Structural and Optical Characterization of g-C3N4 Nanosheet Integrated PVC/PVP Polymer Nanocomposites
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safarpour, M.; Safikhani, A.; Vatanpour, V. Polyvinyl chloride-based membranes: A review on fabrication techniques, applications and future perspectives. Sep. Purif. Technol. 2021, 279, 119678. [Google Scholar] [CrossRef]
- Aghamohammadi, H.; Amousa, N.; Eslami-Farsani, R. Recent advances in developing the MXene/polymer nanocomposites with multiple properties: A review study. Synth. Met. 2021, 273, 116695. [Google Scholar] [CrossRef]
- Kouser, S.; Prabhu, A.; Prashantha, K.; Nagaraja, G.; D’Souza, J.N.; Navada, K.M.; Qurashi, A.; Manasa, D. Modified halloysite nanotubes with Chitosan incorporated PVA/PVP bionanocomposite films: Thermal, mechanical properties and biocompatibility for tissue engineering. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127941. [Google Scholar] [CrossRef]
- Sudharsan, J.; Khare, S.K. Role of nanocomposite additives in well bore stability during shale formation drilling with water based mud–A comprehensive review. Mater. Today Proc. 2022, 62, 6412–6419. [Google Scholar] [CrossRef]
- Raj, D.R.; Prasanth, S.; Vineeshkumar, T.V.; Sudarsanakumar, C. Ammonia sensing properties of tapered plastic optical fiber coated with silver nanoparticles/PVP/PVA hybrid. Opt. Commun. 2015, 340, 86–92. [Google Scholar]
- Najafi-Ashtiani, H. Low temperature processing of BaTiO3-PMMA-PVP hybrid films as transparent dielectric gate. J. Mater. Sci. Mater. Electron. 2019, 30, 7087–7094. [Google Scholar] [CrossRef]
- Munj, H.R.; Nelson, M.T.; Karandikar, P.S.; Lannutti, J.J.; Tomasko, D.L. Biocompatible electrospun polymer blends for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Bhavsa, V.; Tripathi, D. Structural, optical, and aging studies of biocompatible PVC-PVP blend films. J. Polym. Eng. 2018, 38, 419–426. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Feng, Y.; Yin, J. Recent progress in polymer/two-dimensional nanosheets composites with novel performances. Prog. Polym. Sci. 2022, 126, 101505. [Google Scholar]
