Impact of Isonicotinic Acid Blending in Chitosan/Polyvinyl Alcohol on Ripening-Dependent Changes of Green Stage Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Tomatoes
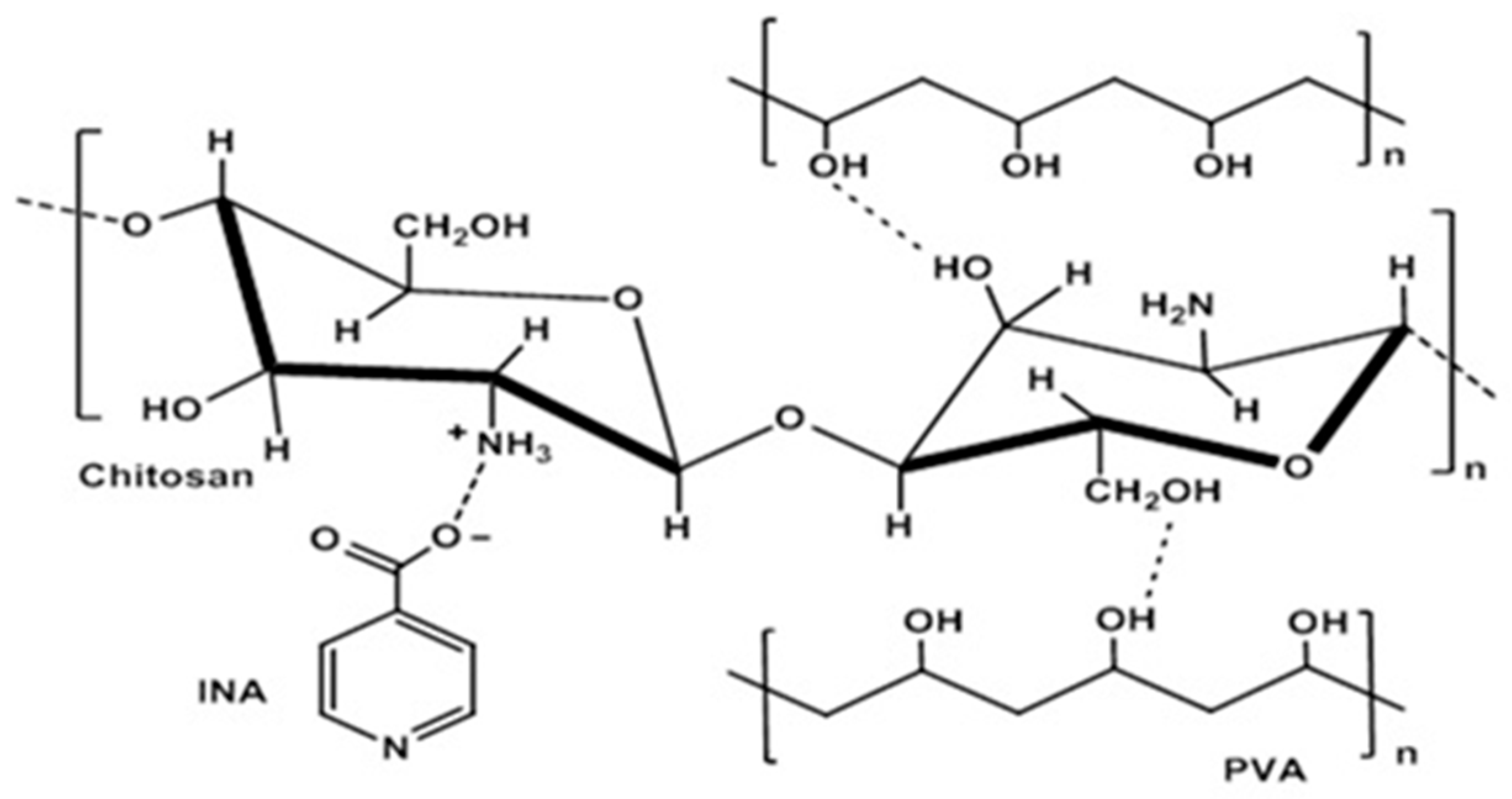
2.3. Coating Solutions
2.4. Characterization of Biodegradable Films
2.4.1. Preparation of Samples
2.4.2. UV/Visible Scanning Spectrophotometry
2.4.3. Fourier-Transform Infrared Analysis (FT-IR)
2.5. Antifungal Activity
2.6. Application of Biodegradable Coating Treatments
2.7. Physical and Chemical Properties
2.7.1. Fruit Firmness
2.7.2. Titratable Acidity
2.7.3. Total Carotenoid Content (TC)
2.7.4. Extraction and Determination of Lycopene
2.7.5. Determination of Ascorbic Acid
2.7.6. Extraction and Determination of Total Phenolic Content
2.7.7. Malondialdehyde (MDA) Content
2.8. Activity of Antioxidant and Browning Enzymes
2.9. Statistical Analysis
3. Results and Discussion
3.1. Optical Properties
3.2. Fourier-Transform Infrared Analysis (FTIR)
3.3. Antifungal Activity
3.4. Physical and Chemical Properties of Tomatoes
3.4.1. Fruit Firmness
3.4.2. Titratable Acidity
3.4.3. Total Carotenoids
3.4.4. Lycopene
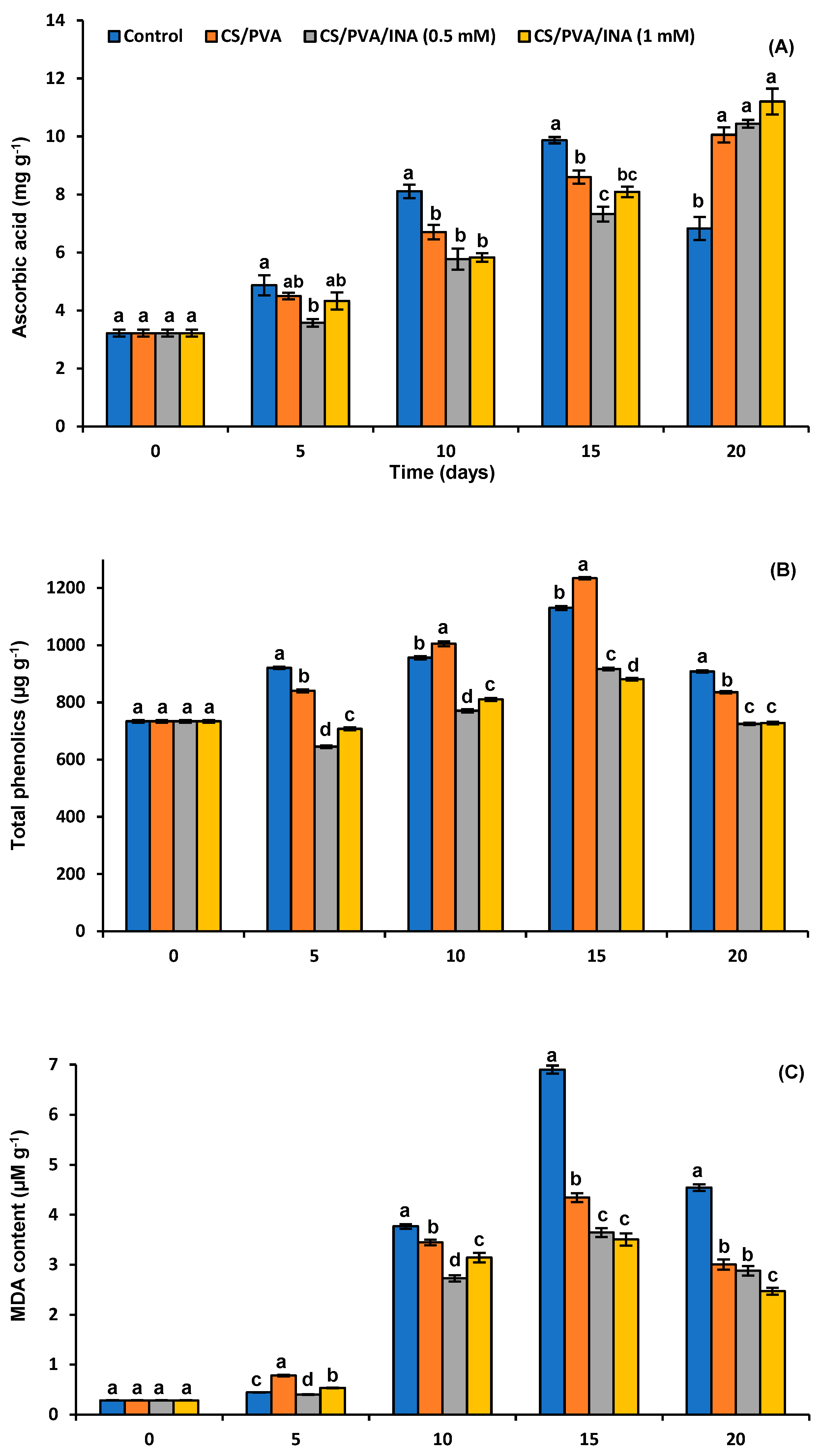
3.4.5. Ascorbic Acid Content
3.4.6. Total Polyphenols
3.4.7. Malondialdehyde (MDA)
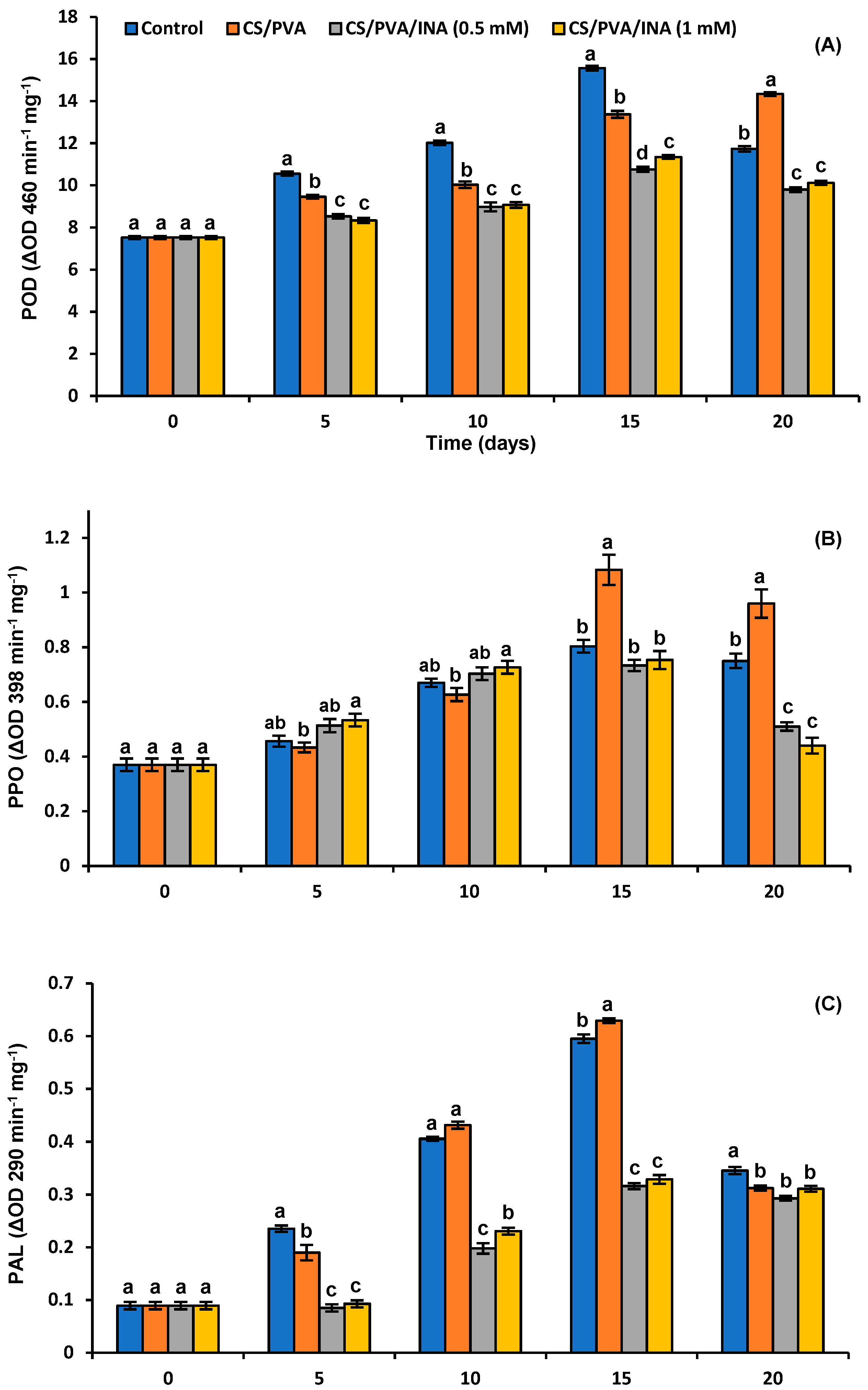
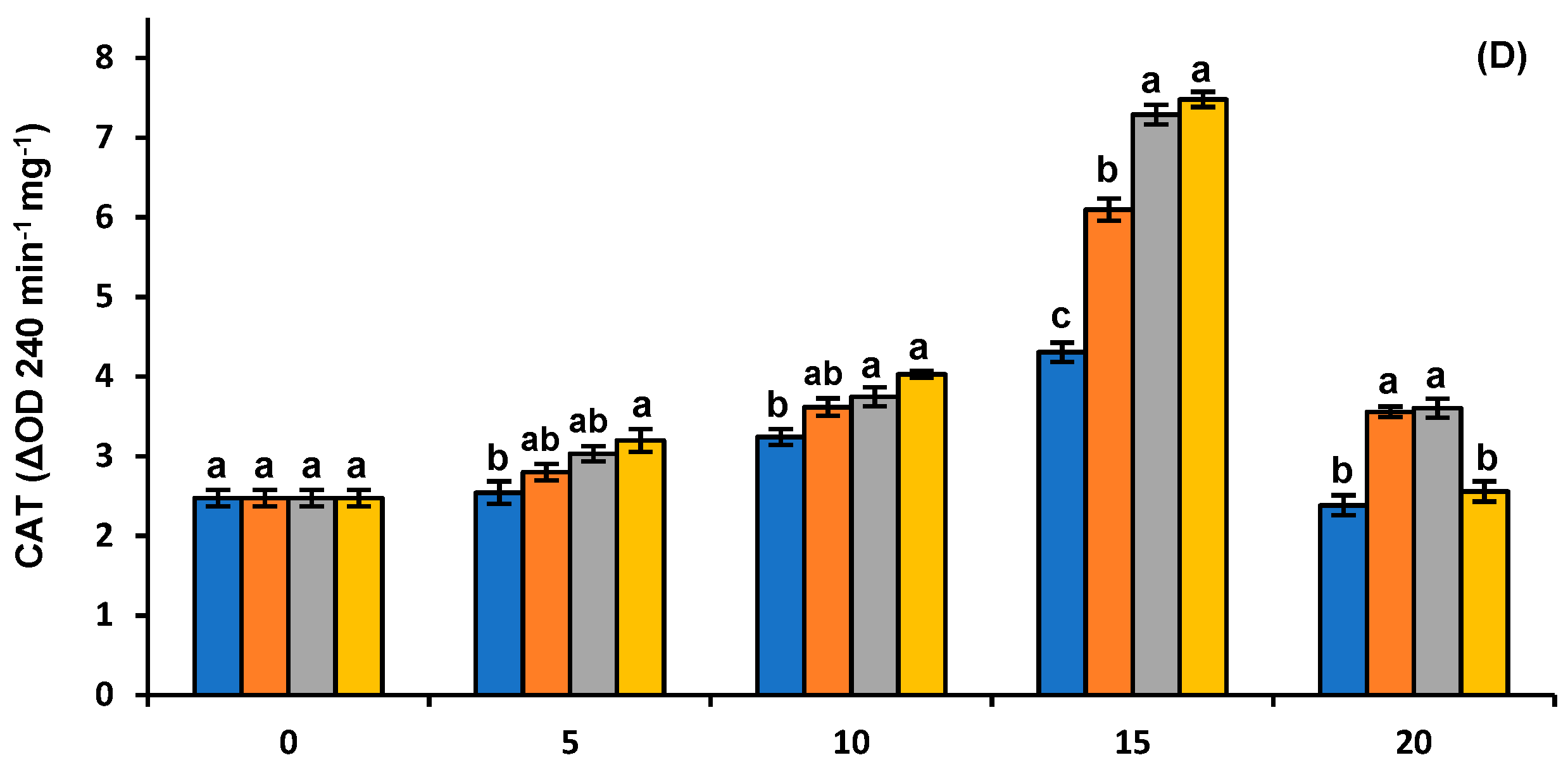
3.5. Antioxidant and Browning Enzymes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Taher, M.A.; MennatAllah, E.A.; Tadros, L.K.; Sanad, M.I. The effects of new formulations based on Gum Arabic on antioxidant capacity of tomato (Solanum lycopersicum L.) fruit during storage. J. Food Meas. Charact. 2020, 14, 2489–2502. [Google Scholar] [CrossRef]
- Karpova, N.; Shagdarova, B.; Lunkov, A.; Ilina, A.; Varlamov, V. Antifungal action of chitosan in combination with fungicides in vitro and chitosan conjugate with gallic acid on tomatoes against Botrytis cinerea. Biotechnol. Lett. 2021, 43, 1565–1574. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Taher, M.A.; Abd El-Aziz, M.H.; Mohamed, S.Y. Action mechanisms and biocontrol of Purpureocillium lilacinum against green mould caused by Penicillium digitatum in orange fruit. J. Appl. Microbiol. 2021, 131, 1378–1390. [Google Scholar] [CrossRef]
