Multifunctional Heterogeneous Ion-Exchange Membranes for Ion and Microbe Removal in Low-Salinity Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Modification of Heterogeneous Cation-Exchange Membranes
2.3. Surface Modification of Heterogeneous AEMs
2.4. Membrane Characterization
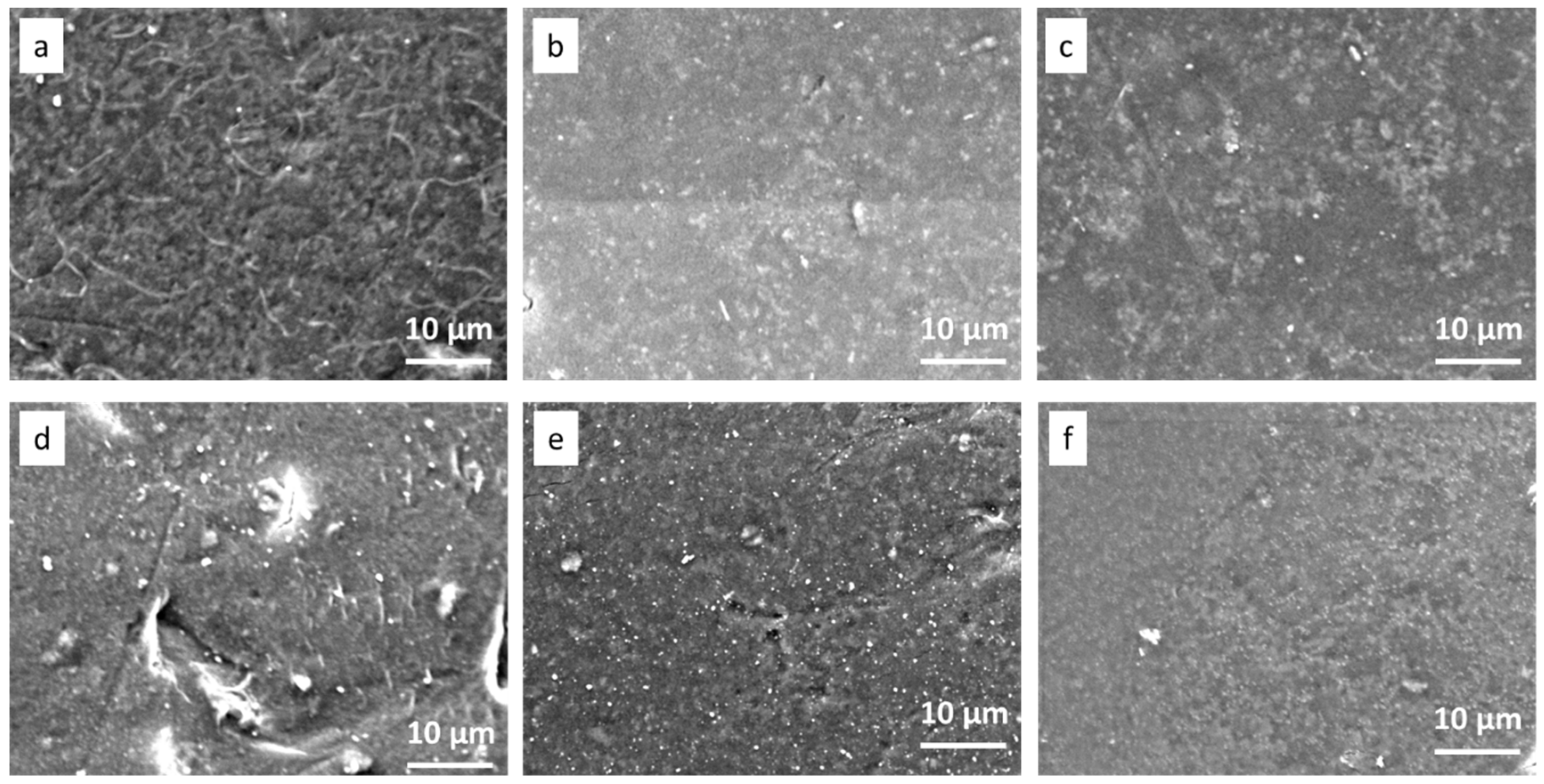
2.4.1. Membrane Morphology
2.4.2. Membrane Metal Content Determination
2.4.3. Metal NP Leaching Measurements
2.5. Membrane Testing and Application
2.6. Antimicrobial Studies
2.6.1. Bacterial Filtration Studies
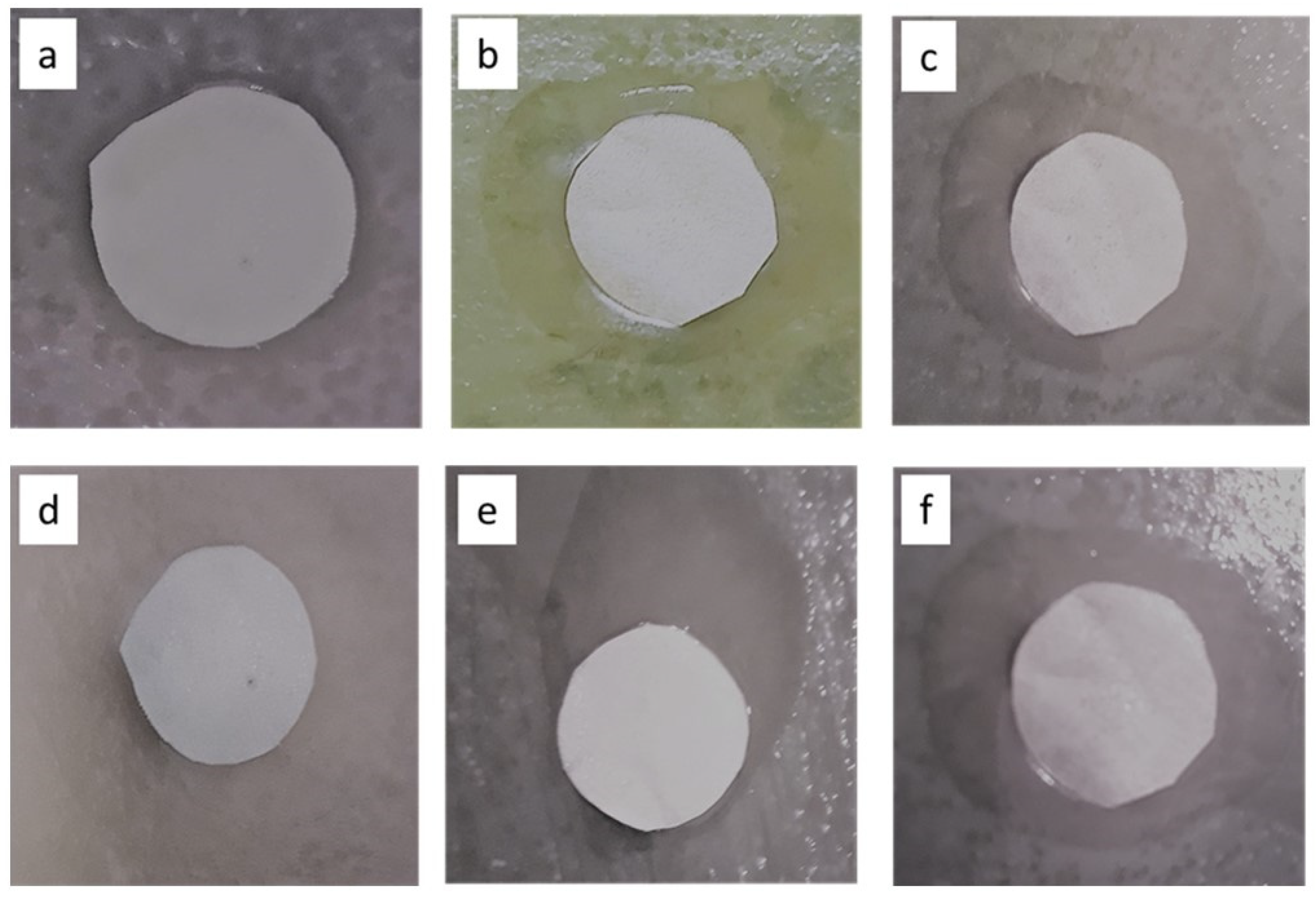
2.6.2. Biofouling Inhibition (Disc Diffusion Method)
3. Results and Discussion
3.1. Membrane Characterization
3.1.1. Membrane Morphology
3.1.2. Nanocomposite Membrane Metal Content
3.1.3. Metal NP Leaching
3.2. Membrane Application
3.2.1. Pure Water Flux
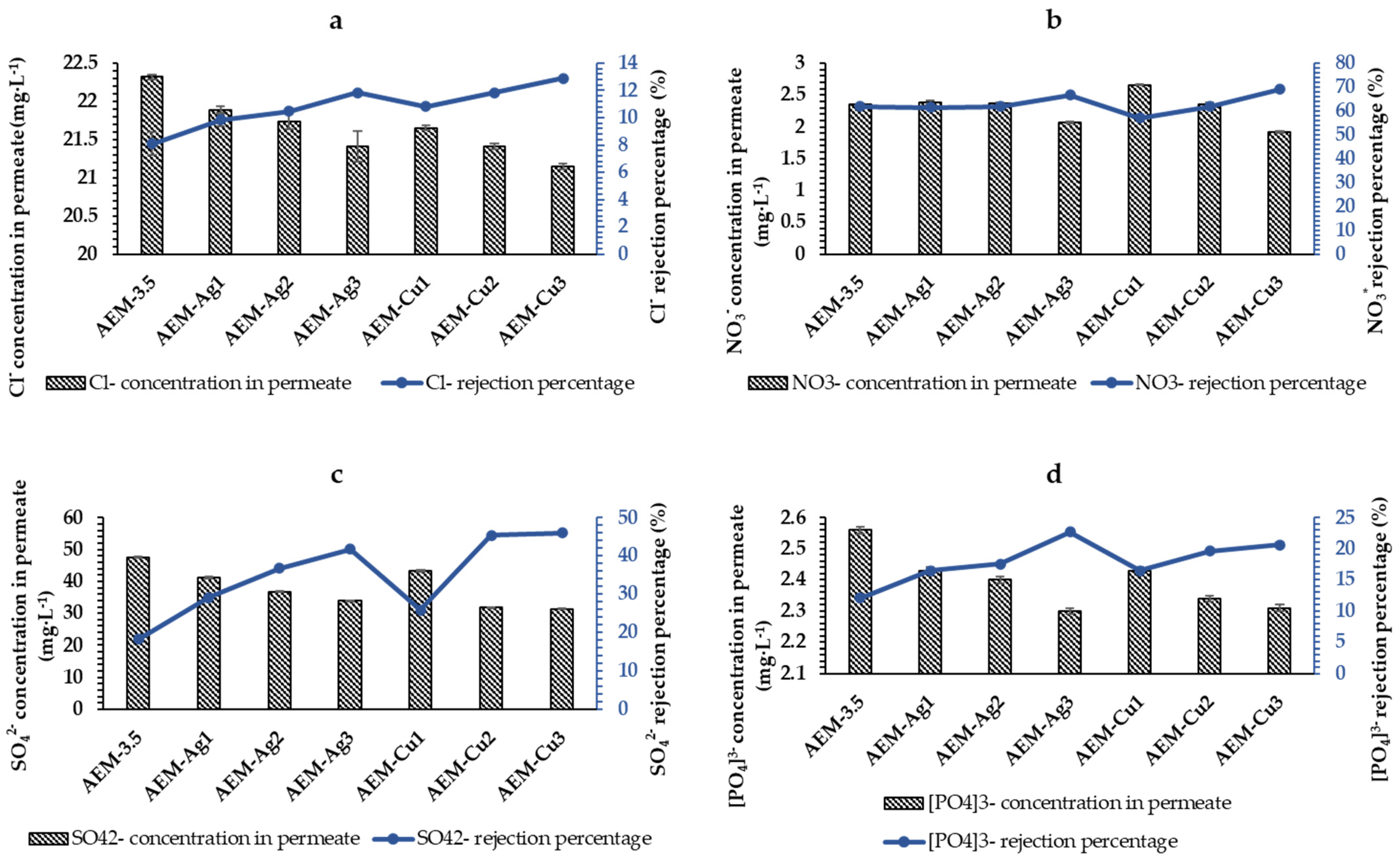
3.2.2. Salt Rejection Studies
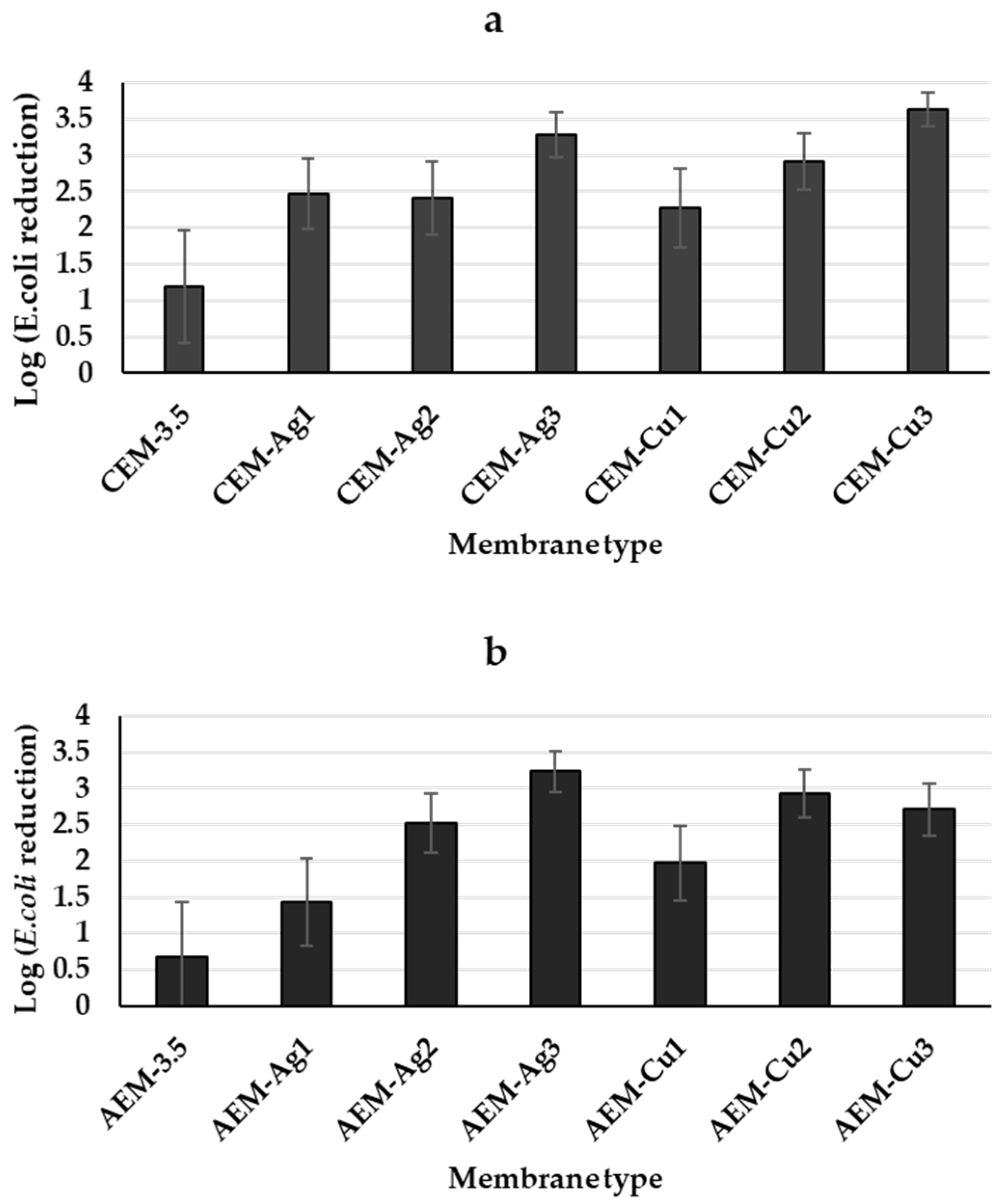
3.3. Antimicrobial Studies
Bacterial Filtration Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Meybeck, M.; Kuusisto, E.; Makela, A.; Malkki, E. Water quality. In Water Quality Monitoring—A Practical Guide to the Design and Implementation of Freshwater; Bartrman, J., Ballance, R., Eds.; United Nations Environment Programme and the World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Bwire, G.; Sack, D.; Kagirita, A.; Obala, T.; Debes, A.; Ram, M.; Komakech, H.; George, C.; Orach, C.G. The quality of drinking and domestic water from the surface water sources (lakes, rivers, irrigation canals and ponds) and springs in cholera prone communities of Uganda: An analysis of vital physicochemical parameters. BMC Public Health 2020, 20, 1128. [Google Scholar] [CrossRef] [PubMed]
- WHO. Drinking Water: Fact Sheets; World Health Organization: Geneva, Switzerland, 2019.
- Luyt, C.D.; Tandlich, R.; Muller, W.; Wilhelmi, B.S. Microbial monitoring of surface water in South Africa: An overview. Int. J. Environ. Res. Public Health 2012, 9, 2669–2693. [Google Scholar] [CrossRef] [PubMed]
- Peter-Varbanets, M.; Zurbroogg, C.; Swartz, C.; Pronk, W. Decentralised systems for portable water and the potential of membrane technology. Water Res. 2009, 43, 245–265. [Google Scholar] [CrossRef]
- Basson, M.S. Water Development in South Africa. OECD: Zaragoza, Spain, 2011. [Google Scholar]
- Hoslett, J.; Massara, T.; Malamis, S.; Ahmad, D.; van den Boogaert, I.; Katsou, E.; Ahmad, B.; Ghazal, H.; Simons, S.; Wrobel, L.; et al. Surface water fitration using granular media and membranes: A review. Sci. Total Environ. 2018, 639, 1268–1282. [Google Scholar] [CrossRef] [PubMed]
- Musingafi, M.C.; Tom, T. Fresh water sources pollution: A human related threat to fresh water security in South Africa. J. Public Policy Gov. 2014, 1, 72–81. [Google Scholar]
- Othman, N.H.; Alias, N.; Fuzil, N.; Marpani, F.; Shahruddin, M.; Chew, C.; Ng, K.; Lau, W.; Ismail, A.F. A review on the use of membrane technology systems in developing countries. Membranes 2022, 12, 30. [Google Scholar]
- Li, X.; Jiang, L.; Li, H. Application of ultrafiltration technology in water treatment. Earth Environ. Sci. 2018, 186, 012009. [Google Scholar]