- Al-Muntaser, A.A.; Pashameah, R.A.; Sharma, K.; Alzahrani, E.; Hameed, S.T.; Morsi, M.A. Boosting of structural, optical, and dielectric properties of PVA/CMC polymer blend using SrTiO3 perovskite nanoparticles for advanced optoelectronic applications. Opt. Mater. 2022, 132, 112799. [Google Scholar] [CrossRef]
- Alshammari, A.H.; Alshammari, M.; Alshammari, K.; Allam, N.K.; Taha, T. PVC/PVP/SrTiO3 polymer blend nanocomposites as potential materials for optoelectronic applications. Results Phys. 2022, 44, 106173. [Google Scholar] [CrossRef]
- Taha, T.A.; Mahmoud, M.H.; Hayat, A. Dielectric relaxation studies on PVC-Pb3O4 polymer nanocomposites. J. Mater. Sci. Mater. Electron. 2021, 32, 27666–27675. [Google Scholar] [CrossRef]
- Alshammari, A.H.; Taha, T.A. Structure, thermal and dielectric insights of PVC/PVP/ZnFe2O4 polymer nanocomposites. Eur. Phys. J. Plus 2021, 136, 1201. [Google Scholar] [CrossRef]
- Vaya, D.; Kaushik, B.; Surolia, P.K. Recent advances in graphitic carbon nitride semiconductor: Structure, synthesis and applications. Mater. Sci. Semicond. Process. 2022, 137, 106181. [Google Scholar]
- Liu, X.; Zhao, C.; Muhmood, T.; Yang, X. Regulating the Assembly of Precursors of Carbon Nitrides to Improve Photocatalytic Hydrogen Production. Catalysts 2022, 12, 1634. [Google Scholar] [CrossRef]
- Muhmood, T.; Uddin, A. Fabrication of spherical-graphitic carbon nitride via hydrothermal method for enhanced photo-degradation ability towards antibiotic. Chem. Phys. Lett. 2020, 753, 137604. [Google Scholar] [CrossRef]
