Magnetic Bacterial Cellulose Biopolymers: Production and Potential Applications in the Electronics Sector
Abstract
:1. Introduction
2. Bacterial Cellulose
2.1. Characteristics
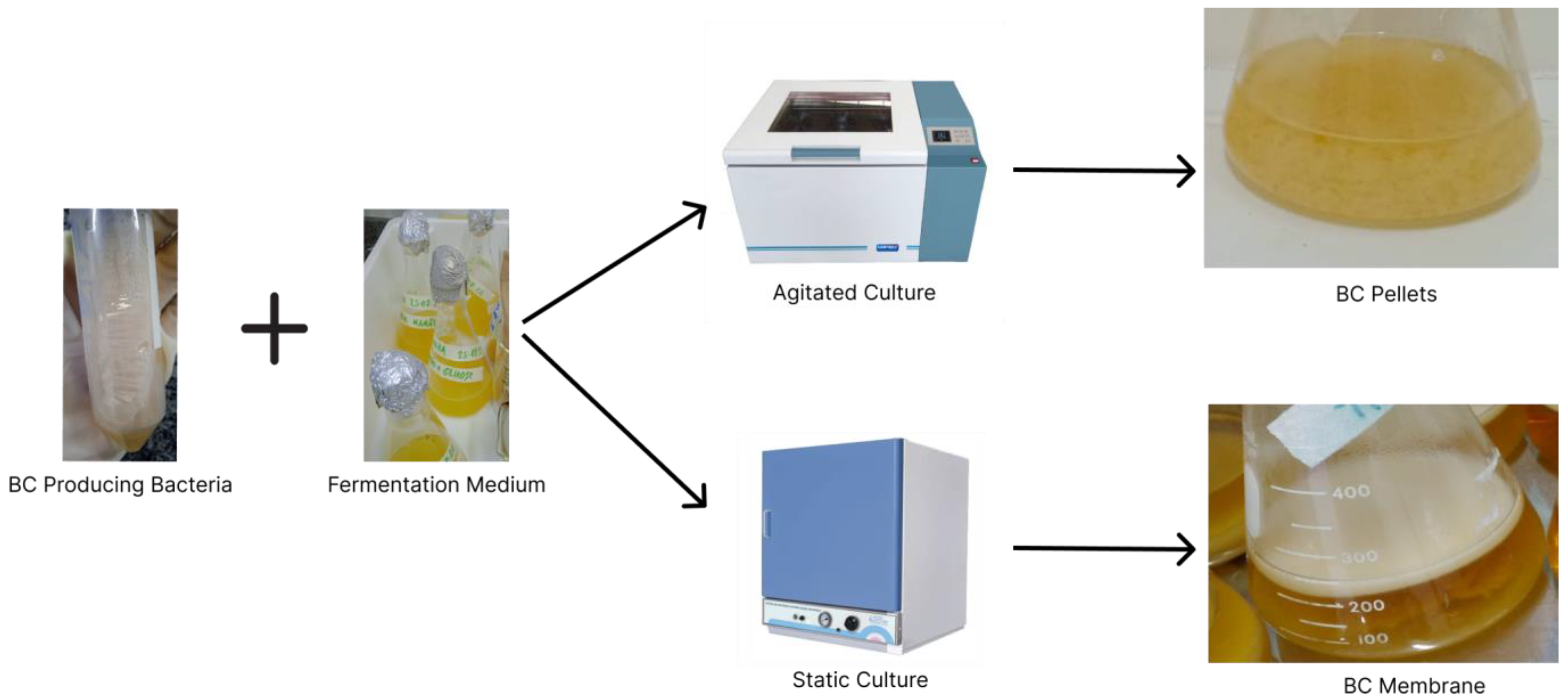
2.2. Production
2.3. Modifications and Dopants
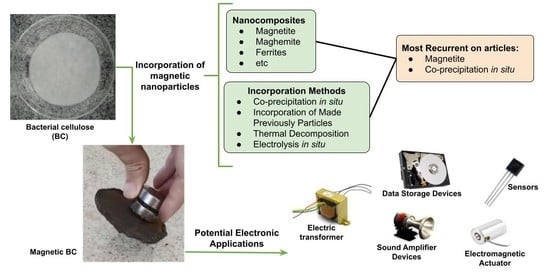
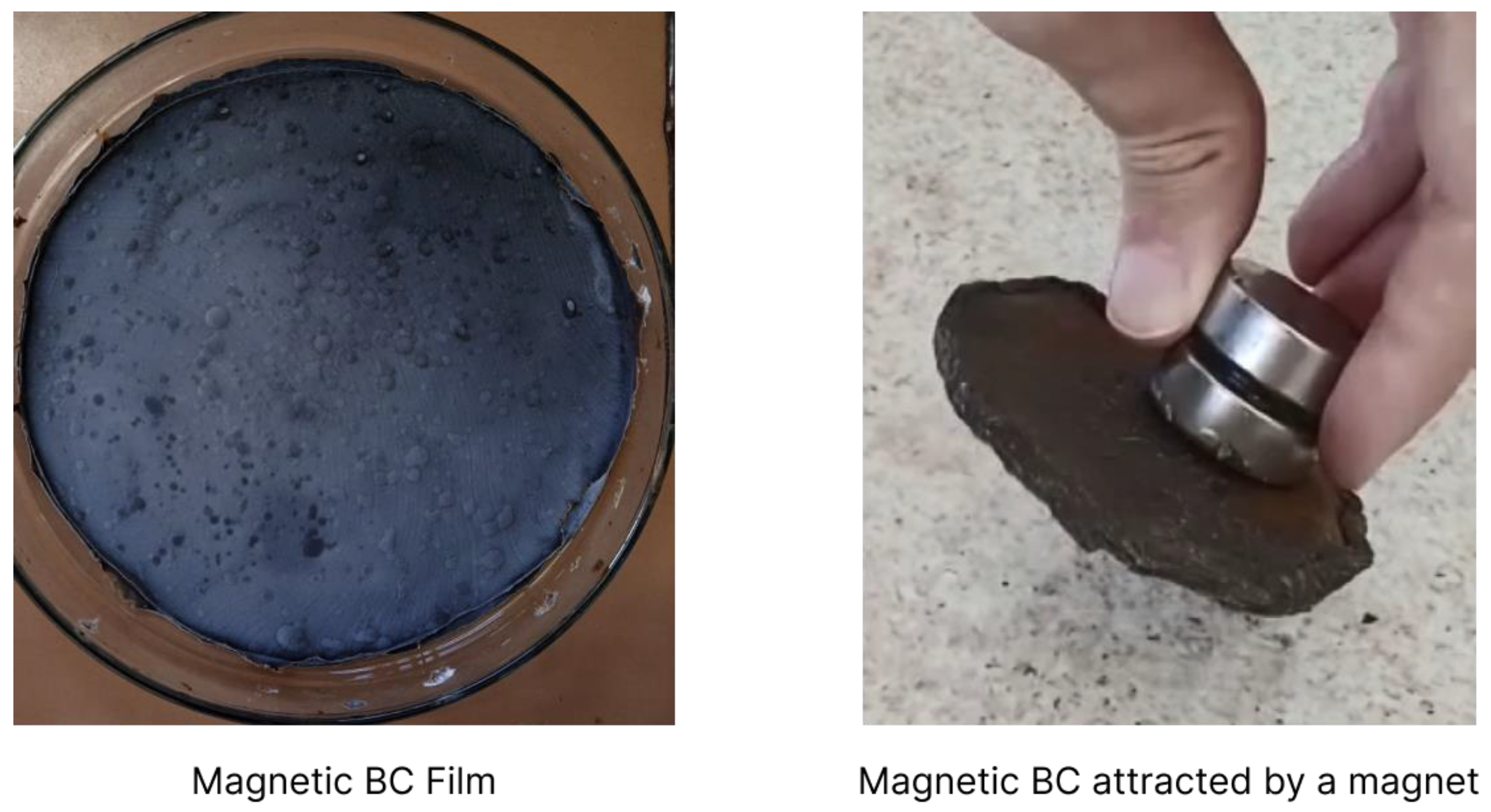
3. Magnetic Bacterial Cellulose Biopolymers
3.1. Magnetic Particles Added to BC
3.2. Forms of BC Magnetic Doping
3.3. Applications
3.4. Electro-Electronic Applications of Magnetic BC
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, M.; Zhang, J.; Xu, W.; Xiong, R.; Huang, C. Cellulose-based fibrous materials for self-powered wearable pressure sensor: A mini review. Cellulose 2023, 63, 1–18. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, m.; Bhattacharya, M.; Mandal, T.; Saswata, G. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Betlej, I.; Zakaria, S.; Krajewski, K.J.; BoruszewskIi, P. Bacterial cellulose–properties and its potential application. Sains Malays 2021, 50, 493–505. [Google Scholar] [CrossRef]
- Romero-Montero, A.; Valencia-Bermúdez, J.L.; Rosas-Meléndez, S.A.; Núñez-Tapia, I.; Piña-Barba, M.C.; Leyva-Gómez, G.; Del Prado-Audelo, M.L. Biopolymeric Fibrous Aerogels: The Sustainable Alternative for Water Remediation. Polymers 2023, 15, 262. [Google Scholar] [CrossRef] [PubMed]
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the fermentation process and properties of bacterial cellulose: A review. Cellulose 2016, 23, 57–91. [Google Scholar] [CrossRef]
- Amorim, J.D.P.; Souza, K.C.; Duarte, C.R.; Duarte, I.S.; Ribeiro, F.A.S.; Silva, G.S.; Farias, P.M.A.; Sting, A.; Costa, A.F.S.; Vinhas, G.M.; et al. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. A review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Costa, A.F.S.; Almeida, F.C.G.; Vinhas, G.M.; Sarubbo, L.A. Production of Bacterial Cellulose by Gluconacetobacter Hansenii Using Corn Steep Liquor as Nutrient Sources. Front. Microbiol. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Costa, A.F.S.; Silva, C.J.G., Jr.; Meira, H.M.; Amorim, J.D.P.; Silva, I.; Gomes, E.S.; Sarubbo, L.A. Production of Paper Using Bacterial Cellulose and Residue From The Sugar and Alcohol Industry. Chem. Eng. Trans. 2020, 79, 85–90. [Google Scholar] [CrossRef]
- Silva, C.J.G., Jr.; Medeiros, A.D.M.; Amorim, J.D.P.; Nascimento, H.A.; Converti, A.; Costa, A.F.S.; Sarubbo, L.A. Bacterial cellulose biotextiles for the future of sustainable fashion: A review. Environ. Chem. Lett. 2021, 19, 2967–2980. [Google Scholar] [CrossRef]
- Nascimento, H.A.; Amorim, J.D.P.; Filho, L.E.P.T.M.; Costa, A.F.S.; Sarubbo, L.A.; Napoleão, D.C.; Vinhas, G.M. Production of Bacterial Cellulose with Antioxidant Additive from Grape Residue with Promising Cosmetic Applications. Polym. Eng. Sci. 2022, 62, 2826–2839. [Google Scholar] [CrossRef]
- Albuquerque, R.M.P.; Meir, H.M.; Silva, I.D.L.; Silva, C.J.G., Jr.; Almeida, F.C.G.; Amorim, J.D.P.; Vinhas, G.M.; Costa, A.F.S.; Sarubbo, L.A. Production of A Bacterial Cellulose/Poly (3-Hydroxybutyrate) Blend Activated with Clove Essential Oil For Food Packaging. Polym. Polym. Compos. 2021, 29, 259–270. [Google Scholar] [CrossRef]
- Amorim, J.D.P.; Nascimento, H.A.; Silva, C.J.G., Jr.; Medeiros, A.D.M.; Silva, I.D.L.; Costa, A.F.S.; Vinhas, G.M.; Sarubbo, L.A. Obtainment Of Bacterial Cellulose With Added Propolis Extract For Cosmetic Applications. Polym. Eng. Sci. 2021, 62, 565–575. [Google Scholar] [CrossRef]
- Huo, Y.; Guo, D.; Yang, J.; Chang, Y.; Wang, B.; Mu, C.; Xiang, J.; Nie, A.; Zhai, K.; Xue, T.; et al. Multifunctional Bacterial Cellulose Nanofibers/Polypyrrole (Ppy) Composite Films for Joule Heating and Electromagnetic Interference Shielding. ACS Appl. Electron. Mater. 2022, 4, 2552–2560. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 4, 1–54. [Google Scholar] [CrossRef]