- Chen, C.; Guo, L.; Yang, Y.; Oguma, K.; Hou, L. Comparative effectiveness of membrane technologies and disinfection methods for virus elimination in water: A review. Sci. Total Environ. 2021, 801, 149678. [Google Scholar] [CrossRef]
- Zularisam, A.W.; Ismail, A.; Sakinah, M. Application and challenges of membrane in surface water treatment. J. Appl. Sci. 2010, 10, 380–390. [Google Scholar]
- Chen, M.; Shen, S.; Zhang, F.; Zhang, C.; Xiong, J. Biodegradable dissolved organic carbon (BDOC) removal from micro-polluted water sources using ultrafiltration: Comparison with conventional processes, operation conditions and membrane fouling control. Polymers 2022, 14, 4689. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Asante-Sackey, D.; Rathilal, S.; Tetteh, E.; Ezugbe, E.; Pillay, L.V. Donnan membrane process for the seletive recovery and removal of target metal ions. Membranes 2021, 11, 358. [Google Scholar] [PubMed]
- Vobecka, L.; Bellon, T.; Slouka, Z. Behaviour of embedded cation exchange particles in a DC electric field. Int. J. Mol. Sci. 2019, 20, 3579. [Google Scholar]
- Kakihana, Y.; Hashim, N.; Mizuno, T.; Anno, M.; Higa, M. Ionic transport properties of cation exchange membranes prepared from poly (vinyl alcohol-b-sodium sytrene sulfonate). Membranes 2021, 11, 452. [Google Scholar] [CrossRef]
- Hassanvand, A.; Wei, K.; Talebi, S.; Chen, G.; Kentish, S.E. The role of ion exchange membranes in membrane capacitive deionization. Membranes 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geise, G.M.; Cassady, H.; Paul, D.; Logan, B.; Hickner, M.A. Specific ion effects on membranes potential and permselectivity of ion exchange membranes. R. Soc. Chem. 2014, 16, 21673–21681. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and application. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Siekierka, A.; Smolinska-Kempisty, K.; Wolska, J. Enhanced specific mechanism of separation by polymeric membrane modification: A short review. Membranes 2021, 11, 942. [Google Scholar] [PubMed]
- Nebavskaya, X.; Sarapulova, V.; Butylskii, D.; Larchet, C.; Pismenskaya, N. Electrochemical properties of homogeneous and heterogeneous anion exchange membranes coated with cation exchange polyelectrolyte. Membranes 2019, 9, 13. [Google Scholar]
- Mikhaylin, S.; Bazinet, L. Fouling on ion exchange membranes: Classification charaterization and strategies of preventation and control. Adv. Colloid Interface Sci. 2016, 229, 34–56. [Google Scholar] [CrossRef]
- Dammak, L.; Fouilloux, J.; Bdiri, M.; Larchet, C.; Renard, E.; Baklouti, L.; Sarapulova, V.; Kozmai, A.; Pismenskaya, N. A review on ion exchange membrane fouling during electrodialysis process in the food industry, Part 1: Types, effects, charaterization, methods, fouling mechanisms and interactions. Membranes 2021, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, M.; Fang, F.; Cui, L.; Wu, J.; Field, R.; Zhang, K. Biogenic silver TFC nanofiltration membrane with antifouling properties. Desalination Water Treat. 2016, 57, 10560–10571. [Google Scholar]
- Zhang, M.; Field, R.; Zhang, K. Biogenic silver nanocomposites polyethersulfone UF membranes with antifouling properties. J. Membr. Sci. 2014, 471, 274–284. [Google Scholar] [CrossRef]