- Muhmood, T.; Cai, Z.; Lin, S.; Xiao, J.; Hu, X.; Ahmad, F. Graphene/graphitic carbon nitride decorated with AgBr to boost photoelectrochemical performance with enhanced catalytic ability. Nanotechnology 2020, 31, 505602. [Google Scholar] [CrossRef] [PubMed]
- Hoh, H.Y.; Zhang, Y.; Zhong, Y.L.; Bao, Q. Harnessing the Potential of Graphitic Carbon Nitride for Optoelectronic Applications. Adv. Opt. Mater. 2021, 9, 2100146. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, Y.; Elnaggar, A.Y.; El Azab, I.H.; Huang, M.; Mahmoud, M.H.H.; El-Bahy, S.M.; Guo, M. Physical vapor deposition of graphitic carbon nitride (g-C3N4) films on biomass substrate: Optoelectronic performance evaluation and life cycle assessment. Adv. Compos. Hybrid Mater. 2022, 5, 813–822. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, K.; Jiao, E.; Chen, B.; Shi, J.; Lu, M. Greatly improved thermal conductivity and electrical insulation for PVA composites via introducing functional carbon nitride nanosheets. Eur. Polym. J. 2021, 159, 110718. [Google Scholar] [CrossRef]
- Shikinaka, K.; Aizawa, K.; Fujii, N.; Osada, Y.; Tokita, M.; Watanabe, J.; Shigehara, K. Flexible, transparent nanocomposite film with a large clay component and ordered structure obtained by a simple solution-casting method. Langmuir 2010, 26, 12493–12495. [Google Scholar] [CrossRef]
- Ahmed, F.; Kumar, S.; Arshi, N.; Anwar, M.S.; Su-Yeon, L.; Kil, G.S.; Park, D.W.; Koo, B.H.; Lee, C.G. Preparation and characterizations of polyaniline (PANI)/ZnO nanocomposites film using solution casting method. Thin Solid Film. 2011, 519, 8375–8378. [Google Scholar] [CrossRef]
- Bidadi, H.; Olad, A.; Parhizkar, M.; Aref, S.M.; Ghafouri, M. Nonlinear properties of ZnO-polymer composites prepared by solution-casting method. Vacuum 2013, 87, 50–54. [Google Scholar] [CrossRef]
- Hayat, A.; Taha, T.A.; Alenad, A.M.; Ullah, I.; Shah, S.J.A.; Uddin, I.; Ullah, I.; Hayat, A.; Khan, W.U. A simplistic molecular agglomeration of carbon nitride for optimized photocatalytic performance. Surf. Interfaces 2021, 25, 101166. [Google Scholar] [CrossRef]