- Urbina, L.; Corcuera, M.A.; Gabilondo, N.; Eceiza, A.; Retegi, A. A review of bacterial cellulose: Sustainable production from agricultural waste and applications in various fields. Cellulose 2021, 28, 8229–8253. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Du, Z.; Hu, S.; Huang, J.; Shi, Z.; Su, B.; Yang, G. Cofe2O4 Embedded Bacterial Cellulose for Flexible, Biodegradable, and Self-Powered Electromagnetic Sensor. Nano Energy 2022, 102, 107740. [Google Scholar] [CrossRef]
- Safarik, I.; Safarikova, M. Magnetic nano-and microparticles in biotechnology. Chem. Zvesti 2009, 63, 497–505. [Google Scholar] [CrossRef]
- Merazzo, K.J.; Lima, A.C.; Rincón-Iglesias, M.; Fernandes, L.C.; Pereira, N.; Lanceros-Mendez, S.; Martins, P. Magnetic materials: A journey from finding north to an exciting printed future. Mater. Horiz. 2021, 8, 2654–2684. [Google Scholar] [CrossRef]
- Sriplai, N.; Pinitsoontorn, S. Bacterial cellulose-based magnetic nanocomposites: A review. Carbohydr. Polym. 2020, 254, 117228. [Google Scholar] [CrossRef]
- Kumar, A.; Sood, A.; Han, S.S. Potential of magnetic nano cellulose in biomedical applications: Recent Advances. Biomater. Polym. Horiz. 2022, 1, 32–47. [Google Scholar] [CrossRef]
- Jin, K.; Jin, C.; Wu, Y. Synthetic Biology-Powered Microbial Co-Culture Strategy and Application of Bacterial Cellulose-Based Composite Materials. Carbohydr. Polym. 2022, 283, 119171. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Tsai, M.L.; Chen, C.W.; Dong, C.D. Genetic modification for enhancing bacterial cellulose production and its applications. Bioengineered 2021, 12, 6793–6807. [Google Scholar] [CrossRef]
- Yu, C. Natural textile fibres: Vegetable fibres. In Textiles and Fashion, 1st ed.; Sinclair, R., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 29–56. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods–A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.D.L.M.; Silva, C.J.G., Jr.; Amorim, J.D.P.; Durval, I.J.B.; Costa, A.F.S.; Sarubbo, L.A. Oily Wastewater Treatment: Methods, Challenges, and Trends. Processes 2022, 10, 743. [Google Scholar] [CrossRef]
- Wasin, M.; Shi, F.; Liu, J.; Khan, M.R.; Farooq, A.; Sanbha, N.; Alfred, M.; Xin, L.; Yajun, C.; Zhao, X. Extraction of cellulose to progress in cellulosic nanocomposites for their potential applications in supercapacitors and energy storage devices. J. Mater. Sci. 2021, 56, 14448–14486. [Google Scholar] [CrossRef]
- Manimaran, P.; Pillai, G.P.; Vignesh, V.; Prithiviraj, M. Characterization of natural cellulosic fibers from Nendran Banana Peduncle plants. Int. J. Biol. Macromol. 2020, 162, 1807–1815. [Google Scholar] [CrossRef]
- Sriplai, N.; Mongkolthanaruk, W.; Eichhorn, S.J.; Pinitsoontorn, S. Magnetic bacterial cellulose and carbon nanofiber aerogel by simple immersion and pyrolysis. J. Mater. Sci. 2020, 55, 4113–4126. [Google Scholar] [CrossRef]
- Zhang, X.; Kang, S.; Adstedt, K.; Kim, M.; Xiong, R.; Yu, J.; Chen, X.; Zhao, X.; Ye, C.; Tsukruk, V.V. Uniformly Aligned Flexible Magnetic Films from Bacterial Nanocelluloses for Fast Actuating Optical Materials. Nat. Commun. 2022, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Biocompatibility of bacterial cellulose-based biomaterials. J. Funct. Biomater. 2012, 3, 864–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portela, R.; Leal, C.R.; Pedro, L. Minireview Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef]
- Liu, W.; Du, H.; Zhang, M.; Liu, K.; Liu, H.; Xie, H.; Zhang, X.; Si, C. Bacterial cellulose-based composite scaffolds for biomedical applications: A review. ACS Sustain. Chem. Eng. 2020, 8, 7536–7562. [Google Scholar] [CrossRef]