- Herzberg, M.; Pandit, S.; Mauter, M.; Oren, Y. Bacterial biofilm formation on ion exchange membranes. J. Membr. Sci. 2020, 596, 117564. [Google Scholar]
- Bdiri, M.; Larchet, C.; Dammak, L. A review on ion exchange fouling and antifouling during electrodialysis used in food industry: Cleaning and strategies of prevention. Chem. Afr. 2020, 3, 609–633. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abba, A.; Benigna, I.; Sorlini, S.; Torretta, V. Overview of the main disinfection processes for wastewater and drinking water treatment plants. Sustainability 2018, 10, 86. [Google Scholar] [CrossRef]
- Rzhepishevska, O.; Ekstrand-Hammarstrom, B.; Popp, M.; Bjorn, E.; Bucht, A.; Sjostedt, A.; Antti, H.; Ramstedt, M. The antibacterial activity of Ga3+ is influenced by ligand complexation as well as the bacterial carbon source. Antimicrob. Agents Chemother. 2011, 55, 5568–5580. [Google Scholar]
- Shkodenko, L.; Kassirov, I.; Koshel, E. Metal oxide nanoparticles against bacterial biofilms: Perspectives and limitations. Microorganism 2020, 8, 1545. [Google Scholar]
- Yee, M.S.; Khiew, P.; Tan, Y.; Kok, Y.; Cheong, K.; Chiu, W.; Leong, C. Potent antifouling silver-polymer nanocomposite microsphere using ion exchange resins as templating matrix. Colloids Surf. A: Physicochem. Eng. Asp. 2014, 457, 382–392. [Google Scholar]
- Dankovick, T.A.; Smith, J.A. Incorporation of copper nanopartcles into paper for piont-of use water purification. Water Res. 2014, 63, 245–251. [Google Scholar]
- Jiang, S.; Wang, F.; Cao, X.; Slater, B.; Wang, R.; Sun, H.; Wang, H.; Shen, X.; Yao, Z. Novel application of ion exchange membranes for preparing effective silver and copper based antimicrobial membranes. Chemosphere 2022, 287, 132131. [Google Scholar] [PubMed]
- Nady, N. Modification methods of poly (arylsulfone) membranes: A mini review focusing on surface modification. Desalination 2011, 275, 1–9. [Google Scholar]
- Nady, N. PES surface modification using green chemistry: New generation of antifouling membranes. Membranes 2016, 6, 23. [Google Scholar]
- Kotoka, F.; Merino-Garcia, I.; Velizarov, S. Surface modifiction of anion exchange membrane for improved reverse electrodialysis process performance: A review. Membranes 2020, 10, 160. [Google Scholar]
- Bastos-Arrieta, J.; Munoz, J.; Vigues, N.; Muraviev, D.; Cespedes, F.; Mas, J.; Baeza, M.; Munoz, M. Intermatrix synthesisnof Ag, AgAu and Au nanoparticles by the galvanic replacement strategy for bactericidal and electrocatalytically active nanocomposites. New J. Chem. 2016, 40, 10344–10352. [Google Scholar]
- Bastos-Arrieta, J.; Munoz, J.; Stenbock-Fermor, A.; Munoz, M.; Muraviev, D.; Cespedes, F.; Tsarkova, L.; Baeza, M. Intermatrix synthesis as a rapid, inexpensive and reproducible methodology for in-situ functionalization of nanostructured surfaces with quantum dots. Appl. Surf. Sci. 2016, 368, 417–426. [Google Scholar]
- Domenech, B.; Bastos-Arrieta, J.; Alonso, A.; Macanas, J.; Munoz, M.; Muraviev, D.N. Bifunctional polymer-metal nanocomposite ion exchange materials. In Ion Exchange Technologies; INTECH: Barcelona, Spain, 2012; pp. 35–72. [Google Scholar]
