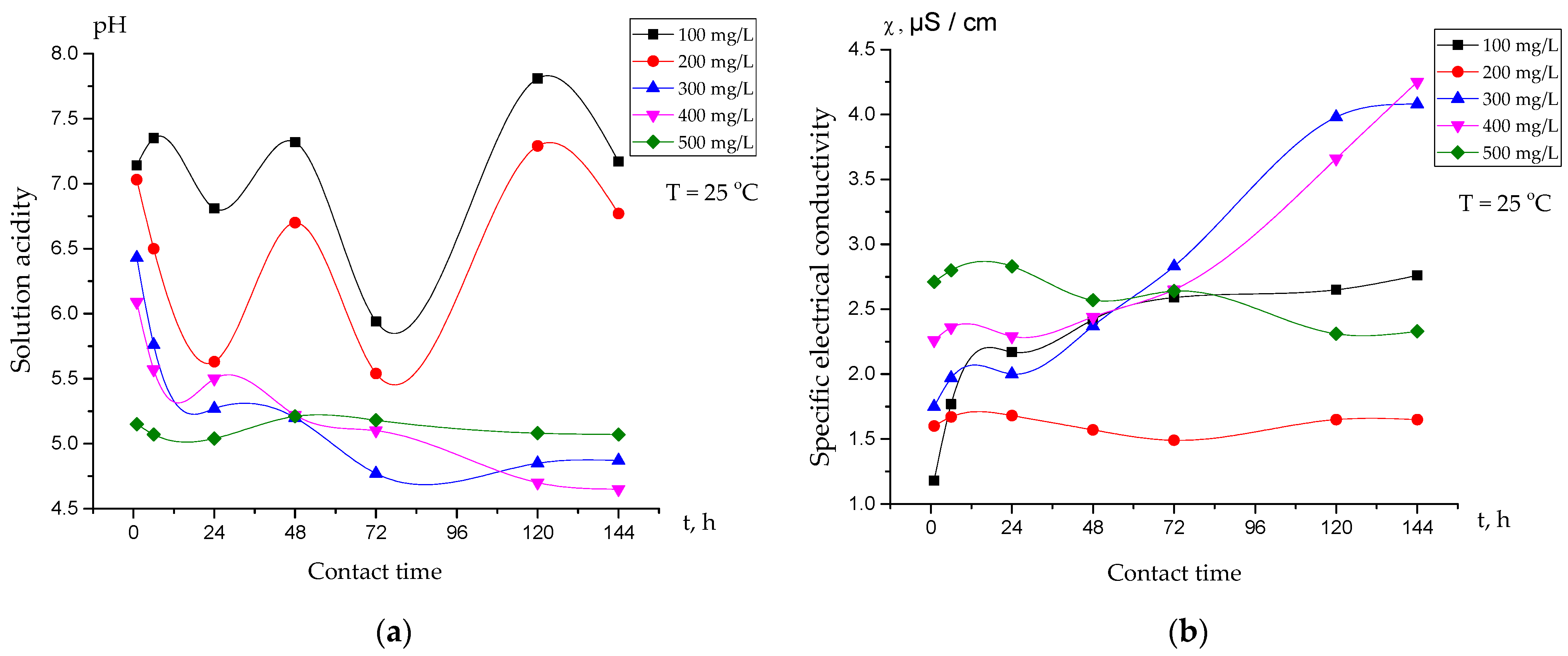
3.2. Electrochemical Studies of the Interpolymer System “Amberlite IR120:AV-17-8” (X:Y) in Aqueous Solutions
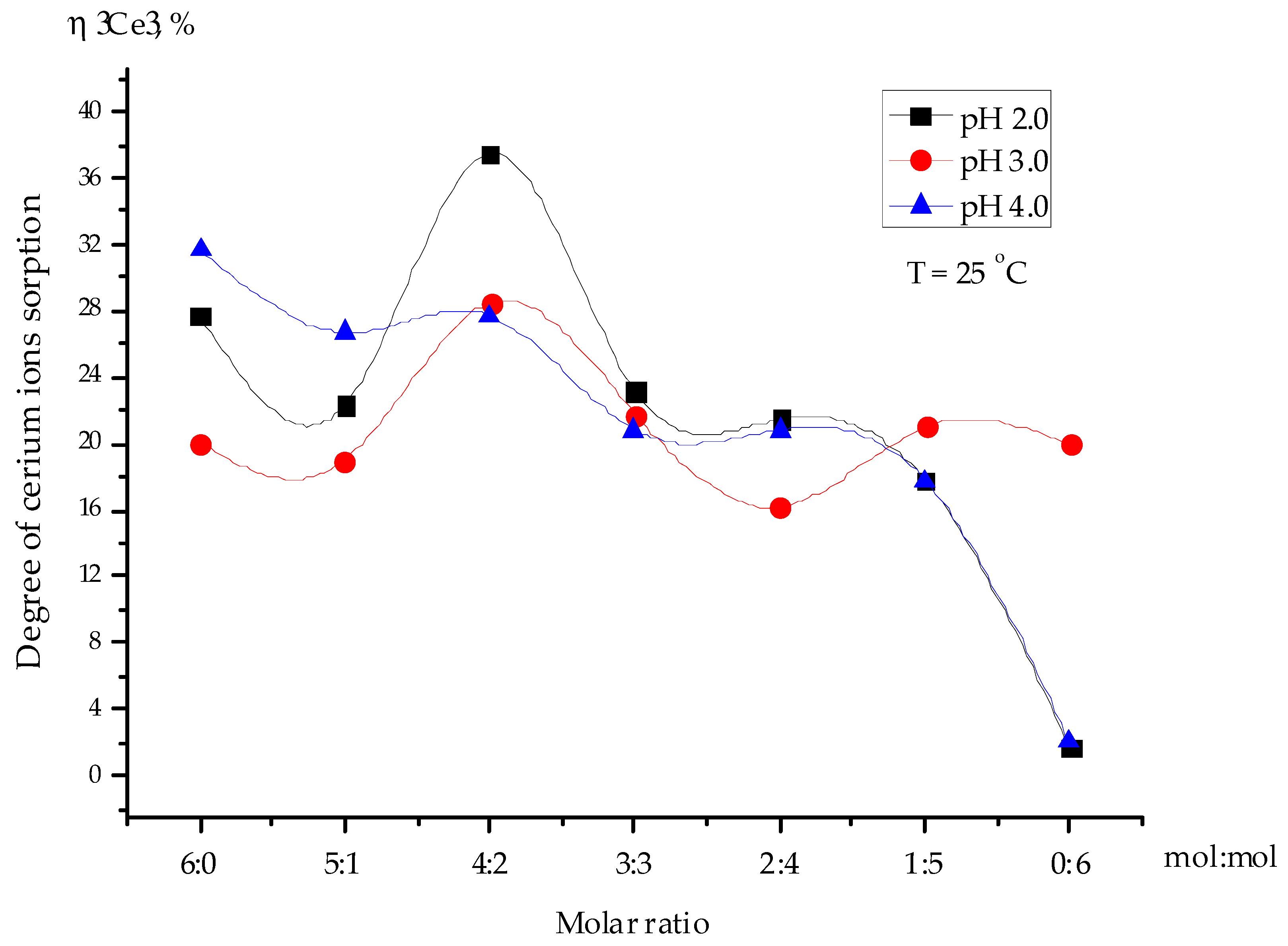
3.3. Influence of the pH on the Sorption Degree of Cerium Ions
3.4. Selective Sorption of Cerium Ions from Uranium-Containing Solution by the Interpolymer System “Amberlite IR120:AV-17-8” (X:Y, Molar Ratio of Ionic Groups)
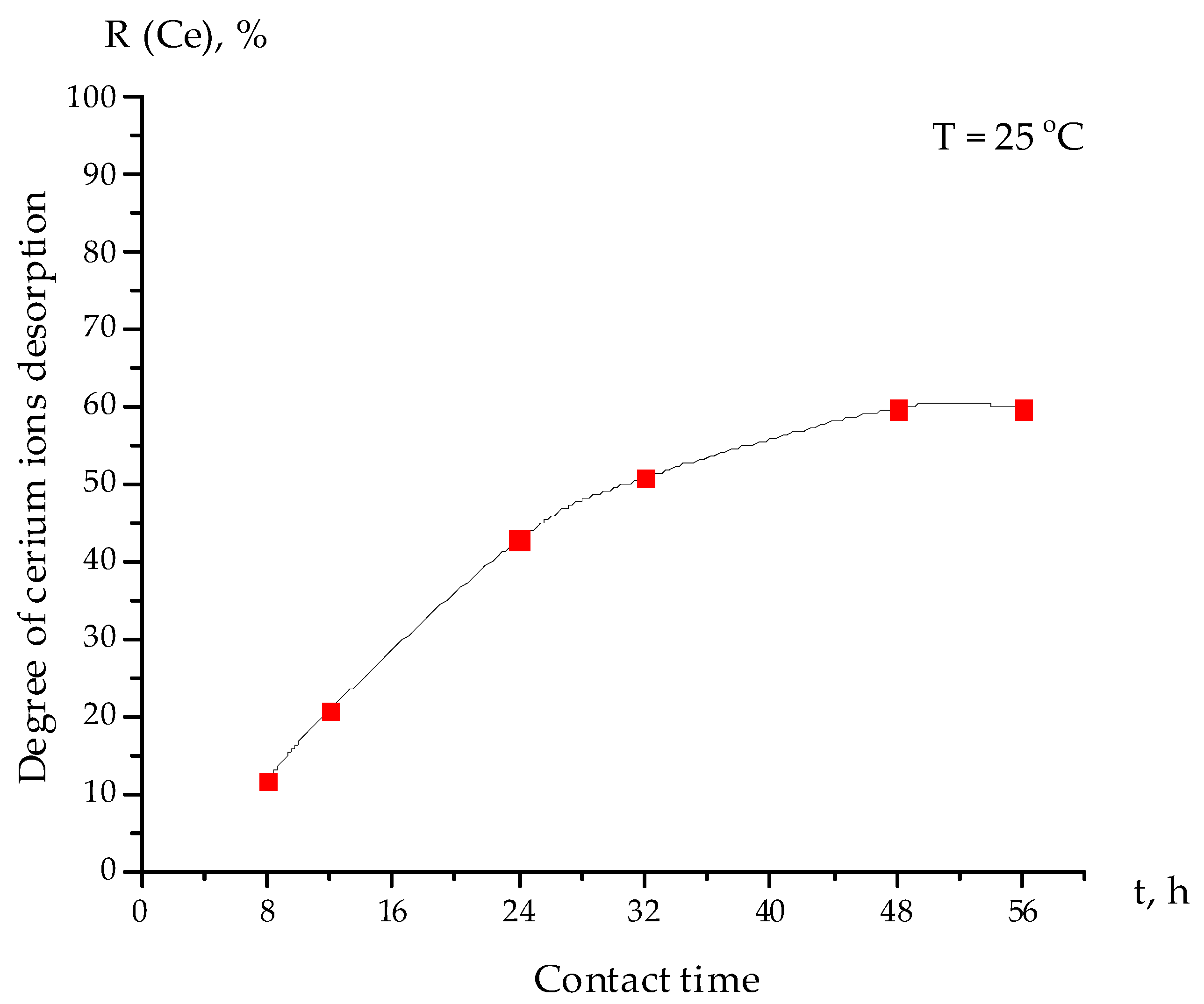
3.5. The Kinetics of Cerium Ion Desorption from the Interpolymer System “Amberlite IR120:AV-17-8” (4:2)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ryan, N.E. Chapter 141. High-temperature corrosion protection. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier B.V.: Amsterdam, The Netherlands, 1995; pp. 93–132. [Google Scholar] [CrossRef]
- Maestro, P.; Huguenin, D. Industrial applications of rare earths: Which way for the end of the Century. J. Alloys Compd. 1995, 225, 520–528. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.K. Innovative Technologies providing enhancement of non-ferrous, precious, rare and rare earth metals extraction. Complex Use Miner. Resour. 2019, 310, 64–75. [Google Scholar] [CrossRef]