- Mahmood, A.; Muhmood, T.; Ahmad, F. Carbon nanotubes heterojunction with graphene like carbon nitride for the enhancement of electrochemical and photocatalytic activity. Mater. Chem. Phys. 2022, 278, 125640. [Google Scholar] [CrossRef]
- Muhmood, T.; Xia, M.; Lei, W.; Wang, F. Erection of duct-like graphitic carbon nitride with enhanced photocatalytic activity for ACB photodegradation. J. Phys. D Appl. Phys. 2018, 51, 065501. [Google Scholar] [CrossRef]
- Lu, X.; Xu, K.; Chen, P.; Jia, K.; Liu, S.; Wu, C. Facile one step method realizing scalable production of g-C3N4 nanosheets and study of their photocatalytic H 2 evolution activity. J. Mater. Chem. A 2014, 2, 18924–18928. [Google Scholar] [CrossRef]
- French, A.D.; Santiago Cintrón, M. Cellulose polymorphy, crystallite size, and the Segal Crystallinity Index. Cellulose 2013, 20, 583–588. [Google Scholar] [CrossRef]
- Taha, T.A.; Saad, R.; Zayed, M.; Shaban, M.; Ahmed, A.M. Tuning the surface morphologies of ZnO nanofilms for enhanced sensitivity and selectivity of CO2 gas sensor. Appl. Phys. A 2023, 129, 115. [Google Scholar] [CrossRef]
- Abbasian, A.R.; Shafiee Afarani, M. One-step solution combustion synthesis and characterization of ZnFe2O4 and ZnFe1.6O4 nanoparticles. Appl. Phys. A 2019, 125, 721. [Google Scholar] [CrossRef]
- Bhran, A.; Shoaib, A.; Elsadeq, D.; El-gendi, A.; Abdallah, H. Preparation of PVC/PVP composite polymer membranes via phase inversion process for water treatment purposes. Chin. J. Chem. Eng. 2018, 26, 715–722. [Google Scholar] [CrossRef]
- Rani, P.; Ahamed, M.B.; Deshmukh, K. Significantly enhanced electromagnetic interference shielding effectiveness of montmorillonite nanoclayand copper oxide nanoparticles basedpolyvinylchloride nanocomposites. Polym. Test. 2020, 91, 106744. [Google Scholar] [CrossRef]