- Eslahi, N.; Mahmoodi, A.; Mahmoudi, N.; Zandi, N.; Simchi, A. Processing and properties of nanofibrous bacterial cellulose-containing polymer composites: A review of recent advances for biomedical applications. Polym. Rev. (Phila Pa) 2019, 60, 144–170. [Google Scholar] [CrossRef]
- Esa, F.; Tasirin, S.M.; Rahman, N.A. Overview of Bacterial Cellulose Production and Application. Ital. Oral Surg. 2014, 2, 113–119. [Google Scholar] [CrossRef]
- Watanabe, K.; Tabuchi, M.; Morinaga, Y.; Yoshinaga, F. Structural features and properties of bacterial cellulose produced in agitated culture. Cellulose 1998, 5, 187–200. [Google Scholar] [CrossRef]
- Sperotto, G.; Stasiak, L.G.; Godoi, J.P.M.G.; Gabiatti, N.C.; Souza, S.S. A review of culture media for bacterial cellulose production: Complex, chemically defined and minimal media modulations. Cellulose 2021, 28, 2649–2673. [Google Scholar] [CrossRef]
- Raut, M.P.; Asare, E.; Syed Mohamed, S.M.D.; Amadi, E.N.; Roy, I. Bacterial Cellulose-Based Blends and Composites: Versatile Biomaterials for Tissue Engineering Applications. Int. J. Mol. Sci. 2023, 24, 986. [Google Scholar] [CrossRef]
- Hestrin, S.; Schramm, M. Synthesis of cellulose by Acetobacterxylinum: II. Preparation of freeze—Dried cells capable of polymerized glucose to cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef]
- Kurosumi, A.; Sasaki, C.; Yamashita, Y.; Nakamura, Y. Utilization of various fruit juices as carbon source for production of bacterial cellulose by Acetobacter xylinum NBRC 13693. Carbohydr. Polym. 2019, 76, 333–335. [Google Scholar] [CrossRef]
- Duarte, E.B.; Andrade, F.K.; Lima, H.L.S.; Nascimento, E.S.; Carneiro, M.J.M.; Borges, M.F.; Luz, E.P.C.G.; Chagas, B.S.; Rosa, M.F. Documentos186—Celulose Bacteriana Propriedades, Meios Fermentativos E Aplicações, 1st ed.; Embrapa Agroindústria Tropical: Fortaleza, Brazil, 2019; pp. 12–19. [Google Scholar]
- Ul-Islam, M.; Ullah, M.W.; Khan, S.; Park, J.K. Strategies for cost-effective and enhanced production of bacterial cellulose. Int. J. Biol. Macromol. 2017, 102, 1166–1173. [Google Scholar]
- Ul-Islam, M.; Ullah, M.W.; Khan, S.; Park, J.K. Production of bacterial cellulose from alternative cheap and waste resources: A step for cost reduction with positive environmental aspects. Korean J. Chem. Eng. 2020, 37, 925–937. [Google Scholar] [CrossRef]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of bacterial cellulose from industrial wastes: A review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Yang, J.; Li, Z.; Wang, H. Functionalized bacterial cellulose derivatives and nanocomposites. Carbohydr. Polym. 2014, 101, 1043–1060. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C. Industrial-scale production and applications of bacterial cellulose. Front. Bioeng. Biotechnol. 2020, 8, 1–19. [Google Scholar] [CrossRef]
- Torres, F.G.; Arroyo, J.J.; Troncoso, O.P. Bacterial cellulose nanocomposites: An all-nano type of material. Mater. Sci. Eng. C 2019, 98, 1277–1293. [Google Scholar] [CrossRef]
- Nascimento, H.A.; Amorim, J.D.P.; Silva, C.J.G., Jr.; Medeiros, A.D.L.M.; Costa, A.F.S.; Napoleão, D.C.; Vinhas, G.M.; Sarubbo, L.A. Influence of gamma irradiation on the properties of bacterial cellulose produced with concord grape and red cabbage extracts. Curr. Res. Biotechnol. 2022, 4, 119–128. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Wang, D.; Grady, T.L. A comparison of kombucha SCOBY bacterial cellulose purification methods. Appl. Sci. 2020, 2, 1–7. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Improving bacterial cellulose films by ex-situ and in-situ modifications: A review. Food Hydrocoll. 2021, 113, 106514. [Google Scholar] [CrossRef]