- Bastos-Arrieta, J.; Munoz, M.; Puiz, P.; Muraviev, D.N. Morphological chnages of gel-type functional polymers after intermatrix synthesis of polymer stabilized silver nanoparticles. Nanoscale Res. Lett. 2013, 8, 255. [Google Scholar]
- Domenech, B.; Ziegler, K.; Carrillo, F.; Munoz, M.; Muraviev, D.; Macanas, J. Developing of novel catalytically active polymer-metal-nanocomposites based on activated foams and textile fibers. Nanoscale Res. Lett. 2013, 8, 238. [Google Scholar] [CrossRef] [Green Version]
- Domenech, B.; Vigues, N.; Mas, J.; Munoz, M.; Muraviev, D.; Macanas, J. Polymer-metal nanocomposites containing dual-function metal nanoparticles: Ion exchange materials modified with catalytically-active and bactericide silver nanoparticles. Solvent Extr. Ion Exch. 2014, 32, 301–315. [Google Scholar] [CrossRef]
- Alonso, A.; Macanas, J.; Davies, G.; Gun, Y.; Munoz, M.; Muraviev, D.N. Environmentally-safe catalytically active active and biocide polymer-metal nanocomposites with enhanced structural parameters. In Advances in Nanocomposite Technology; Hashim, A., Ed.; INTECH: Barcelona, Spain, 2011; pp. 175–200. [Google Scholar]
- Muraviev, D.N.; Macanas, J.; Parrondo, J.; Munoz, M.; Alonso, A.; Alegret, S.; Ortueta, M.; Mijangos, F. Cation exchange membrane as nanoreactor: Intermatrix synthesis of platinum-copper core-shell. React. Funct. Polym. 2007, 67, 1612–1621. [Google Scholar] [CrossRef]
- Alonso, A.; Bastos-Arrieta, J.; Davies, G.; Gun, Y.; Munoz-Berbel, X.; Macanas, J.; Mas, J.; Munoz, M.; Muraveiv, D.N. Ecologically friendly polymer-metal and polymer-metal oxide nanocomposites for complex water treatment. In Nanocomposite-New Trends and Development; INTECH: Barcelona, Spain, 2012; pp. 187–214. [Google Scholar]
- Mudau, F.; Motsa, M.; Hassard, F.; De Kock, L. Resin-loaded heterogeneous polyether sulfone ion exchange membranes for saline groundwater treatment. Membranes 2022, 12, 736. [Google Scholar] [PubMed]
- WHO. Silver in Drinking-Water. Background Document for Development of WHO Guidelines for Drinking-Water Quality. (WHO/HEP/ECH/WSH/2021.7); World Health Organization: Geneva, Switzerland, 2021.
- US EPA. 2018 Edition of Drinking Water Standards and Health Advisories. EPA 822-F-18-001; Office of Water U.S. Environmental Protection Agency: Washington, DC, USA, 2018.
- Smith, E.J.; Davison, W.; Hamilton-Taylor, J. Methods for preparing synthetic freshwaters. Water Res. 2002, 36, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.; Macanas, J.; Munoz, M.; Muraviev, D.N. Intermatrix synthesis: Easy technique permitting preparation of polymer-stabilized nanoparticles with desired composition and structure. Nanoscale Res. Lett. 2011, 6, 343. [Google Scholar]
- Alonso, A.; Munoz-Berbel, X.; Vigues, M.; Rodriguez-Rodriguez, R.; Macanas, J.; Munoz, M.; Mas, J.; Muraviev, D.N. Superparamagnetic Ag@Co-nanocomposite on granulated cation exchange polymeric matrices with enhanced antibacterial activity for the enviromentally safe purification of water. Adv. Funct. Mater. 2013, 23, 2450–2458. [Google Scholar]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effect of size and aggregation/agglomeration of nanoparticles on the interfacial/interphase properties and tensile strength polymer nanocomposite. Nanoscale Res. Lett. 2018, 13, 214. [Google Scholar] [CrossRef]