- Taha, T.A.; Alzara, M.A.A. Synthesis, thermal and dielectric performance of PVA-SrTiO3 polymer nanocomposites. J. Mol. Struct. 2021, 1238, 130401. [Google Scholar] [CrossRef]
- Zidan, H.M.; Abdelrazek, E.M.; Abdelghany, A.M.; Tarabiah, A.E. Characterization and some physical studies of PVA/PVP filled with MWCNTs. J. Mater. Res. Technol. 2019, 8, 904–913. [Google Scholar] [CrossRef]
- Darkwah, W.K.; Ao, Y. Mini review on the structure and properties (photocatalysis), and preparation techniques of graphitic carbon nitride nano-based particle, and its applications. Nanoscale Res. Lett. 2018, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Solodovnichenko, V.S.; Polyboyarov, V.A.; Zhdanok, A.A.; Arbuzov, A.B.; Zapevalova, E.S.; Kryazhev, Y.G.; Likholobov, V.A. Synthesis of carbon materials by the short-term mechanochemical activation of polyvinyl chloride. Procedia Eng. 2016, 152, 747–752. [Google Scholar] [CrossRef]
- Smith, M.W.; Dallmeyer, I.; Johnson, T.J.; Brauer, C.S.; McEwen, J.-S.; Espinal, J.F.; Garcia-Perez, M. Structural analysis of char by Raman spectroscopy: Improving band assignments through computational calculations from first principles. Carbon 2016, 100, 678–692. [Google Scholar] [CrossRef]
- Dong, J.; Fredericks, P.M.; George, G.A. Studies of the structure and thermal degradation of poly (vinyl chloride)—Poly (N-vinyl-2-pyrrolidone) blends by using Raman and FTIR emission spectroscopy. Polym. Degrad. Stab. 1997, 58, 159–169. [Google Scholar] [CrossRef]
- Moustapha, M.E.; Friedrich, J.F.; Farag, Z.R.; Krüger, S.; Geesi, M. Potentiometric studies on the influence of poly (N-vinylpyrrolidone) on the thermal degradation behavior of poly (vinyl chloride) blends. Mater. Test. 2019, 61, 179–184. [Google Scholar] [CrossRef]
- Haque, M.A.; Paliwal, L.J. Synthesis, spectral characterization and thermal aspects of coordination polymers of some transition metal ions with adipoyl bis (isonicotinoylhydrazone). J. Mol. Struct. 2017, 1134, 278–291. [Google Scholar] [CrossRef]