- Barud, H.S.; Regiani, T.; Marques, R.F.C.; Lustri, W.R.; Messaddeq, Y.; Ribeiro, S.J.L. Antimicrobial Bacterial Cellulose-Silver Nanoparticles Composite Membranes. J. Nanomater. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, L.; Zheng, S.; Chai, S.; Wei, J.; Zhong, L.; He, Y.; Xue, J. Bacteriostatic Activity and Cytotoxicity of Bacterial Cellulose-Chitosan Film Loaded with In-Situ Synthesized Silver Nanoparticles. Carbohydr. Polym. 2022, 281, 119017. [Google Scholar]
- Audtarat, S.; Hongsachart, P.; Dasri, T.; Chio-Srichan, S.; Soontaranon, S.; Wongsinlatam, W.; Sompech, S. Green synthesis of silver nanoparticles loaded into bacterial cellulose for antimicrobial application. Nanocomposites 2022, 8, 34–46. [Google Scholar] [CrossRef]
- Anwar, Y.; Ul-Islam, M.; Mohammed Ali, H.S.H.; Ullah, I.; Khalil, A.; Kamal, T. Silver impregnated bacterial cellulose-chitosan composite hydrogels for antibacterial and catalytic application. J. Mater. Res. Technol. 2022, 18, 2037–3047. [Google Scholar] [CrossRef]
- Rakhimova, B.; Kudaibergenov, K.; Sassykova, L.; Spanova, G.; Aknazarov, S.; Tulepov, M. Preparation of Composites of Antibacterial Materials Based on Bacterial Cellulose and Silver Nanoparticles for Wound Healing. Int. J. Nanosci. Nanotechnol. 2022, 18, 123–133. [Google Scholar]
- Müller, D.; Rambo, C.R.; Recouvreu, D.O.S.; Porto, L.M.; Barra, G.M.O. Chemical in Situ Polymerization of Polypyrrole on Bacterial Cellulose Nanofibers. Synth. Met. 2011, 161, 106–111. [Google Scholar] [CrossRef]
- Li, S.; Huang, D.; Yang, J.; Zhang, B.; Zhang, X.; Yang, G.; Wang, M.; Shen, Y. Freestanding Bacterial Cellulose–Polypyrrole Nanofibres Paper Electrodes for Advanced Energy Storage Devices. Nano Energy 2014, 9, 309–317. [Google Scholar] [CrossRef]
- Krathumkhet, N.; Imae, T.; Paradee, N. Electrically controlled transdermal ibuprofen delivery consisting of pectin-bacterial cellulose/polypyrrole hydrogel composites. Cellulose 2021, 28, 11451–11463. [Google Scholar] [CrossRef]
- Fraser, S.A.; Van Zyl, W.E. In situ polymerization and electrical conductivity of polypyrrole/cellulose nanocomposites using Schweizer’s reagent. RSC Adv. 2022, 12, 22031–22043. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, H.J.; Yang, H.S. Polymerization of Aniline on Bacterial Cellulose and Characterization Of Bacterial Cellulose/Polyaniline Nanocomposite Films. Curr. Appl. Phys. 2012, 12, 75–80. [Google Scholar] [CrossRef]
- Rana, A.K.; Scarpa, F.; Thakur, V.K. Cellulose/polyaniline hybrid nanocomposites: Design, fabrication, and emerging multidimensional applications. Ind. Crops Prod. 2022, 187, 115356. [Google Scholar] [CrossRef]
- Hosseini, H.; Mousavi, S.M.; Wurm, F.R.; Goodarzi, V. Display of hidden properties of flexible aerogel based on bacterial cellulose/polyaniline nanocomposites with helping of multiscale modeling. Eur. Plym. J. 2021, 146, 110251. [Google Scholar] [CrossRef]
- Guan, F.; Chen, S.; Sheng, N.; Chen, Y.; Yao, J.; Pei, Q.; Wang, H. Mechanically Robust Reduced Graphene Oxide/Bacterial Cellulose Film Obtained Via Biosynthesis for Flexible Supercapacitor. Chem. Eng. J. 2019, 360, 829–837. [Google Scholar] [CrossRef]
- Gabryś, T.; Fryczkowska, B.; Fabia, J.; Biniaś, D. Preparation of an Active Dressing by In Situ Biosynthesis of a Bacterial Cellulose–Graphene Oxide Composite. Polymers 2022, 14, 2864. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, Y.; Zheng, S.; Zhong, L.; Xue, J. Preparation and properties of conductive bacterial cellulose-based graphene oxide-silver nanoparticles antibacterial dressing. Carbohydr. Polym. 2021, 257, 117671. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.A.A.; Pedro, A.C.; Ribeiro, V.R.; Bortolini, D.G.; Ozaki, M.S.C.; Maciel, G.M.; Haminiuk, C.W.I. Bacterial cellulose: From production optimization to new applications. Int. J. Biol. Macromol. 2020, 164, 2598–2611. [Google Scholar] [CrossRef]