- Taher, M.A.; Lo’ay, A.A.; Gouda, M.; Limam, S.A.; Abdelkader, M.F.M.; Osman, S.O.; Fikry, M.; Ali, E.F.; Mohamed, S.Y.; Khalil, H.A.; et al. Impacts of Gum Arabic and polyvinylpyrrolidone (PVP) with salicylic acid on peach fruit (Prunus persica) shelf life. Molecules 2022, 27, 2595. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, D.; Belwal, T.; Li, L.; Chen, H.; Xu, T.; Luo, Z. Effect of nano-SiOx/chitosan complex coating on the physicochemical characteristics and preservation performance of green tomato. Molecules 2019, 24, 4552. [Google Scholar] [CrossRef]
- Dominguez-Martinez, B.M.; Martínez-Flores, H.E.; Berrios, J.D.; Otoni, C.G.; Wood, D.F.; Velazquez, G. Physical characterization of biodegradable films based on chitosan, polyvinyl alcohol and Opuntia mucilage. J. Polym. Environ. 2017, 25, 683–691. [Google Scholar] [CrossRef]
- Liu, H.; Tian, W.; Li, B.; Wu, G.; Ibrahim, M.; Tao, Z.; Wang, Y.; Xie, G.; Li, H.; Sun, G. Antifungal effect and mechanism of chitosan against the rice sheath blight pathogen, Rhizoctonia solani. Biotechnol. Lett. 2012, 34, 2291–2298. [Google Scholar] [CrossRef]
- Guo, X.; Yu, Z.; Zhang, M.; Tang, W.; Sun, Y.; Li, X. Enhancing the production of phenolic compounds during barley germination by using chitooligosaccharides to improve the antioxidant capacity of malt. Biotechnol. Lett. 2018, 40, 1335–1341. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Dawood, H.D. Active chitosan/PVA with ascorbic acid and berry quality of ‘Superior seedless’ grapes. Sci. Hortic. 2017, 224, 286–292. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Dawood, H.D. Minimize browning incidence of banana by postharvest active chitosan/PVA combines with oxalic acid treatment to during shelf-life. Sci. Hortic. 2017, 226, 208–215. [Google Scholar] [CrossRef]
- Ogata, S.; Takeuchi, M.; Okumura, K.; Taguchi, H. Apoptosis induced by niacin-related compounds in HL-60 cells. Biosci. Biotechnol. Biochem. 1998, 62, 2351–2356. [Google Scholar] [CrossRef]
- Ogata, S.; Akeuchi, M.T.; Teradaira, S.; Amamoto, N.Y.; Wata, K.I.; Okumura, K.; Aguchi, H.T. Radical scavenging activities of niacin-related compounds. Biosci. Biotechnol. Biochem. 2002, 66, 641–645. [Google Scholar] [CrossRef]