- Baraker, B.M.; Lobo, B. Spectroscopic analysis of CdCl2 doped PVA–PVP blend films. Can. J. Phys. 2017, 95, 738–747. [Google Scholar] [CrossRef]
- Zirzlmeier, J.; Schrettl, S.; Brauer, J.C.; Contal, E.; Vannay, L.; Brémond, É.; Jahnke, E.; Guldi, D.M.; Corminboeuf, C.; Tykwinski, R.R.; et al. Optical gap and fundamental gap of oligoynes and carbyne. Nat. Commun. 2020, 11, 4797. [Google Scholar] [CrossRef] [PubMed]
- Horti, N.C.; Kamatagi, M.D.; Patil, N.R.; Nataraj, S.K.; Sannaikar, M.S.; Inamdar, S.R. Synthesis and photoluminescence properties of titanium oxide (TiO2) nanoparticles: Effect of calcination temperature. Optik 2019, 194, 163070. [Google Scholar] [CrossRef]
- Qasem, A.; Mostafa, M.S.; Yakout, H.; Mahmoud, M.; Shaaban, E.R. Determination of optical bandgap energy and optical characteristics of Cd30Se50S20 thin film at various thicknesses. Opt. Laser Technol. 2022, 148, 107770. [Google Scholar] [CrossRef]
- El-naggar, A.M.; Heiba, Z.K.; Mohamed, M.B.; Kamal, A.M.; Osman, M.M.; Albassam, A.A.; Lakshminarayana, G. Improvement of the optical characteristics of PVA/PVP blend with different concentrations of SnS2/Fe. J. Vinyl Addit. Technol. 2022, 28, 82–93. [Google Scholar] [CrossRef]
- Taha, T.A.; Hendawy, N.; El-Rabaie, S.; Esmat, A.; El-Mansy, M.K. Effect of NiO NPs doping on the structure and optical properties of PVC polymer films. Polym. Bull. 2019, 76, 4769–4784. [Google Scholar] [CrossRef]
- Aziz, S.B.; Rasheed, M.A.; Hussein, A.M.; Ahmed, H.M. Fabrication of polymer blend composites based on [PVA-PVP](1 − x):(Ag2S) x (0.01≤ x ≤ 0.03) with small optical band gaps: Structural and optical properties. Mater. Sci. Semicond. Process. 2017, 71, 197–203. [Google Scholar] [CrossRef]