- Gregory, D.A.; Tripathi, L.; Fricker, A.T.R.; Asare, E.; Orlando, I.; Raghavendran, V.; Roy, I. Bacterial cellulose: A smart biomaterial with diverse applications. Mater. Sci. Eng. R Rep. 2021, 145, 1–27. [Google Scholar] [CrossRef]
- Usawattanakul, N.; Torgbo, S.; Sukyai, P.; Khantayanuwong, S.; Puangsin, B.; Srichola, P. Development of nanocomposite film comprising of Polyvinyl Alcohol (PVA) incorporated with bacterial cellulose nanocrystals and magnetite nanoparticles. Polymers 2021, 13, 1778. [Google Scholar] [CrossRef]
- Shu, Y.; Bai, Q.; Fu, G.; Xiong, Q.; Li, C.; Ding, H.; Uyama, H. Hierarchical Porous Carbons from Polysaccharides Carboxymethyl Cellulose, Bacterial Cellulose, and Citric Acid for Supercapacitor. Carbohydr. Polym. 2020, 227, 115346. [Google Scholar] [CrossRef]
- Bai, Q.; Shen, Y.; Asoh, T.A.; Li, C.; Dan, Y.; Uyama, H. Controlled preparation of interconnected 3D hierarchical porous carbons from bacterial cellulose-based composite monoliths for supercapacitors. Nanoscale. 2020, 28, 1–14. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, K.; Yao, X.; Jiang, J.; Wang, M.; Yuan, S. Ultrasound-assisted preparation of Fe(OH)3@bacterial cellulose aerogel for efficient removal of organic contamination in water. Appl. Surf. Sci. 2023, 607, 154959. [Google Scholar] [CrossRef]
- Choi, S.M.; Shin, E.J. The Nanofication and Functionalization of Bacterial Cellulose and Its Applications. Nanomaterials 2020, 10, 406. [Google Scholar] [CrossRef]
- Safae, M.; Taran, M. Preparation of Bacterial Cellulose Fungicide Nanocomposite Incorporated with MgO Nanoparticles. J. Polym. Environ. 2021, 30, 2066–2076. [Google Scholar] [CrossRef]
- Mirtalebi, S.S.; Almasi, H.; Khaledabad, M.A. Physical, morphological, antimicrobial and release properties of novel MgO-bacterial cellulose nanohybrids prepared by in-situ and ex-situ methods. Int. J. Biol. Macromol. 2019, 128, 848–857. [Google Scholar] [CrossRef]
- Coey, J.M. Magnetism and Magnetic Materials, 1st ed.; Cambridge University Press: New York, NY, USA, 2010; pp. 1–40. [Google Scholar]
- Shackelford, J.F. Introdução à Ciencia Dos Materiais, 6th ed.; Pearson Prentice Hall: São Paulo, Brazil, 2008; pp. 416–432. [Google Scholar]
- Spaldin, N.A. Magnetic Materials: Fundamentals and Applications, 2nd ed.; Cambridge University Press: Cambridge, UK, 2012; pp. 87–91. [Google Scholar]
- Callister, W.D.; Rethwisch, D.G. Ciência E Engenharia De Materiais: Uma Introdução, 9th ed.; LTV: Rio De Janeiro, Brazil, 2016; pp. 740–767. [Google Scholar]
- Sheng, P.; Wang, B.; Li, R. Flexible Magnetic Thin Films and Devices. J. Semicond. 2018, 39, 1–14. [Google Scholar] [CrossRef]
- Fatima, H.; Charinpanitkul, T.; Kim, K.S. Fundamentals to Apply Magnetic Nanoparticles for Hyperthermia Therapy. Nanomaterials 2021, 11, 1203. [Google Scholar] [CrossRef]
- Kianfar, E. Magnetic Nanoparticles in Targeted Drug Delivery: A Review. J. Supercond. Nov. Magn. 2021, 34, 1709–1735. [Google Scholar] [CrossRef]
- Salidkul, N.; Mongkolthanaruk, W.; Faungnawakij, K.; Pinitsoontorn, S. Hard magnetic membrane based on bacterial cellulose–barium ferrite nanocomposites. Carbohydr. Polym. 2021, 264, 118016. [Google Scholar] [CrossRef]
- Chanthiwong, M.; Mongkolthanaruk, W.; Eichhorn, S.J.; Pinitsoontorn, S. Controlling the processing of co-precipitated magnetic bacterial cellulose/iron oxide nanocomposites. Mater. Des. 2020, 196, 109148. [Google Scholar] [CrossRef]