- Ahmad, N.; Ang, B.; Amalina, M.; Bong, C.W. Influence of precursor concentration and temperature on the formation of nanosilver in chemical reduction method. Sains Malays. 2018, 47, 157–168. [Google Scholar]
- Sibiya, P.N.; Moloto, M.J. Effect of precursor concentration and pH on the shape and size of starch capped silver selenide (Ag2Se) nanoparticles. Chalcogenide Lett. 2014, 11, 577–588. [Google Scholar]
- Lah, N.A.C.; Johan, M.R. Facile shape control synthesis and optical properties of silver nanoperticles by Daxad 19 surfactant. Appl. Surf. Sci. 2011, 257, 7494–7500. [Google Scholar]
- Saito, Y.; Morimura, W.; Kuratani, R.; Nishikawa, S. Factors controlling the ionic mobility of lithium electrolyte solutions in separator membranes. J. Phys. Chem. 2016, 120, 3619–3624. [Google Scholar] [CrossRef]
- Banerjee, P.; Bagchi, B. Ion’s motion in water. J. Chem. Phys. 2019, 150, 190901. [Google Scholar] [CrossRef]
- Li, X.; Pang, R.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. In situ formation of Ag nanoparticles in PVDF ultrafiltration membrane to mitigate organic and bacterial fouling. Desalination 2013, 324, 48–56. [Google Scholar]
- Wen, Y.; Yuan, J.; Ma, X.; Wang, S.; Liu, Y. Polymeric nanocomposite membranes for water treatment. Environ. Chem. Lett. 2019, 17, 1539–1551. [Google Scholar]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar]
- Palza, H.; Quijada, R.; Delgado, K. Antimicrobial polymer composites with micro- and nanoparticles: Effects of particle size and poymer matrix. J. Bioact. Compat. Polym. 2015, 30, 1–15. [Google Scholar] [CrossRef]
- Mukherjee, M.; Bandyopadhyaya, R. Silver nanoparticle impregnated polyethersulfone ultrafiltration membrane: Optimization of degree of grafting of acrylic acid for biofouling prevention and improved water permeability. J. Environ. Chem. Eng. 2020, 8, 103711. [Google Scholar]
- Hosseini, S.M. Fabrication of novel mixed matrix electrodialysis heterogeneous ion-exchanhe membranes modified by ilmenite (FeTiO3): Electrochemical and ionic transport characteristics. Ionics 2015, 21, 437–447. [Google Scholar]
- Gohil, J.M.; Ray, P. Preparation of heterogeneous cation and anion exchange membranes by eco-friendly method: Electrochemical charaterization and desalination performance. J. Membr. Sci. Res. 2020, 7, 102–110. [Google Scholar]
- Nujic, M.; Milinkovic, D.; Habuda-Stanic, M. Nitrate removal from water by ion exchange. Croat. J. Food Sci. Technol. 2017, 9, 182–186. [Google Scholar]
- Ma, Y.; Shi, F.; Ma, S.; Wu, M.; Zhang, S.; Gao, C. Effect of PEG additive on the morphology and performance of polysulfone ultrafiltration membrane. Desalination 2011, 272, 51–58. [Google Scholar]
- Taka, A.L.; Pillay, K.; Mbianda, X.Y. Nanosponge cyclodetrix polyurethanes and their modification with nanomaterials for the removal pollutants from waste water: A review. Carbohydr. Polym. 2017, 159, 94–107. [Google Scholar]
- WHO. Guidelines for Drinking Water Quality: Fourth Addition; World Health Organization: Geneva, Switzerland, 2011.