- Dhatarwal, P.; Sengwa, R.J. Investigation on the optical properties of (PVP/PVA)/Al2O3 nanocomposite films for green disposable optoelectronics. Phys. B Condens. Matter 2021, 613, 412989. [Google Scholar] [CrossRef]
- Sakr, G.B.; Yahia, I.S.; Fadel, M.; Fouad, S.S.; Romčević, N. Optical spectroscopy, optical conductivity, dielectric properties and new methods for determining the gap states of CuSe thin films. J. Alloys Compd. 2010, 507, 557–562. [Google Scholar] [CrossRef]
- Aly, K.A. Optical band gap and refractive index dispersion parameters of As x Se70Te30− x (0≤ x ≤ 30 at.%) amorphous films. Appl. Phys. A 2010, 99, 913–919. [Google Scholar] [CrossRef]
- Taha, T.A. Optical properties of PVC/Al2O3 nanocomposite films. Polym. Bull. 2019, 76, 903–918. [Google Scholar] [CrossRef]
- Mondal, R.; Biswas, D.; Paul, S.; Das, A.S.; Chakrabarti, C.; Roy, D.; Bhattacharya, S.; Kabi, S. Investigation of microstructural, optical, physical properties and dielectric relaxation process of sulphur incorporated selenium–tellurium ternary glassy systems. Mater. Chem. Phys. 2021, 257, 123793. [Google Scholar] [CrossRef]
- Soliman, T.S.; Vshivkov, S.A.; Elkalashy, S.I. Structural, linear and nonlinear optical properties of Ni nanoparticles–Polyvinyl alcohol nanocomposite films for optoelectronic applications. Opt. Mater. 2020, 107, 110037. [Google Scholar] [CrossRef]
- Emshary, C.A.; Ali, I.M.; Hassan, Q.M.; Sultan, H.A. Linear and nonlinear optical properties of potassium dichromate in solution and solid polymer film. Phys. B Condens. Matter 2021, 613, 413014. [Google Scholar] [CrossRef]
- Ali, H.E.; Khairy, Y. Synthesis, characterization, refractive index-bandgap relations, and optical nonlinearity parameters of CuI/PVOH nanocomposites. Opt. Laser Technol. 2021, 136, 106736. [Google Scholar]
- Alrowaili, Z.A.; Taha, T.A.; El-Nasser, K.S.; Donya, H. Significant enhanced optical parameters of PVA-Y2O3 polymer nanocomposite films. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3101–3110. [Google Scholar] [CrossRef]