- Svedmyr, N.; Harthon, L.; Lundholm, L. The relationship between the plasma concentration of free nicotinic acid and some of its pharmacologic effects in man. Clin. Pharmacol. Ther. 1969, 10, 559–570. [Google Scholar] [CrossRef]
- Landy, M. Effect of nicotinic acid, Its isomers and related compounds upon nutrition of Staphylococcus aureus. Proc. Soc. Exp. Biol. Med. 1938, 38, 504–507. [Google Scholar] [CrossRef]
- Hoffman, C.; Schweitzer, T.R.; Dalby, G. The fungistatic properties of pyridine carboxylic and aminobenzoic acids, a resonance effect. J. Am. Pharm. Assoc. 1942, 31, 97–99. [Google Scholar] [CrossRef]
- Chakraborty, N.; Ghosh, S.; Chandra, S.; Sengupta, S.; Acharya, K. Abiotic elicitors mediated elicitation of innate immunity in tomato: An ex vivo comparison. Physiol. Mol. Biol. Plants. 2016, 22, 307–320. [Google Scholar] [CrossRef]
- Taher, M.A.; Khojah, E.; Darwish, M.S.; Elsherbiny, E.A.; Elawady, A.A.; Dawood, D.H. Biosynthesis of silver nanoparticles by polysaccharide of Leucaena leucocephala seeds and their anticancer, antifungal properties and as preservative of composite milk sample. J. Nanomater. 2022, 2022, 7490221. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Taher, M.A. Effectiveness salicylic acid blending in chitosan/PVP biopolymer coating on antioxidant enzyme activities under low storage temperature stress of ‘Banati’ guava fruit. Sci. Hortic. 2018, 238, 343–349. [Google Scholar] [CrossRef]
- Carvalho, J.D.L.M.; Gomes, P.B.; Godoy, R.L.O.; Pacheco, S.; Fernandes do Monte, P.H.; de Carvalho, J.L.V.; Nutti, M.R.; Neves, A.C.L.; Vieira, A.C.; Ramos, S.R.R. Total carotenoid content, α-carotene and β-carotene, of landrace pumpkins (Cucurbita moschata Duch): A preliminary study. Food Res. Int. 2012, 47, 337–340. [Google Scholar] [CrossRef]
- Fish, W.W.; Perkins-Veazie, P.; Collins, J.K.A. Quantitative assay for lycopene that utilizes reduced volumes of organic solvents. J. Food Compos. Anal. 2002, 15, 309–317. [Google Scholar] [CrossRef]
- Zhang, Z.; Huber, D.J.; Qu, H.; Yun, Z.; Wang, H.; Huang, Z.; Huang, H.; Jiang, Y. Enzymatic browning and antioxidant activities in harvested litchi fruit as influenced by apple polyphenols. Food Chem. 2015, 171, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, E.A.; Taher, M.A. Silicon induces resistance to postharvest rot of carrot caused by Sclerotinia sclerotiorum and the possible of defense mechanisms. Postharvest. Biol. Technol. 2018, 140, 11–17. [Google Scholar] [CrossRef]