- Lapidus, G.T.; Doyle, F.M. Selective thorium and uranium extraction from monazite: II. Approaches to enhance the removal of radioactive contaminants. Hydrometallurgy 2015, 155, 161–167. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, Q.; Hou, Y.; Wang, H.; Zhou, A.; Wang, L. Ground-state structures, physical properties and phase diagram of carbon-rich nitride C5N. J. Phys. Condens. Matter 2018, 30, 385402. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Z.; Chen, J.; Kallem, P.; Banat, F.; Qiu, H. Recent advances in selective separation technologies of rare earth elements: A Review. Environ. Chem. Eng. 2022, 10, 107104. [Google Scholar] [CrossRef]
- Allahkarami, E.; Rezai, B. Removal of cerium from different aqueous solutions using different adsorbents: A Review. Process Saf. Environ. Prot. 2019, 124, 345–362. [Google Scholar] [CrossRef]
- de Farias, A.B.; da Costa, T.B.; da Silva, M.G.; Vieira, M.G. Cerium recovery from aqueous solutions by Bio/adsorption: A review in a circular economy context. J. Clean. Prod. 2021, 326, 129395. [Google Scholar] [CrossRef]
- Ferella, F. A review on management and recycling of spent selective catalytic reduction catalysts. J. Clean. Prod. 2020, 246, 118990. [Google Scholar] [CrossRef]
- Brazdil, J.F. The emergence of the ubiquity of cerium in heterogeneous oxidation catalysis science and Technology. Catalysts 2022, 12, 959. [Google Scholar] [CrossRef]
- Vorobyev, P.; Mikhailovskaya, T.; Yugay, O.; Serebryanskaya, A.; Chukhno, N.; Imangazy, A. Catalytic oxidation of 4-methylpyridine on modified vanadium oxide catalysts. Iran. J. Chem. Chem. Eng. 2018, 37, 81–89. [Google Scholar] [CrossRef]
- Christmann, P. A Forward Look into Rare Earth Supply and Demand: A Role for Sedimentary Phosphate Deposits? Procedia Eng. 2014, 83, 19–26. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Kazatomprom. Available online: https://www.kazatomprom.kz/en/page/dobicha_prirodnogo_urana (accessed on 30 December 2022).