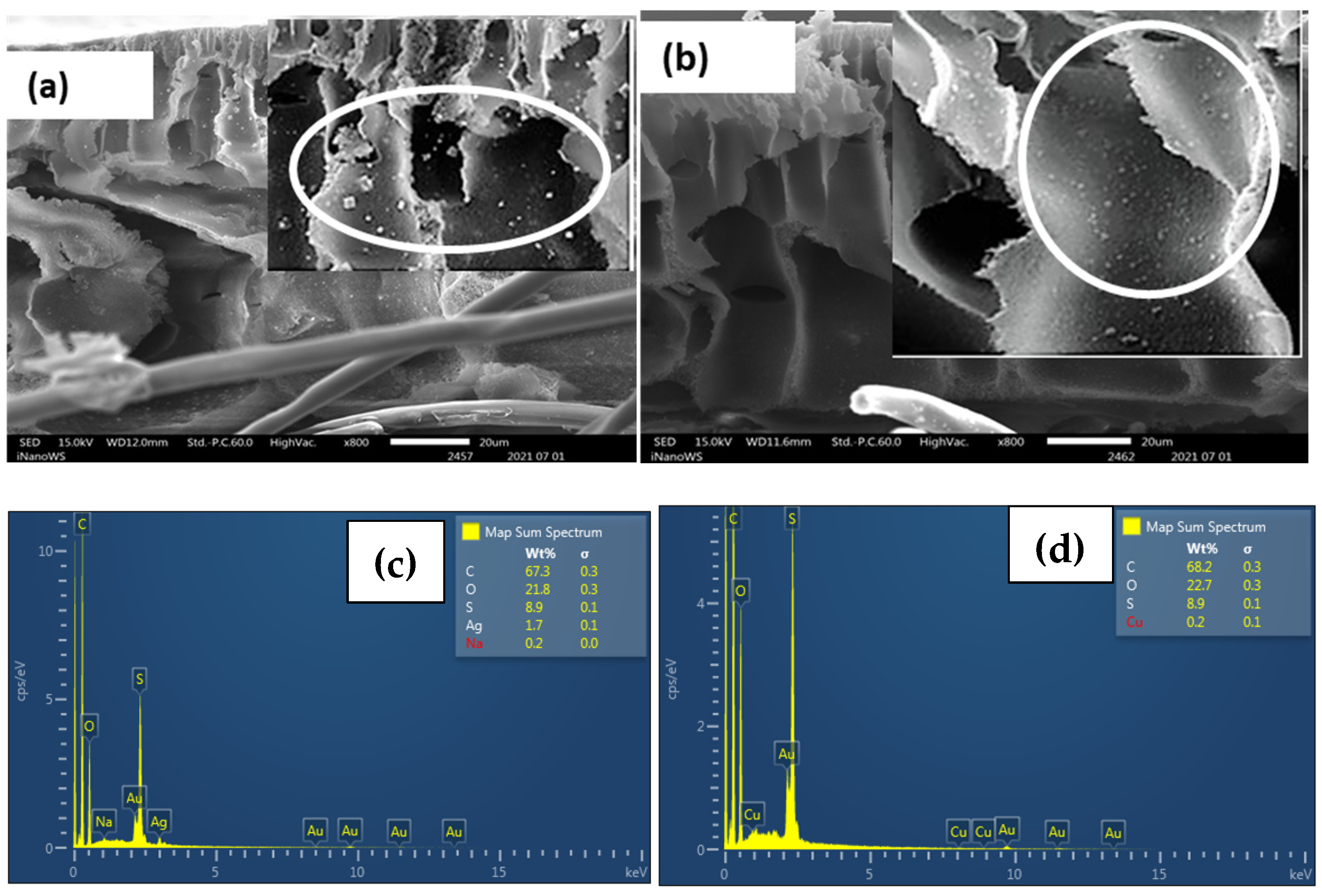
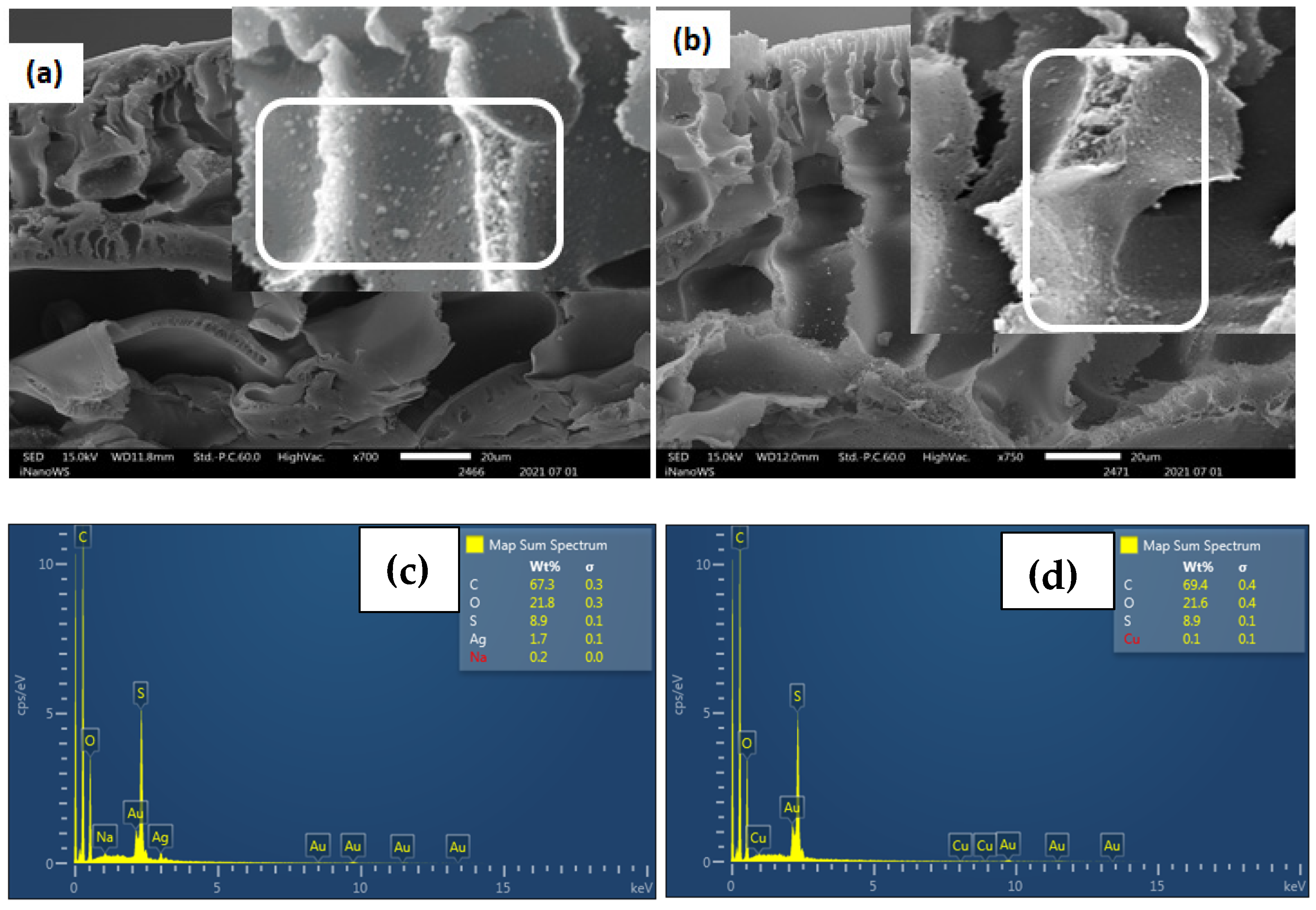

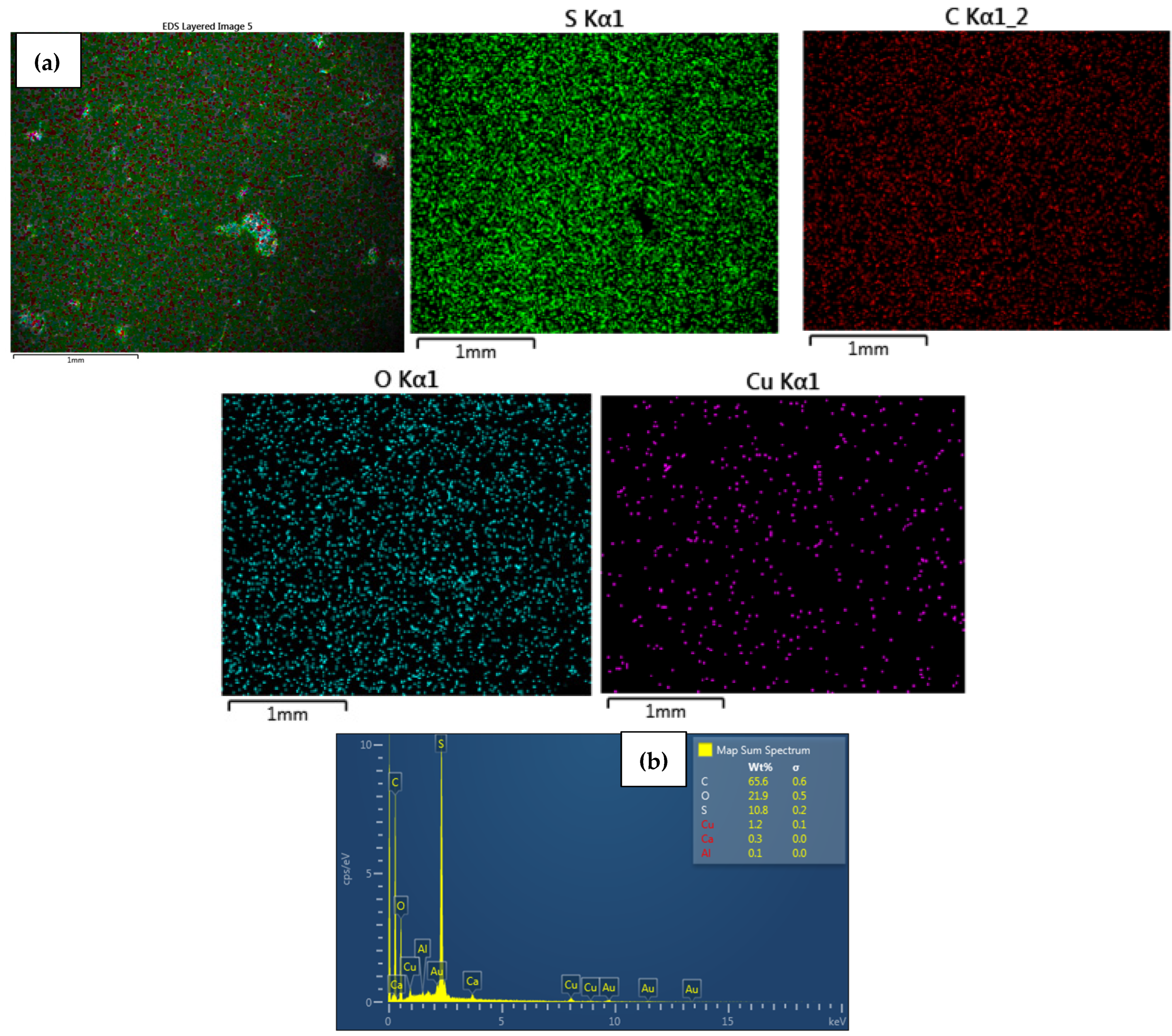



| Functionality | Cation Exchange Membrane (CEM) | Anion Exchange Membrane (AEM) |
|---|---|---|
| Ion-exchange resin | Amberlyst 15 | Amberlite IRA 900 |
| Functional Group | Sulfonic acid | Quaternary ammonium |
| Resin loading | 3.5 wt.% | |
| Polymer binder | PES (15 wt.%) | |
| Pore former | PEG 10,000 (2 wt.%) | |
| Solvent used in membrane preparation | NMP (79.5 wt.%) | |
| Membrane-supporting fabric | Polyester nonwoven fabric | |
| Preparation method | Phase inversion | |
| Ion-exchange capacity (IEC) (meq·g−1) | 0.58 | 1 |
| Water content (%) | 65.55 ± 0.52 | 63.96 ± 0.32 |
| Contact angle (°) | 76.26 ± 2.68 | 74.49 ± 4.50 |
| Membrane potential (mV) | 18.97 | 26.0 |
| Permselectivity and transport number | 0.66 and 0.44 | 0.72 and 0.54 |
| Membrane thickness (µm) | 216.8 | 223.2 |
| Water permeability (L·m−2·h−1) @ 0.05 MPa | 14.90 ± 0.45 | 17.79 ± 0.29 |
| Membrane Type | Functionalized Membranes | AgNO3 (mol·L−1) | CuSO4 (mol·L−1) |
|---|---|---|---|
| CEM-3.5 | CEM-Ag1 | 0.01 | |
| CEM-Ag2 | 0.025 | ||
| CEM-Ag3 | 0.05 | ||
| CEM-Cu1 | 0.01 | ||
| CEM-Cu2 | 0.025 | ||
| CEM-Cu3 | 0.05 | ||
| AEM-3.5 | AEM-Ag1 | 0.01 | |
| AEM-Ag2 | 0.025 | ||
| AEM-Ag2 | 0.05 | ||
| AEM-Cu1 | 0.01 | ||
| AEM-Cu2 | 0.025 | ||
| AEM-Cu3 | 0.05 |
| Stock Solutions (Compound Formula) | Final Concentration (mg·L−1) | Mass of Salt Per Volume of Water (g) | Volume (mL) of Stock Solution to Make 5 L of Synthetic Surface Water | |