- Luo, Z.; Xu, X.; Yan, B. Accumulation of lignin and involvement of enzymes in bamboo shoot during storage. Eur. Food Res. Technol. 2008, 226, 635–640. [Google Scholar] [CrossRef]
- Assis, J.S.; Maldonado, R.; Munoz, T.; Escribano, M.I.; Merodio, C. Effect of high carbondioxide concentration on PAL activity and phenolic contents in ripening Cherimoya fruit. Postharvest. Biol. Technol. 2001, 23, 33–39. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Safwat, N.A.; Elaasser, M.M. Fungitoxicity of organic extract of Ocimum basilicum on growth and morphogenesis of Bipolaris species (teleomorph Cochliobolus). J. Appl. Microbiol. 2017, 123, 841–852. [Google Scholar] [CrossRef]
- Abdolrahimi, M.; Seifi, M.; Ramezanzadeh, M.H. Study the effect of acetic acid on structural, optical and mechanical properties of PVA/chitosan/MWCNT films. Chin. J. Phys. 2018, 56, 221–230. [Google Scholar] [CrossRef]
- Chen, W.T. Preparation, structure and properties of [Tb(HIA)2(IA)(H2O)2(HgCl2)]n(nHgCl4)·3nH2O (IA = Isonicotinate Anion). J. Chem. Crystallogr. 2021, 51, 116–123. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Phan, N.T.; Trinh, C.D.; Tran, T.V.; Pham, B.T.; Quynh, B.T.P.; Phung, T.K. Glycerol-plasticized chitosan film for the preservation of orange. J. Food Saf. 2021, 42, e12943. [Google Scholar] [CrossRef]
- Rudko, G.; Kovalchuk, A.; Fediv, V.; Chen, W.M.; Buyanova, I.A. Enhancement of polymer endurance to UV light by incorporation of semiconductor nanoparticles. Nanoscale Res. Lett. 2015, 10, 81. [Google Scholar] [CrossRef]
- Rubilar, J.F.; Cruz, R.M.; Silva, H.D.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Physico-mechanical properties of chitosan films with carvacrol and grape seed extract. J. Food Eng. 2013, 115, 466–474. [Google Scholar] [CrossRef]
- Tantala, J.; Rachtanapun, C.; Tongdeesoontorn, W.; Jantanasakulwong, K.; Rachtanapun, P. Moisture sorption isotherms and prediction models of carboxymethyl chitosan films from different sources with various plasticizers. Adv. Mater. Sci. Eng. 2019, 2019, 4082439. [Google Scholar] [CrossRef]