- Houbi, A.; Aldashevich, Z.A.; Atassi, Y.; Bagasharova Telmanovna, Z.; Saule, M.; Kubanych, K. Microwave Absorbing Properties of Ferrites and Their Composites: A Review. J. Magn. Magn. Mater. 2021, 529, 167839. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Chircov, C.; Grumezescu, A.M.I. Magnetite Nanoparticles: Synthesis Methods–A Comparative Review. Methods 2021, 199, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Marins, J.A.; Soares, B.G.; Barud, H.S.; Ribeiro, S.J. L Flexible magnetic membranes based on bacterial cellulose and its evaluation as electromagnetic interference shielding material. Materi. Sci. Eng C 2013, 33, 3994–4001. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, S.; Wan, T.; Jia, Y.; Yang, H.; Li, J.; Yan, L.; Zhong, C. Biosynthesis of spherical Fe3O4/bacterial cellulose nanocomposites as adsorbents for heavy metal ions. Carbohydr. Polym. 2011, 86, 1558–1564. [Google Scholar] [CrossRef]
- Sriplai, N.; Mongkolthanaruk, W.; Eichhorn, S.J.; Pinitsoontorn, S. Magnetically responsive and flexible bacterial cellulose membranes. Carbohydr. Polym. 2018, 192, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, R.; Liu, S.; Li, Q.; Zhang, L.; Zhang, L.; Guan, J. Structure and magnetic properties of regenerated cellulose/Fe3O4 nanocomposite films. J. Appl. Polym. Sci. 2009, 111, 2477–2484. [Google Scholar] [CrossRef]
- Pavón, J.J.; Allain, J.P.; Verma, D.; Echeverry-Rendón, M.; Cooper, C.L.; Reece, L.M.; Shetty, A.R.; Tomar, V. In situ Study Unravels Bio-Nanomechanical Behavior in a Magnetic Bacterial Nano-cellulose (MBNC) Hydrogel for Neuro-Endovascular Reconstruction. Macromol. Biosci. 2019, 19, 1800225. [Google Scholar] [CrossRef]
- Chaabane, L.; Chahdoura, H.; Mehdaoui, R.; Snoussi, M.; Beyou, E.; Lahcini, M.; Baouab, M.H.V. Functionalization of developed bacterial cellulose with magnetite nanoparticles for nanobiotechnology and nanomedicine applications. Carbohydr. Polym. 2020, 247, 116707. [Google Scholar] [CrossRef] [PubMed]
- Yingkamhaeng, N.; Intapan, I.; Sukyai, P. Fabricação e caracterização de nanocelulose bacteriana superparamagnética funcionalizada usando síntese in situ assistida por ultra-som. Fibers Polym. 2018, 19, 489–497. [Google Scholar] [CrossRef]
- Mira-Cuenca, C.; Meslier, T.; Roig-Sanchez, S.; Laromaine, A.; Roig, A. Patterning Bacterial Cellulose Films with Iron Oxide Nanoparticles and Magnetic Resonance Imaging Monitoring. ACS Appl. Polym. Mater. 2021, 3, 4959–4965. [Google Scholar] [CrossRef]
- Qiao, W.; Zhang, Z.; Qian, Y.; Xu, L.; Guo, H. Bacterial laccase immobilized on a magnetic dialdehyde cellulose without cross-linking agents for decolorization. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127818. [Google Scholar] [CrossRef]
- da Rosa Salles, T.; da Silva Bruckamann, F.; Viana, A.R.; Krause, L.M.F.; Mortari, S.R.; Rhoden, C.R.B. Magnetic nanocrystalline cellulose: Azithromycin adsorption and in vitro biological activity against melanoma cells. J. Polym. Environ. 2022, 30, 2695–2713. [Google Scholar] [CrossRef]
- Olsson, R.T.; Azizi, M.A.S.; Salazar-Alvarez, G.; Belova, L.; Ström, V.; Berglund, L.A.; Ikkala, O.; Nogués, J.; Gedde, U.W. Making flexible magnetic aerogels and stiff magnetic nanopaper using cellulose nanofibrils as templates. Nat. Nanotechnol. 2010, 5, 584–588. [Google Scholar] [CrossRef]
- Zeng, M.; Laromaine, A.; Feng, W.; Levkin, P.A.; Roig, A. Origami magnetic cellulose: Controlled magnetic fraction and patterning of flexible bacterial cellulose. J. Mater. Chem. C 2014, 2, 6312–6318. [Google Scholar] [CrossRef]
- Sriplai, N.; Mangayil, R.; Pammo, A.; Santala, V.; Tuukkanen, S.; Pinitsoontorn, S. Enhancing piezoelectric properties of bacterial cellulose films by incorporation of MnFe2O4 nanoparticles. Carbohydr. Polym. 2020, 231, 1–21. [Google Scholar] [CrossRef]
- Vitta, S.; Drillon, M.; Derory, A. Magnetically responsive bacterial cellulose: Synthesis and magnetic studies. J. Appl. Phys. 2010, 108, 053905. [Google Scholar] [CrossRef]
- Silva, M.F.; Pineda, E.A.G.; Bergamasco, R. Aplicação de óxidos de ferro nanoestruturados como adsorventes e fotocatalisadores na remoção de poluentes de águas residuais. Quím. Nova 2015, 38, 393–398. [Google Scholar] [CrossRef]