- Andrès, Y.; Texier, A.C.; Le Cloirec, P. Rare earth elements removal by microbial biosorption: A Review. Environ. Technol. 2003, 24, 1367–1375. [Google Scholar] [CrossRef]
- Zou, D.; Chen, J.; Li, D.Q. Separation chemistry and clean technique of cerium(iv): A Review. J. Rare Earths 2014, 32, 681–685. [Google Scholar] [CrossRef]
- Miller, D.D.; Siriwardane, R.; McIntyre, D. Anion structural effects on interaction of rare earth element ions with Dowex 50W X8 cation exchange resin. J. Rare Earths 2018, 36, 879–890. [Google Scholar] [CrossRef]
- Kerimkulova, A.R.; Azat, S.; Velasco, L.; Mansurov, Z.A.; Lodewyckx, P.; Tulepov, M.I.; Kerimkulova, M.R.; Berezovskaya, I.; Imangazy, A. Granular rice husk-based sorbents for sorption of vapors of organic and inorganic matters. J. Chem. Technol. Metall. 2019, 54, 578–584. Available online: https://dl.uctm.edu/journal/node/j2019-3/16_18-55_p_578-584.pdf (accessed on 30 December 2022).
- Atamanov, M.; Yelemessova, Z.; Imangazy, A.; Kamunur, K.; Lesbayev, B.; Mansurov, Z.; Yue, T.; Shen, R.; Yan, Q.-L. The Catalytic Effect of CuO-Doped Activated Carbon on Thermal Decomposition and Combustion of AN/Mg/NC Composite. J. Phys. Chem. C 2019, 123, 22941–22948. [Google Scholar] [CrossRef]
- Yelemessova, Z.; Imangazy, A.; Tulepov, M.; Mansurov, Z. Energetic Metal–Organic Frameworks: Thermal Behaviors and Combustion of Nickel Oxide (II) Based on Activated Carbon Compositions. J. Eng. Phys. Thermophys. 2021, 94, 804–811. [Google Scholar] [CrossRef]
- Cardoso, C.E.; Almeida, J.C.; Lopes, C.B.; Trindade, T.; Vale, C.; Pereira, E. Recovery of rare earth elements by carbon-based nanomaterials—A review. Nanomaterials 2019, 9, 814. [Google Scholar] [CrossRef]
- Imangazy, A.; Smagulova, G.; Kaidar, B.; Mansurov, Z.; Kerimkulova, A.; Umbetkaliev, K.; Zakhidov, A.; Vorobyev, P.; Jumadilov, T. Compositional Fibers Based on Coal Tar Mesophase Pitch Obtained by Electrospinning Method. Chem. Chem. Technol. 2021, 15, 403–407. [Google Scholar] [CrossRef]
- Kamunur, K.; Jandosov, J.; Abdulkarimova, R.; Hori, K.; Yelemessova, Z. Combustion study of different transitional metal oxide based on AN/MgAl composites gas generators. Eurasian Chem.—Technol. J. 2017, 19, 341. [Google Scholar] [CrossRef]
- Jumadilov, T.; Yskak, L.; Imangazy, A.; Suberlyak, O. Ion Exchange Dynamics in Cerium Nitrate Solution Regulated by Remotely Activated Industrial Ion Exchangers. Materials 2021, 14, 3491. [Google Scholar] [CrossRef] [PubMed]
- Amberlite IR120 H. Available online: https://www.lenntech.com/Data-sheets/Amberlite-IR-120-H-L.pdf (accessed on 30 November 2022).