- Chaiwong, N.; Leelapornpisid, P.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Sakdatorn, V.; Leksawasdi, N.; Phimolsiripol, Y. Antioxidant and moisturizing properties of carboxymethyl chitosan with different molecular weights. Polymers 2020, 12, 1445. [Google Scholar] [CrossRef] [PubMed]
- Rachtanapun, P.; Klunklin, W.; Jantrawut, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Seesuriyachan, P.; Leksawasdi, N.; Chaiyaso, T.; Ruksiriwanich, W.; Phongthai, S.; et al. Characterization of chitosan film incorporated with curcumin Extract. Polymers 2021, 13, 963. [Google Scholar] [CrossRef]
- Marzano, M.; Borbone, N.; Amato, F.; Oliviero, G.; Fucile, P.; Russo, T.; Sannino, F. 3D chitosan-gallic acid complexes: Assessment of the chemical and biological properties. Gels 2022, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Abureesh, M.A.; Oladipo, A.A.; Gazi, M. Facile synthesis of glucose-sensitive chitosan–poly(vinyl alcohol) hydrogel: Drug release optimization and swelling properties. Macromolecules 2016, 90, 75–80. [Google Scholar] [CrossRef]
- Li, D.; Zhong, G. Synthesis, Crystal structure, and thermal decomposition of the cobalt (II) Complex with 2-picolinic acid. Sci. World J. 2014, 2014, 641608. [Google Scholar] [CrossRef]
- Świderski, G.; Kalinowska, M.; Malejko, J.; Lewandowski, W. Spectroscopic (IR, Raman, UV and fluorescence) study on lanthanide complexes of picolinic acid. Vib. Spectrosc. 2016, 87, 81–87. [Google Scholar] [CrossRef]
- Mukherjeea, G.; Biradha, K. 1D, 2D and 3D coordination polymers of 1,3-phenylene diisonicotinate with Cu(i)/Cu(ii): Cu2I2 building block, anion influence and guest inclusions. Cryst. Eng. Comm. 2014, 16, 4701–4705. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Co-Ordination Compounds, 3rd ed.; John Wiley: New York, NY, USA, 1978. [Google Scholar]
- Tella, A.C.; Mehlana, G.; Alimi, L.O.; Bourne, S.A. Solvent-free synthesis, characterization and solvent-vapor interaction of Zinc(II) and Copper(II) coordination polymers containing nitrogen-donor ligands. Z. Anorg. Allg. Chem. 2017, 643, 523–530. [Google Scholar] [CrossRef]