|---|---|---|---|---|
| Cation | Anion | |||
| H1 CaCl2·6H2O Ca (NO3)2·4H2O | 13.727 2.004 | 24.285 6.200 | 1 L 7.491 1.181 | 50 |
| H2 CaCO3 | 33.367 | 58.233 | 5 L 0.458 | 4545 |
| H3 KHCO3 KH2PO4 NaHCO3 Na2SO4 | 2.932 1.173 6.207 9.081 | 4.577 2.910 16.473 18.971 | 1 L 0.751 0.408 2.268 2.805 | 50 |
| H4 MgSO4·7H2O | 9.904 | 39.143 | 1 L 10.044 | 50 |
| Water | 305 | |||
| Ag Membrane Type | Hydrodynamic Particle Diameter (nm) of Ag | Cu Membrane Type | Hydrodynamic Particle Diameter (nm) of Cu |
|---|---|---|---|
| CEM-Ag1 | 62.42 ± 18.8 | CEM-Cu1 | 54.2 ± 14.7 |
| CEM-Ag2 | 89.90 ± 5.1 | CEM-Cu2 | 60.9 ± 11.4 |
| CEM-Ag3 | 101.0 ± 5.4 | CEM-Cu3 | 77.2 ± 3.2 |
| AEM-Ag1 | 89.4 ± 5.3 | AEM-Cu1 | 79.8 ± 11.0 |
| AEM-Ag2 | 93.1 ± 3.5 | AEM-Cu2 | 87.2 ± 1.0 |
| AEM-Ag3 | 121.1 ± 10.6 | AEM-Cu3 | 125.7± 21.1 |
| Ag Membrane Type | Metal Content (mg·cm−2) | Cu Membrane Type | Metal Content (mg·cm−2) |
|---|---|---|---|
| CEM-Ag1 | 0.020 ± 0.003 | CEM-Cu1 | 0.031 ± 0.003 |
| CEM-Ag2 | 0.029 ± 0.001 | CEM-Cu2 | 0.048 ± 0.02 |
| CEM-Ag3 | 0.034 ± 0.002 | CEM-Cu3 | 0.062 ± 0.01 |
| AEM-Ag1 | 0.052 ± 0.01 | AEM-Cu1 | 0.053 ± 0.007 |
| AEM-Ag2 | 0.077 ± 0.03 | AEM-Cu2 | 0.087 ± 0.008 |
| AEM-Ag3 | 0.084 ± 0.01 | AEM-Cu3 | 0.218 ± 0.02 |
| Ag Membrane Type | Ag Metal Loss | Cu Membrane Type | Cu Metal Loss | ||
|---|---|---|---|---|---|
| Concentration (ppb) | R (%) | Concentration (ppb) | R (%) | ||
| CEM-Ag1 | 4.82 ± 0.23 | 24.10 ± 0.23 | CEM-Cu1 | 2.02 ± 0.01 | 6.51 ± 0.01 |
| CEM-Ag2 | 4.85 ± 0.01 | 16.72 ± 0.01 | CEM-Cu2 | 3.44 ± 0.02 | 7.16 ± 0.02 |