- Ion Exchanger AV-17-8. Available online: http://smoly.com.ua/silnoosnovnyiy-anionit-av-17-8 (accessed on 30 November 2022).
- Jumadilov, T.; Kondaurov, R.; Imangazy, A.; Myrzakhmetova, N.; Saparbekova, I. Phenomenon of remote interaction and sorption ability of rare cross-linked hydrogels of polymethacrylic acid and poly-4-vinylpyridine in relation to erbium ions. Chem. Chem. Technol. 2019, 13, 451–458. [Google Scholar] [CrossRef]
- Jumadilov, T.K.; Yermukhambetova, B.; Panchenko, S.; Suleimenov, I. Long-distance electrochemical interactions and anomalous ion exchange phenomenon. AASRI Procedia 2012, 3, 553–558. [Google Scholar] [CrossRef]
- Bekturov, E.; Tolendina, A.; Shaikhutdinov, Y.; Dzhumadilov, T. Complexation of poly(ethylene glycol) with some salts of alkali-earth metals. Polym. Adv. Technol. 1993, 4, 564–566. [Google Scholar] [CrossRef]
- Bekturganova, G.K.; Dzhumadilov, T.K.; Bekturov, E.A. Electroconductivity and viscosity of complexes of poly(vinylpyridines) with alkali metal salts in organic solvents. Macromol. Chem. Phys. 1996, 197, 105–111. [Google Scholar] [CrossRef]
- Jumadilov, T.; Malimbayeva, Z.; Khimersen, K.; Saparbekova, I.; Imangazy, A.; Suberlyak, O. Specific features of praseodymium extraction by intergel system based on polyacrylic acid and poly-4-vinylpyridine hydrogels. Bull. Karaganda Univ. Chem. Ser. 2021, 103, 53–59. [Google Scholar] [CrossRef]
- Nesterov, Y.V. Ion exchangers and ion exchange. In Sorption Technology for the Extraction of Uranium and Other Metals by In-Situ Leaching; Yunikor-Izdat: Moscow, Russia, 2007; p. 480. [Google Scholar]
- Utesheva, A.A.; Grazulevicius, J.V. Specific features of uranyl ions extraction by interpolymer system based on polyacrylic acid and polyethyleneimine hydrogels. Complex Use Miner. Resour. 2021, 4, 65–71. [Google Scholar] [CrossRef]
- Zhou, S.; Li, X.; Shi, Y.; Alshameri, A.; Yan, C. Preparation, characterization, and ce(III) adsorption performance of Poly(allylamine)/silica composite. Desalin. Water Treat. 2014, 56, 1321–1334. [Google Scholar] [CrossRef]
- Lin, C.; Luo, W.; Chen, J.; Zhou, Q. Rice husk grafted PMAA by ATRP in aqueous phase and its adsorption for Ce3+. Chem. Phys. Lett. 2017, 690, 68–73. [Google Scholar] [CrossRef]
- Goneam Hamed, M.; Breky, M.M.E.; Ghazy, O.; Borai, E.H. Separation and preconcentration of cerium (III) and iron (III) on magnetic nanocomposite hydrogel. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129779. [Google Scholar] [CrossRef]
- Lankapati, H.M.; Dankhara, P.M.; Lathiya, D.R.; Shah, B.; Chudasama, U.V.; Choudhary, L.; Maheria, K.C. Removal of lanthanum, cerium and thorium metal ions from aqueous solution using ZrT hybrid ion exchanger. Sustain. Energy Technol. Assess. 2021, 47, 101415. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, T.; Zhang, L.; Ke, Z.; Kovarik, L.; Dong, H. Resource recovery: Adsorption and biomineralization of cerium by bacillus licheniformis. J. Hazard. Mater. 2022, 426, 127844. [Google Scholar] [CrossRef] [PubMed]
- Iftekhar, S.; Srivastava, V.; Ramasamy, D.L.; Naseer, W.A.; Sillanpää, M. A novel approach for synthesis of exfoliated biopolymeric-LDH hybrid nanocomposites via in-situ coprecipitation with Gum Arabic: Application towards Rees Recovery. J. Chem. Eng. 2018, 347, 398–406. [Google Scholar] [CrossRef]