- Rana, V.; Babita, K.; Goyal, D.; Gorea, R.; Tiwary, A. Optimization of chitosan films as a substitute for animal and human epidermal sheets for in vitro permeation of polar and non-polar drugs. Acta Pharm. 2004, 54, 287–299. [Google Scholar] [PubMed]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Palma-Guerrero, J.; Lopez-Jimenez, J.A.; Perez-Berna, A.J.; Huang, I.C.; Jansson, H.B.; Salinas, J. Membrane fluidity determines sensitivity of filamentous fungi to chitosan. Mol. Microbiol. 2010, 75, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Judge, V.; Narasimhan, B.; Ahuja, M.; Sriram, D.; Clercq, E.D.; Yogeeswari, P.; Pannecouque, C.; Balzarini, J. Synthesis, antimycobacterial, antiviral, antimicrobial activities, and QSAR studies of isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazides. Med. Chem. Res. 2012, 21, 1935–1952. [Google Scholar] [CrossRef]
- Ali, A.; Maqbool, M.; Ramachandran, S.; Alderson, P.G. Gum Arabic as a novel edible coating for enhancing shelf-life and improving postharvest quality of tomato (Solanum lycopersicum L.) fruit. Postharvest. Biol. Technol. 2010, 58, 42–47. [Google Scholar] [CrossRef]
- Nkolisa, N.; Magwaza, L.S.; Workneh, T.S.; Chimphango, A. Evaluating evaporative cooling system as an energy-free and cost-effective method for postharvest storage of tomatoes (Solanum lycopersicum L.) for smallholder farmers. Sci. Hortic. 2018, 241, 131–143. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdelrahman, S.Z.; Megahed, M.M.A.; Abdeldaym, E.A.; El-Mogy, M.M.; Abdelgawad, K.F. Extending shelf life and maintaining quality of tomato fruit by calcium chloride, hydrogen peroxide, chitosan, and ozonated water. Horticulturae 2021, 7, 309. [Google Scholar] [CrossRef]
- Meena, M.; Pilania, S.; Pal, A.; Mandhania, S.; Bhushan, B.; Kumar, S.; Gohari, G.; Saharan, V. Cu-chitosan nano-net improves keeping quality of tomato by modulating physio-biochemical responses. Sci. Rep. 2020, 10, 21914. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Nikou, A.; Tzortzakis, N. Effectiveness of Aloe vera gel coating for maintaining tomato fruit quality. N. Z. J. Crop Hortic. Sci. 2016, 44, 203–217. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y.; He, W.; Ji, C.; Xia, P.; Wang, Y.; Du, S.; Li, H.; Raikhel, N.; Xiao, J.; et al. Pyrazinamide and derivatives block ethylene biosynthesis by inhibiting ACC oxidase. Nat. Commun. 2017, 12, 15758. [Google Scholar] [CrossRef]