| CEM-Ag3 | 8.41 ± 0.07 | 24.73 ± 0.07 | CEM-Cu3 | 2.94 ± 0.03 | 4.74 ± 0.03 |
| AEM-Ag1 | 5.21 ± 0.15 | 10.00 ± 0.15 | AEM-Cu1 | 3.93 ± 0.02 | 6.40 ± 0.02 |
| AEM-Ag2 | 6.23 ± 0.10 | 8.09 ± 0.10 | AEM-Cu2 | 4.85 ± 0.14 | 5.77 ± 0.14 |
| AEM-Ag3 | 8.72 ± 0.12 | 10.02 ± 0.12 | AEM-Cu3 | 5.32 ± 0.05 | 2.44 ± 0.05 |
| Membrane Type | Pure Water Flux (L·m−2·h−1) |
|---|---|
| CEM-3.5 | 14.90 ± 0.45 |
| CEM-Ag1 | 13.31 ± 0.13 |
| CEM-Ag2 | 12.67 ± 0.16 |
| CEM-Ag3 | 12.00 ± 0.25 |
| CEM-Cu1 | 12.97 ± 0.23 |
| CEM-Cu2 | 12.55 ± 0.10 |
| CEM-Cu3 | 11.92 ± 0.18 |
| AEM-3.5 | 17.79 ± 0.29 |
| AEM-Ag1 | 16.15 ± 0.28 |
| AEM-Ag2 | 15.50 ± 0.18 |
| AEM-Ag3 | 14.94 ± 0.44 |
| AEM-Cu1 | 16.27 ± 0.38 |
| AEM-Cu2 | 15.41 ± 0.29 |
| AEM-Cu3 | 14.69 ± 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mudau, F.H.; Hassard, F.; Motsa, M.M.; De Kock, L.-A. Multifunctional Heterogeneous Ion-Exchange Membranes for Ion and Microbe Removal in Low-Salinity Water. Polymers 2023, 15, 843. https://doi.org/10.3390/polym15040843
Mudau FH, Hassard F, Motsa MM, De Kock L-A. Multifunctional Heterogeneous Ion-Exchange Membranes for Ion and Microbe Removal in Low-Salinity Water. Polymers. 2023; 15(4):843. https://doi.org/10.3390/polym15040843
Chicago/Turabian StyleMudau, Fulufhelo Hope, Francis Hassard, Machawe Mxolisi Motsa, and Lueta-Ann De Kock. 2023. "Multifunctional Heterogeneous Ion-Exchange Membranes for Ion and Microbe Removal in Low-Salinity Water" Polymers 15, no. 4: 843. https://doi.org/10.3390/polym15040843