- He, J.; Li, Y.; Xue, X.; Ru, H.; Huang, X.; Yang, H. Separation of fluorine/cerium from fluorine-bearing rare earth sulfate solution by selective adsorption using hydrous zirconium oxide. RSC Adv. 2016, 6, 43814–43822. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Shahat, A.; Naushad, M.; Shiwaku, H.; Yaita, T. Investigation of ligand immobilized nano-composite adsorbent for efficient cerium(III) detection and Recovery. J. Chem. Eng. 2015, 265, 210–218. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Alfarra, A.; Frackowiak, E.; Béguin, F. The HSAB concept as a means to interpret the adsorption of metal ions onto activated carbons. Appl. Surf. Sci. 2004, 228, 84–92. [Google Scholar] [CrossRef]

| Molar Ratio of Amberlite IR120:AV-17-8 (X:Y) | Weight Ratio of Amberlite IR120:AV-17-8 (X:Y) a | θ at pH 2.0 | θ at pH 3.0 | θ at pH 4.0 |
|---|---|---|---|---|
| 6:0 | 0.207 g:0.000 g | 5.93% | 4.28% | 6.78% |
| 5:1 | 0.172 g:0.024 g | 4.77% | 4.06% | 5.71% |
| 4:2 | 0.138 g:0.049 g | 8.02% | 6.10% | 5.93% |
| 3:3 | 0.103 g:0.073 g | 4.96% | 4.66% | 4.45% |
| 2:4 | 0.069 g:0.098 g | 4.60% | 3.47% | 4.45% |
| 1:5 | 0.034 g:0.122 g | 3.81% | 4.49% | 3.81% |
| 0:6 | 0.000 g:0.146 g | 0.36% | 4.28% | 0.43% |
| Sorbents | PH Range | Optimum PH | Maximum Sorption Capacity (mg/g) | Reference |
|---|---|---|---|---|
| Poly(allylamine)/silica composite | 1.0–6.0 | 4.0 | 111.80 | [34] |
| Rice husk grafted poly (methyl acrylic acid) | 1.0–7.0 | 6.0 | 122.51 | [35] |
| Magnetic nanocomposite hydrogel | 1.0–4.0 | 4.0 | 151.00 | [36] |
| ZrT hybrid ion exchanger | 1.0–5.0 | 5.0 | 112.00 | [37] |
| Bacillus licheniformis | 1.0–6.0 | 6.0 | 38.93 | [38] |
| Biopolymeric-layered double hydroxides hybrid nanocomposites | 2.0–7.0 | 4.0 | 116.82 | [39] |
| Hydrous zirconium oxide | 0.0–1.0 | ~0.5 | 184.50 | [40] |
| Ligand immobilized nano-composite | 1.0–7.0 | 2.5 | 150.37 | [41] |
| Interpolymer system “Amberlite IR120:AV-17-8” (4:2) | 2.0–4.0 | 2.0 | 210.70 | Current study |
| Ion | Amount of Ions (mg/g) a | ||
|---|---|---|---|
| pH = 2.0 | pH = 3.0 | pH = 4.0 | |
| Ce | 1.293 | 1.138 | 1.112 |
| U | 0.001> | 0.001> | 0.001> |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jumadilov, T.; Utesheva, A.; Grazulevicius, J.; Imangazy, A. Selective Sorption of Cerium Ions from Uranium-Containing Solutions by Remotely Activated Ion Exchangers. Polymers 2023, 15, 816. https://doi.org/10.3390/polym15040816
Jumadilov T, Utesheva A, Grazulevicius J, Imangazy A. Selective Sorption of Cerium Ions from Uranium-Containing Solutions by Remotely Activated Ion Exchangers. Polymers. 2023; 15(4):816. https://doi.org/10.3390/polym15040816
Chicago/Turabian StyleJumadilov, Talkybek, Ainamgul Utesheva, Juozas Grazulevicius, and Aldan Imangazy. 2023. "Selective Sorption of Cerium Ions from Uranium-Containing Solutions by Remotely Activated Ion Exchangers" Polymers 15, no. 4: 816. https://doi.org/10.3390/polym15040816
APA StyleJumadilov, T., Utesheva, A., Grazulevicius, J., & Imangazy, A. (2023). Selective Sorption of Cerium Ions from Uranium-Containing Solutions by Remotely Activated Ion Exchangers. Polymers, 15(4), 816. https://doi.org/10.3390/polym15040816