- Silva, B.W.; Silva, C.G.M.; Santana, D.B.; Salvador, A.R.; Medeiros, D.B.; Belghith, I.; da Silva, N.M.; Cordeiro, M.H.M.; Misobutsi, G.P. Chitosan delays ripening and ROS production in guava (Psidium guajava L.) fruit. Food Chem. 2018, 1, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, G.; Bartley, G.E.; Scolnik, P.A. Regulation of carotenoid biosynthesis during tomato development. Plant. Cell 1993, 5, 379–387. [Google Scholar] [PubMed]
- Fraser, P.D.; Kiano, J.W.; Truesdale, M.R.; Schuch, W.; Bramley, P.M. Phytoene synthase-2 enzyme activity in tomato does not contribute to carotenoid synthesis in ripening fruit. Plant Mol. Biol. 1999, 40, 687–698. [Google Scholar] [CrossRef]
- Ali, A.; Maqbool, M.; Alderson, P.G.; Zahid, N. Effect of gum Arabic as an edible coating on antioxidant capacity of tomato (Solanum lycopersicum L.) fruit during storage. Postharvest. Biol. Technol. 2013, 76, 119–124. [Google Scholar] [CrossRef]
- Frusciante, L.; Carli, P.; Ercolano, M.R.; Pernice, R.; Matteo, A.D.; Fogliano, V.; Pellegrini, N. Antioxidant nutritional quality of tomato. Mol. Nutr. Food Res. 2007, 51, 609–661. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.N.; Rice-Evans, C.A. Factors influencing the antioxidant activity determined by the ABTS•+ radical cation assay. Free Radi. Res. 1997, 26, 195–199. [Google Scholar] [CrossRef]
- Naeem, A.; Abbas, T.; Ali, T.M.; Hasnain, A. Effect of antioxidant and antibacterial properties of guar gum coating containing spice extracts and its application on tomatoes (Solanum lycopersicum L.). J. Food Meas. Charact. 2018, 12, 2725–2734. [Google Scholar] [CrossRef]
- Zewdie, B.; Shonte, T.T.; Woldetsadik, K. Shelf life and quality of tomato (Lycopersicon esculentum Mill.) fruits as affected by neem leaf extract dipping and beeswax coating. Int. J. Food Prop. 2022, 25, 570–592. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Taher, M.A. Influence of edible coatings chitosan/PVP blending with salicylic acid on biochemical fruit skin browning incidence and shelf life of guava fruits cv. ‘Banati’. Sci. Hortic. 2018, 235, 424–436. [Google Scholar] [CrossRef]