- Krishnaswamy, S.; Panigrahi, P.; Kala, P.P.; Sofini, S.; Nagarajan, G.S. Smart apparel using nano graphitic carbon nitride/PVA in a cotton cloth for military application. Heliyon 2022, 8, e10345. [Google Scholar] [CrossRef] [PubMed]
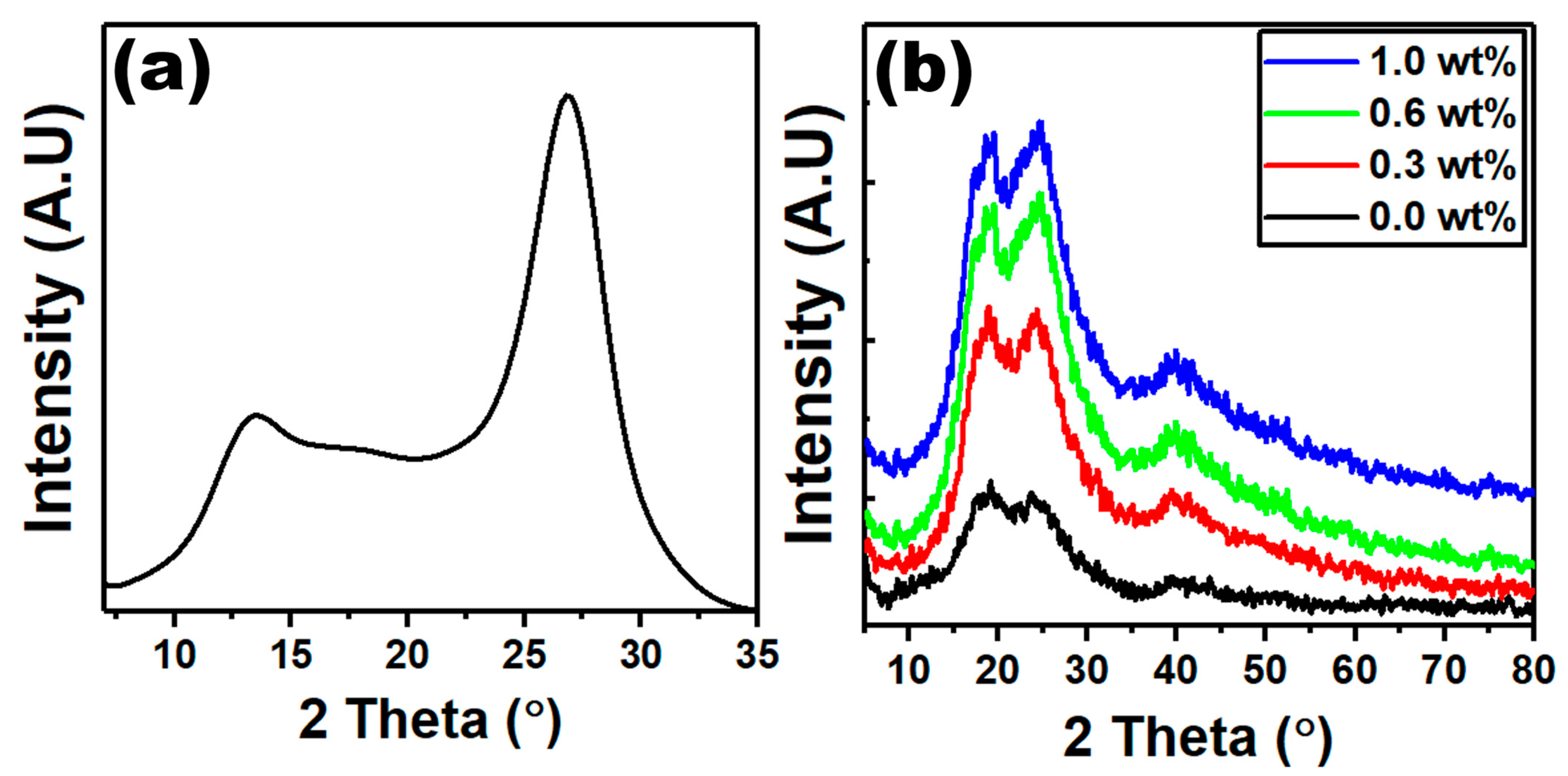
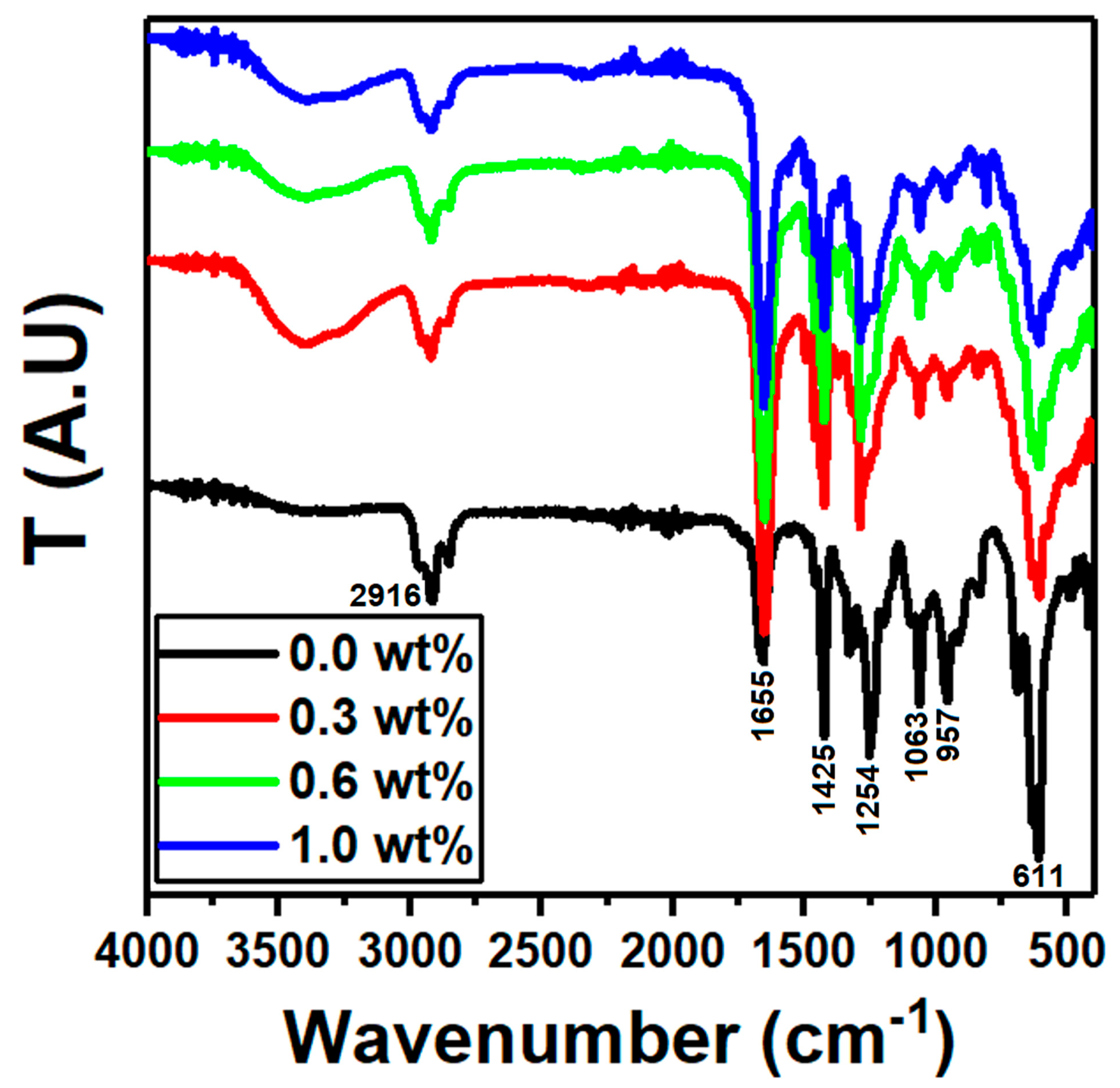
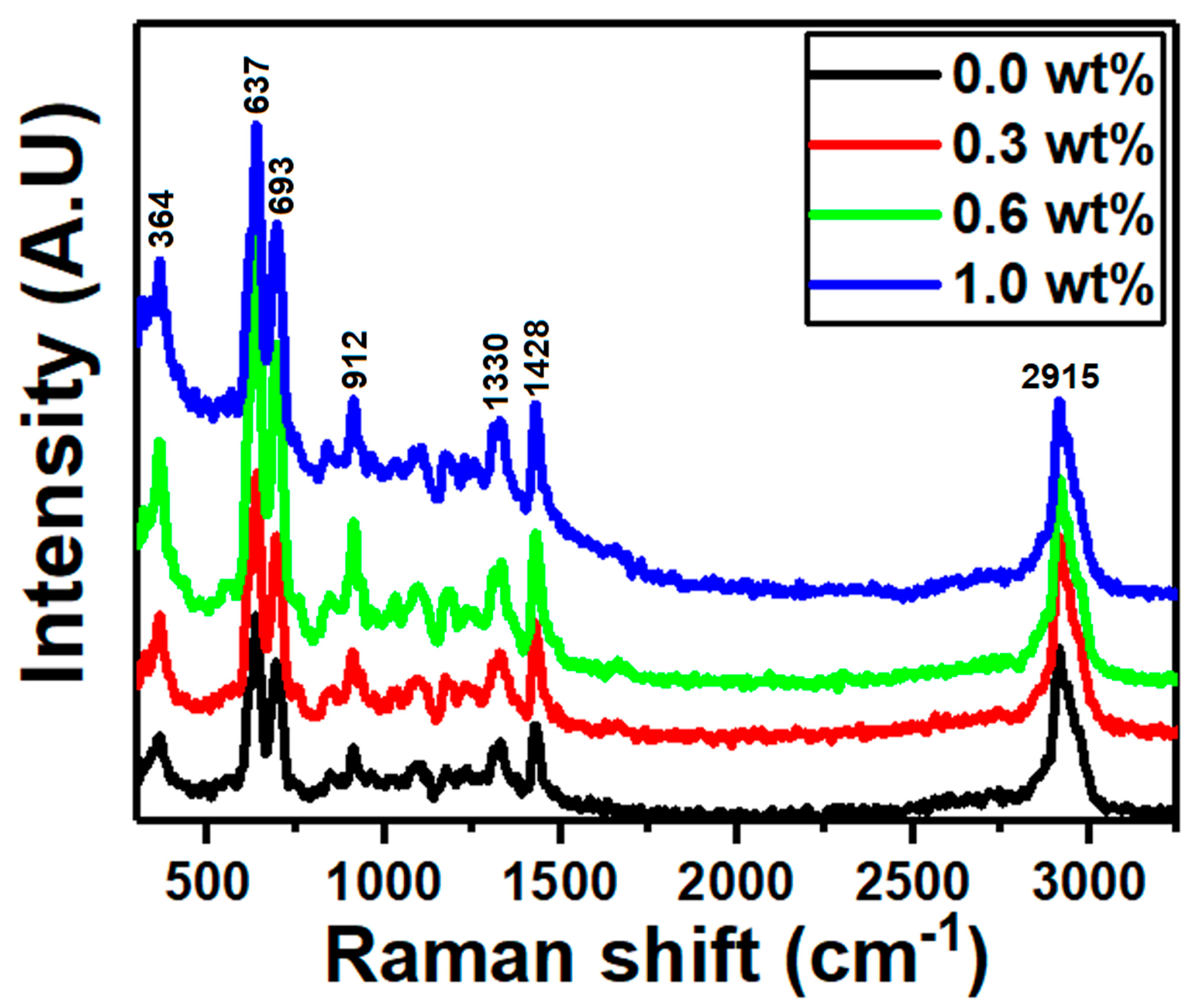
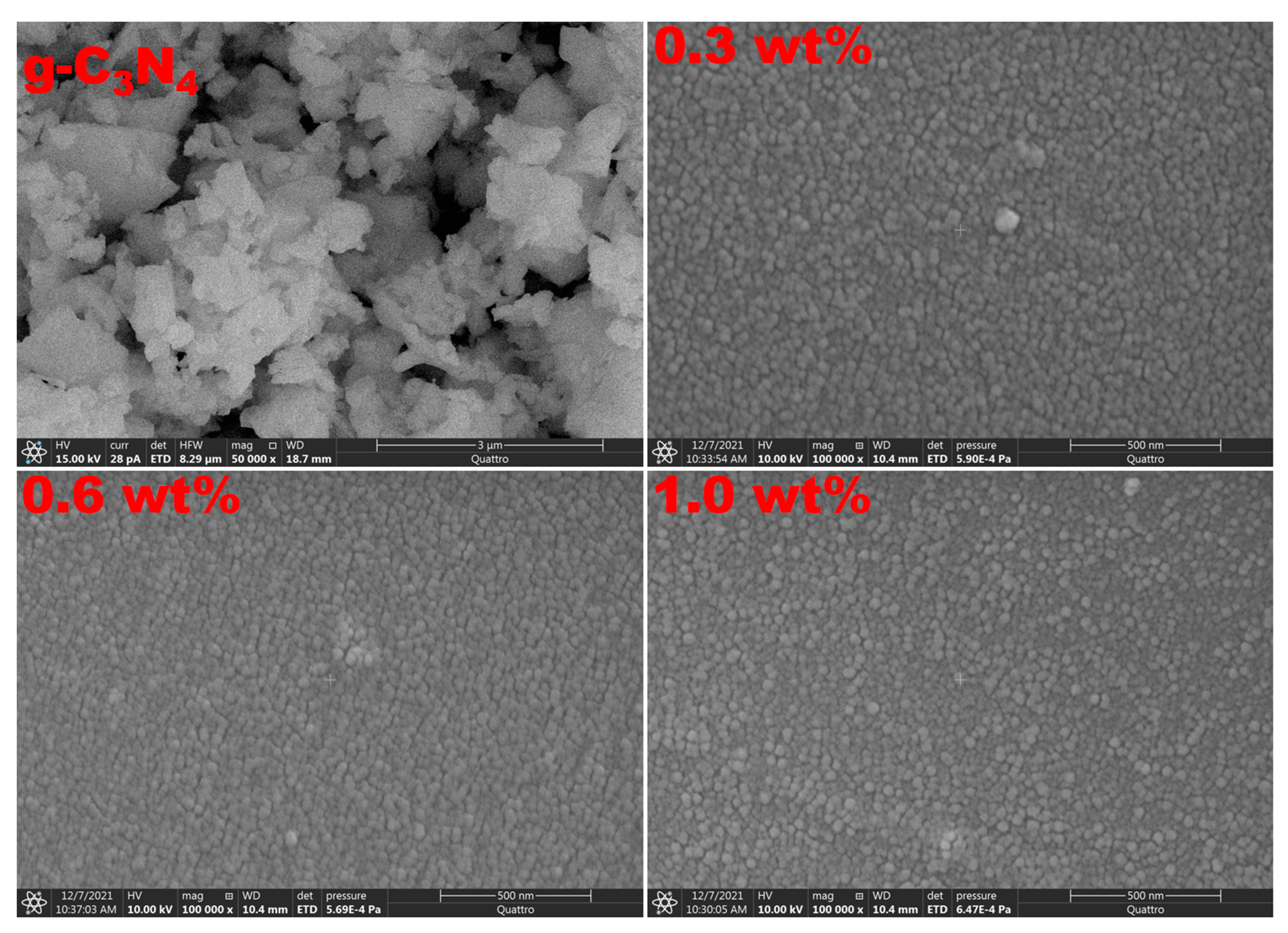
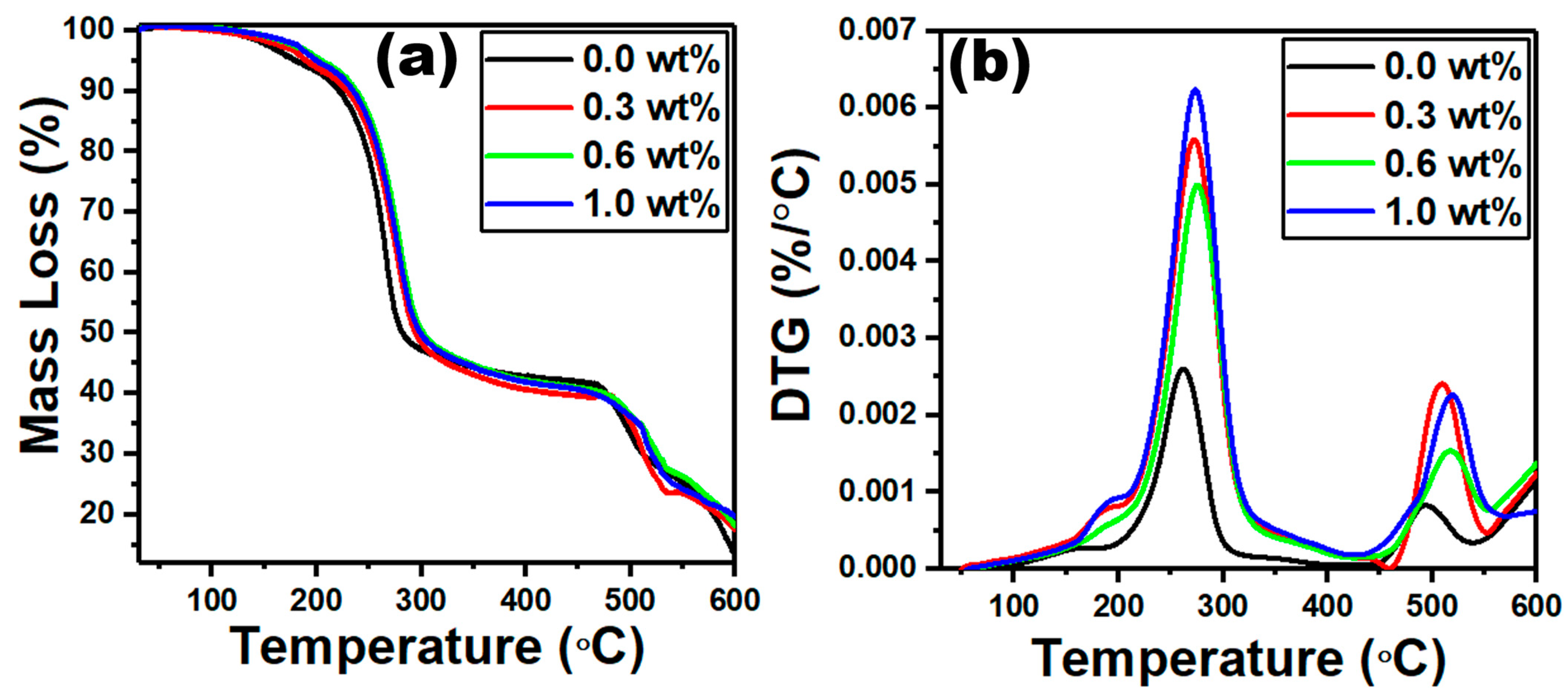


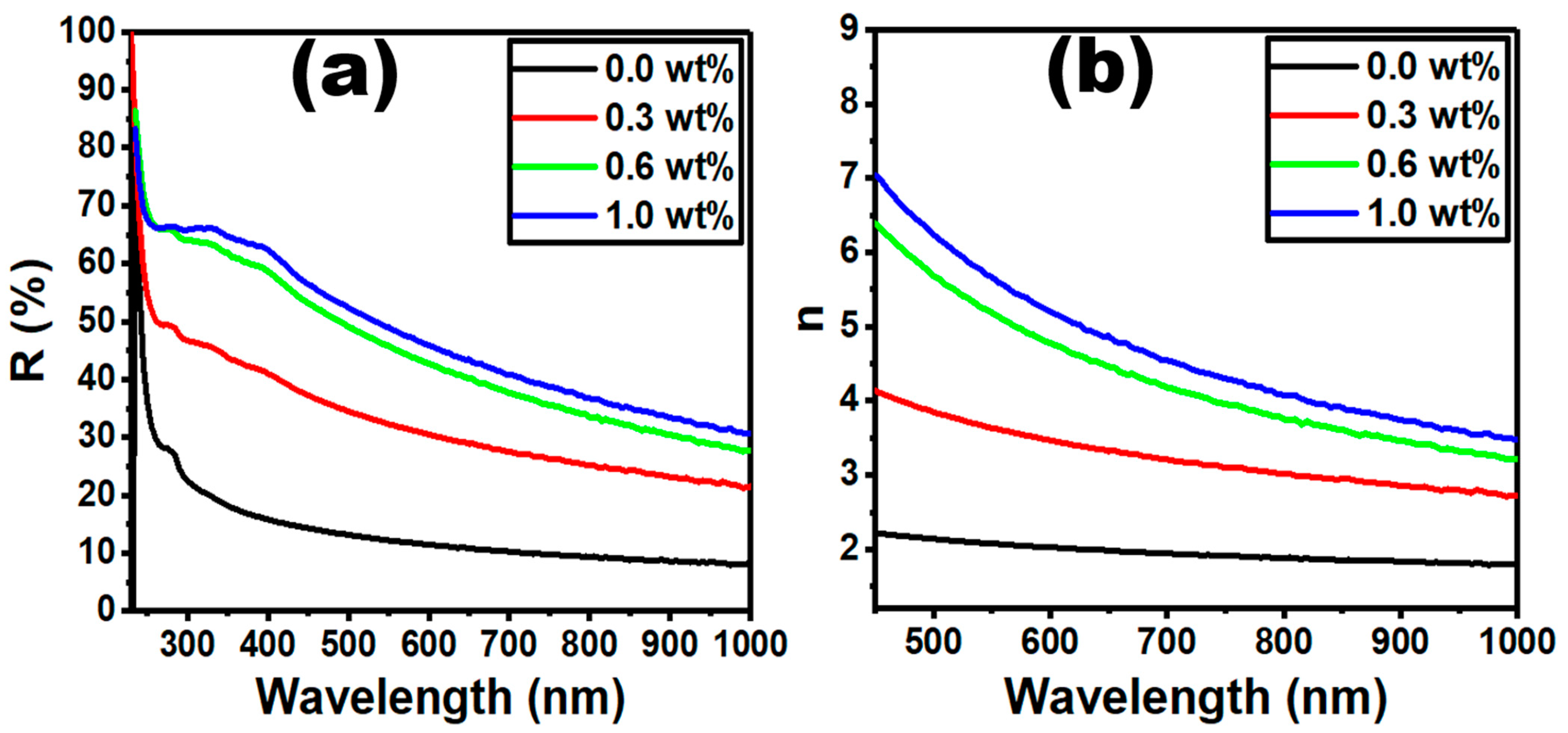
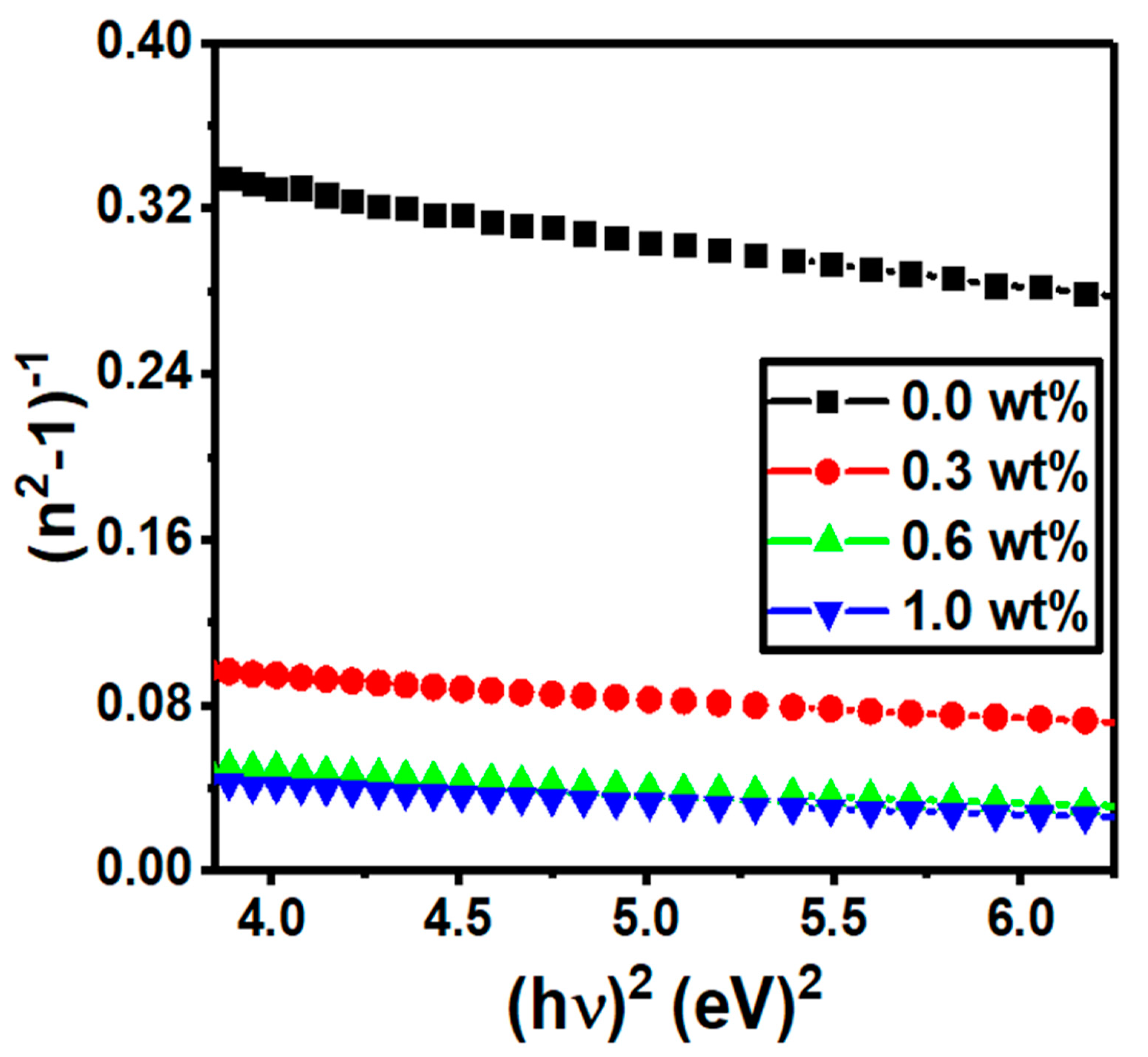

| g-C3N4 Content (wt%) | Edir (eV) | Eind (eV) | E0 (eV) | Ed (eV) | n0 | f (eV2) |
|---|---|---|---|---|---|---|
| 0.0 | 5.29 | 5.27 | 4.21 | 9.88 | 1.83 | 41.56 |
| 0.3 | 5.25 | 5.23 | 3.62 | 26.79 | 2.90 | 96.90 |
| 0.6 | 5.25 | 5.23 | 3.16 | 39.41 | 3.67 | 124.69 |
| 1 | 5.37 | 5.34 | 3.14 | 46.08 | 3.96 | 144.51 |
| g-C3N4 (wt%) | χ(1) (esu) | χ(3) × 10−13 (esu) | n2 × 10−12 (esu) |
|---|---|---|---|
| 0.0 | 0.19 | 2.07 | 4.26 |
| 0.3 | 0.59 | 204.6 | 266.1 |
| 0.6 | 0.99 | 1650 | 1695 |
| 1.0 | 1.17 | 3163 | 3012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshammari, A.H.; Alshammari, K.; Alshammari, M.; Taha, T.A.M. Structural and Optical Characterization of g-C3N4 Nanosheet Integrated PVC/PVP Polymer Nanocomposites. Polymers 2023, 15, 871. https://doi.org/10.3390/polym15040871
Alshammari AH, Alshammari K, Alshammari M, Taha TAM. Structural and Optical Characterization of g-C3N4 Nanosheet Integrated PVC/PVP Polymer Nanocomposites. Polymers. 2023; 15(4):871. https://doi.org/10.3390/polym15040871
Chicago/Turabian StyleAlshammari, Alhulw H., Khulaif Alshammari, Majed Alshammari, and Taha Abdel Mohaymen Taha. 2023. "Structural and Optical Characterization of g-C3N4 Nanosheet Integrated PVC/PVP Polymer Nanocomposites" Polymers 15, no. 4: 871. https://doi.org/10.3390/polym15040871