- Shokrollahi, H. A Review of The Magnetic Properties, Synthesis Methods and Applications of Maghemite. J. Magn. Magn. Mater. 2017, 426, 74–81. [Google Scholar] [CrossRef]
- Vaewbundit, S.; Siriphannon, P. Soft solution growth of magnetite-maghemite nanocrystals in crosslinked chitosan templates and their superparamagnetic properties. Nanocomposites 2022, 8, 142–154. [Google Scholar] [CrossRef]
- Fontanive, V.C.P.; Khalil, N.M.; Cotica, L.F.; Mainardes, R.M. Aspectos Físicos e Biológicos de Nanopartículas de Ferritas Magnéticas. Rev. Ciênc. Farm. Básica E Apl. 2014, 35, 549–558. [Google Scholar]
- Sagayaraj, R.; Aravazhi, S.; Chandrasekaran, G. Review on Structural and Magnetic Properties of (Co–Zn) Ferrite Nanoparticles. Int. Nano Lett. 2021, 11, 307–319. [Google Scholar] [CrossRef]
- Amiri, M.; Salavati-Niasari, M.; Akbari, A. Magnetic Nanocarriers: Evolution of Spinel Ferrites for Medical Applications. Adv. Colloid Interface Sci. 2019, 265, 29–44. [Google Scholar] [CrossRef]
- Narang, S.B.; Pubby, K. Nickel Spinel Ferrites: A Review. J. Magn. Magn. Mater. 2021, 519, 1–115. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. Manganese Ferrite (Mnfe2O4) Nanoparticles: From Synthesis to Application-A Review. J. Ind. Eng. Chem. 2021, 103, 292–304. [Google Scholar] [CrossRef]
- Nypelö, T. Magnetic Cellulose: Does Extending Cellulose Versatility with Magnetic Functionality Facilitate Its Use In Devices? J. Mater. Chem. C 2022, 10, 805–818. [Google Scholar] [CrossRef]
- Andriani, D.; Apriyana, A.Y.; Karina, M. The optimization of bacterial cellulose production and its applications: A review. Cellulose 2020, 27, 6747–6766. [Google Scholar] [CrossRef]
- Moscovici, M. Present and Future Medical Applications of Microbial Exopolysaccharides. Front Microbiol. 2015, 6, 1012. [Google Scholar] [CrossRef] [Green Version]
- Aditya, T.; Allain, J.P.; Jaramillo, C.; Restrepo, A.M. Surface modification of bacterial cellulose for biomedical applications. Int. J. Mol. Sci. 2022, 23, 610. [Google Scholar] [CrossRef]
- Guan, F.; Guo, C.F. Flexible, high-strength, and porous nano-nano composites based on bacterial cellulose for wearable electronics: A review. Soft Sci. 2021, 1, 1–22. [Google Scholar] [CrossRef]
- Choi, S.M.; Rao, K.M.; Zo, S.M.; Shin, E.J.; Han, S.S. Bacterial Cellulose and Its Applications. Polymers 2022, 14, 1080. [Google Scholar] [CrossRef]
- Nakajima, T.; Fujio, Y.; Sugahara, T.; Tsuchiya, T. Flexible Ceramic Film Sensors for Free-Form Devices. Sensors 2022, 22, 1996. [Google Scholar] [CrossRef]
- Galland, S.; Andersson, R.L.; Salajková, M.; Ström, V.; Olsson, R.T.; Berglund, L.A. Cellulose Nanofibers Decorated with Magnetic Nanoparticles–Synthesis, Structure and use in Magnetized High Toughness Membranes for a Prototype Loudspeaker. J. Mater. Chem. C 2013, 1, 7963–7972. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Y.; Song, W.; Luan, J.; Wen, X.; Wu, Z.; Chen, X.; Wang, Q.; Guo, S. In Situ Synthesis of Silver-Nanoparticles/Bacterial Cellulose Composites for Slow-Released Antimicrobial Wound Dressing. Carbohydr. Polym. 2014, 102, 762–771. [Google Scholar] [CrossRef]
- Tarrés, Q.; Deltell, A.; Espinach, F.X.; Pèlach, M.À.; Delgado-Aguilar, M.; Mutjé, P. Magnetic Bionanocomposites from Cellulose Nanofibers: Fast, Simple and Effective Production Method. Int. J. Biol. Macromol. 2017, 99, 29–36. [Google Scholar] [CrossRef]

| Properties | Bacterial Cellulose | Vegetable Cellulose | Reference |
|---|---|---|---|
| Purity (%) | >99 | <80 | [9,24] |
| Crystallinity degree (%) | 60–90 | 40–78 | |
| Fibre diameter size scale | Nanometric | Micrometric | [25,26] |
| Young’s modulus (GPa) | 15–30 | 1.5–203 | [6,27] |