- Dávila-Aviña, J.E.; Villa-Rodríguez, J.A.; Villegas-Ochoa, M.A.; Tortoledo-Ortiz, O.; Olivas, G.I.; Ayala-Zavala, J.F.; González-Aguilar, G.A. Effect of edible coatings on bioactive compounds and antioxidant capacity of tomatoes at different maturity stages. J. Food Sci. Technol. 2014, 51, 2706–2712. [Google Scholar] [CrossRef]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of chitosan coating on the postharvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Pan, L.; Xiao, T.; Ren, X.; Liu, Z. Exogenous niacin treatment increases NADPH oxidase in kiwifruit. Braz. J. Biol. 2018, 78, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Aucique-Pérez, C.E.; Resende, R.S.; Neto, L.B.C.; Dornelas, F.; DaMatta, F.M.; Rodrigues, F.Á. Picolinic acid spray stimulates the antioxidative metabolism and minimizes impairments on photosynthesis on wheat leaves infected by Pyricularia oryzae. Physiol. Plant. 2019, 167, 628–644. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, E.A.; Taher, M.A.; Elsebai, M.F. Activity of Purpureocillium lilacinum filtrates on biochemical characteristics of Sclerotinia sclerotiorum and induction of defense responses in common bean. Eur. J. Plant Pathol. 2019, 155, 39–52. [Google Scholar] [CrossRef]
- Domiciano, G.P.; Cacique, I.S.; Freitas, C.C.; Einhardt, A.M.; Rodrigues, F.Á. Picolinic acid stress imposed on rice leaves is not alleviated by silicon. Trop. Plant Pathol. 2020, 45, 448–453. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taher, M.A.; Elsherbiny, E.A. Impact of Isonicotinic Acid Blending in Chitosan/Polyvinyl Alcohol on Ripening-Dependent Changes of Green Stage Tomato. Polymers 2023, 15, 825. https://doi.org/10.3390/polym15040825
Taher MA, Elsherbiny EA. Impact of Isonicotinic Acid Blending in Chitosan/Polyvinyl Alcohol on Ripening-Dependent Changes of Green Stage Tomato. Polymers. 2023; 15(4):825. https://doi.org/10.3390/polym15040825
Chicago/Turabian StyleTaher, Mohamed A., and Elsherbiny A. Elsherbiny. 2023. "Impact of Isonicotinic Acid Blending in Chitosan/Polyvinyl Alcohol on Ripening-Dependent Changes of Green Stage Tomato" Polymers 15, no. 4: 825. https://doi.org/10.3390/polym15040825
APA StyleTaher, M. A., & Elsherbiny, E. A. (2023). Impact of Isonicotinic Acid Blending in Chitosan/Polyvinyl Alcohol on Ripening-Dependent Changes of Green Stage Tomato. Polymers, 15(4), 825. https://doi.org/10.3390/polym15040825