| Magnetic saturation (emu/g) | 0 | 0 | [28,29] |
| Coercivity (kOe) | 0 | 0 |
| Dopant | Added Properties | References |
|---|---|---|
| Silver nanoparticles | Antimicrobial | [50,51,52,53,54] |
| Polypyrrole | Electrical conductivity | [13,55,56,57,58] |
| Polyaniline | Electrical conductivity | [59,60,61] |
| Graphene/Graphene oxide | Mechanical Strength and electrical conductivity | [62,63,64] |
| Magnetic Dopants | Forms of BC Magnetic Doping | Saturation Magnetization of Magnetic BC (emu/g) * | Coercive Field of Magnetic BC (Oe) * | Applications | References |
|---|---|---|---|---|---|
| Magnetite | In situ co-precipitation | 60.0 | 15 | Application in nonlinear optics, clinical applications such as contrast, agents for magnetic resonance, hyperthermia and cell separation and sensors. | [85] |
| Incorporation of previously made particles | 41.0 | 27 | Absorption of heavy metals | [86] | |
| Incorporation of previously made particles | 53.6 | ** | Actuators, sensors, flexible data storage | [87] | |
| In situ electrolysis | 4.2–21.2 | ** | Electronic and magnetic devices, enzymatic assays, drug delivery systems | [88] | |
| In situ co-precipitation | *** | *** | Tissue reconstruction | [89] | |
| In situ co-precipitation | 23.63 | 0.042 | Drug delivery | [90] | |
| In situ co-precipitation | 5.14–11.56 | ** | Electronic devices | [67] | |
| In situ co-precipitation | 40.57 | ** | Electronic devices | [91] | |
| Thermal decomposition | *** | *** | Magnetic resonance device | [92] | |
| In situ co-precipitation | 34.07 | ** | Device for enzyme immobilization | [93] | |
| Incorporation of previously made particles | *** | *** | Drug delivery | [94] | |
| Incorporation of previously made particles | 0.14 | ** | Optical materials | [29] | |
| Cobalt ferrite | In situ co-precipitation | 3.769–5.026 | 5000 | Electric actuators | [95] |
| Sensors | [16] | ||||
| Maghemite | Thermal decomposition | 60 | ** | Sound amplifier devices | [96] |
| Barium ferrite | Incorporation of previously made particles | 24.1–49.5 | 5.31 | Data storage devices, electromagnetic adsorbers | [81] |
| Magnetite and maghemite | In situ co-precipitation | 55.0–61.0 | ** | Sensors, actuators, and metal adsorbents | [82] |
| Manganese ferrite | In situ co-precipitation | *** | *** | Sensors | [97] |
| Nickel nanoparticles | In situ co-precipitation | 2.8 | 28 | Magnetic ink and magnetic scaffolds for tissue engineering | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, T.C.; Amorim, J.D.P.d.; Silva Junior, C.J.G.d.; de Medeiros, A.D.M.; Santana Costa, A.F.d.; Vinhas, G.M.; Sarubbo, L.A. Magnetic Bacterial Cellulose Biopolymers: Production and Potential Applications in the Electronics Sector. Polymers 2023, 15, 853. https://doi.org/10.3390/polym15040853
de Souza TC, Amorim JDPd, Silva Junior CJGd, de Medeiros ADM, Santana Costa AFd, Vinhas GM, Sarubbo LA. Magnetic Bacterial Cellulose Biopolymers: Production and Potential Applications in the Electronics Sector. Polymers. 2023; 15(4):853. https://doi.org/10.3390/polym15040853
Chicago/Turabian Stylede Souza, Thaís Cavalcante, Julia Didier Pedrosa de Amorim, Claudio José Galdino da Silva Junior, Alexandre D’Lamare Maia de Medeiros, Andréa Fernanda de Santana Costa, Gloria Maria Vinhas, and Leonie Asfora Sarubbo. 2023. "Magnetic Bacterial Cellulose Biopolymers: Production and Potential Applications in the Electronics Sector" Polymers 15, no. 4: 853. https://doi.org/10.3390/polym15040853
APA Stylede Souza, T. C., Amorim, J. D. P. d., Silva Junior, C. J. G. d., de Medeiros, A. D. M., Santana Costa, A. F. d., Vinhas, G. M., & Sarubbo, L. A. (2023). Magnetic Bacterial Cellulose Biopolymers: Production and Potential Applications in the Electronics Sector. Polymers, 15(4), 853. https://doi.org/10.3390/polym15040853