Preparation and In Vitro Evaluation of Chitosan-g-Oligolactide Based Films and Macroporous Hydrogels for Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Films and Macroporous Hydrogel Samples
2.3. Characterization of the Macroporous Hydrogels
2.3.1. Study of the Macroporous Hydrogel Morphology
2.3.2. Macroporous Hydrogel Swelling Degree Measurements
2.3.3. Study of Macroporous Hydrogel Degradation In Vitro
2.4. In Vitro Evaluation
2.4.1. Cell Cultivation
2.4.2. Characterization of Human Adipose Tissue Derived MSCs
2.4.3. Sterilization of the Matrix Samples
2.4.4. Cytotoxicity Study
2.4.5. Cell Cultivation in the Macroporous Hydrogels
2.4.6. Cultivation of Cells on the Films
2.5. Statistics
3. Results and Discussions
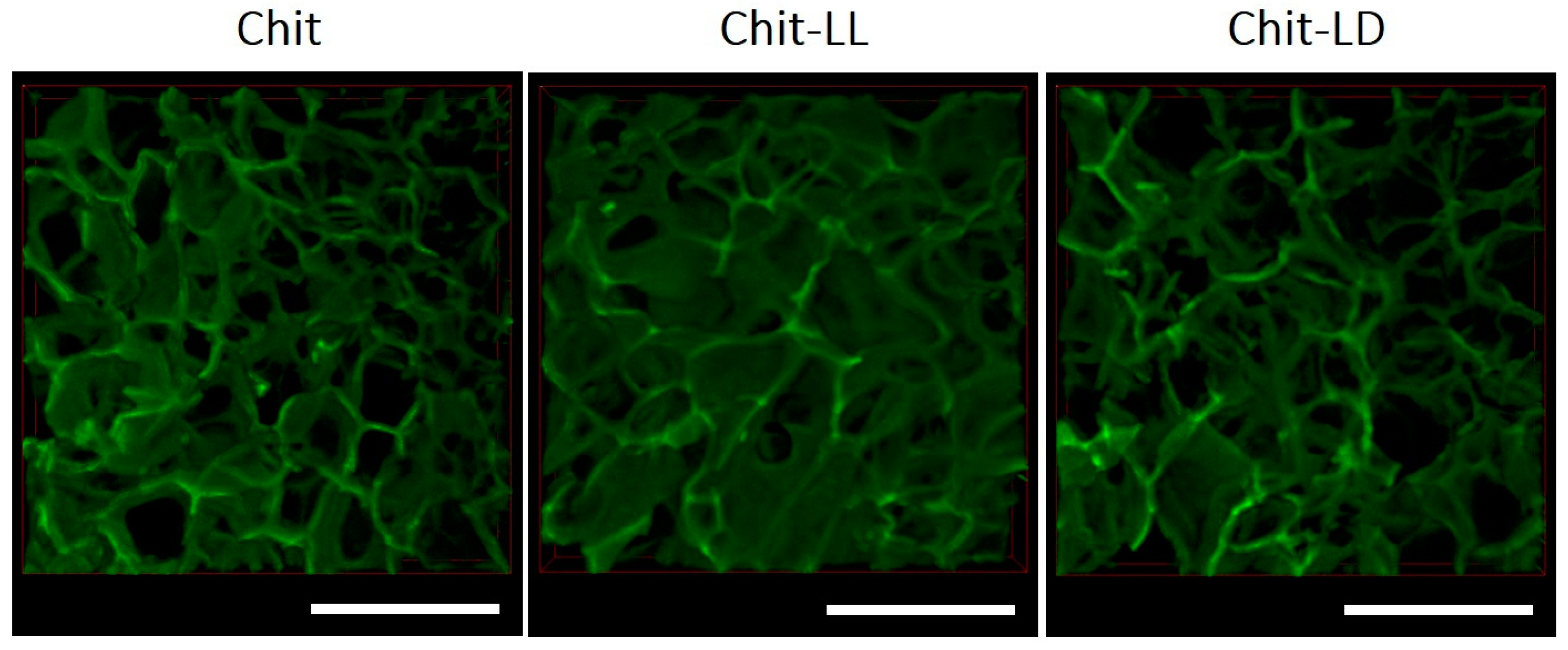
3.1. Study of the Macroporous Hydrogel Structures
3.2. Study of Macroporous Hydrogel Swelling Behavior
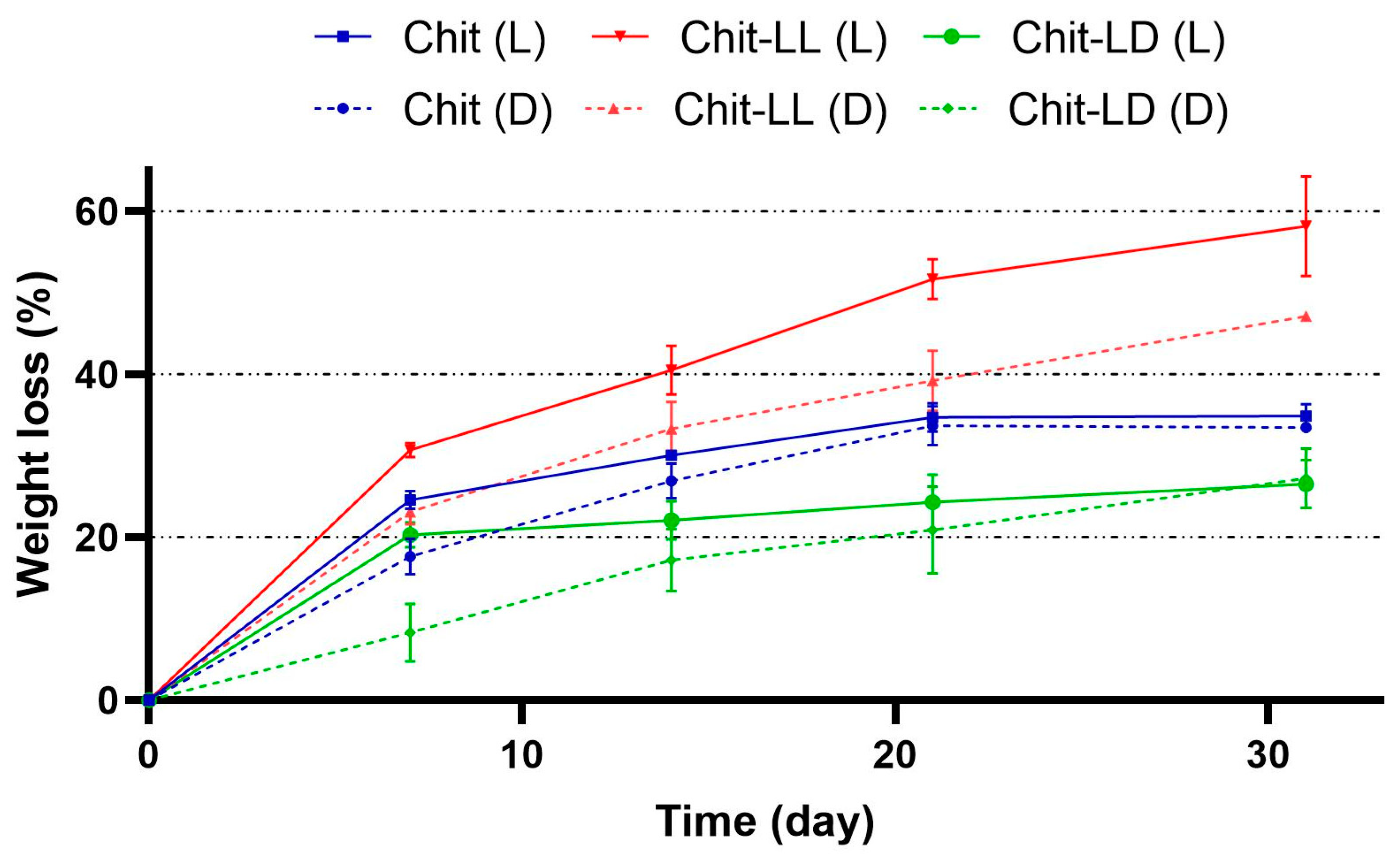
3.3. Study of the Macroporous Hydrogel Degradation
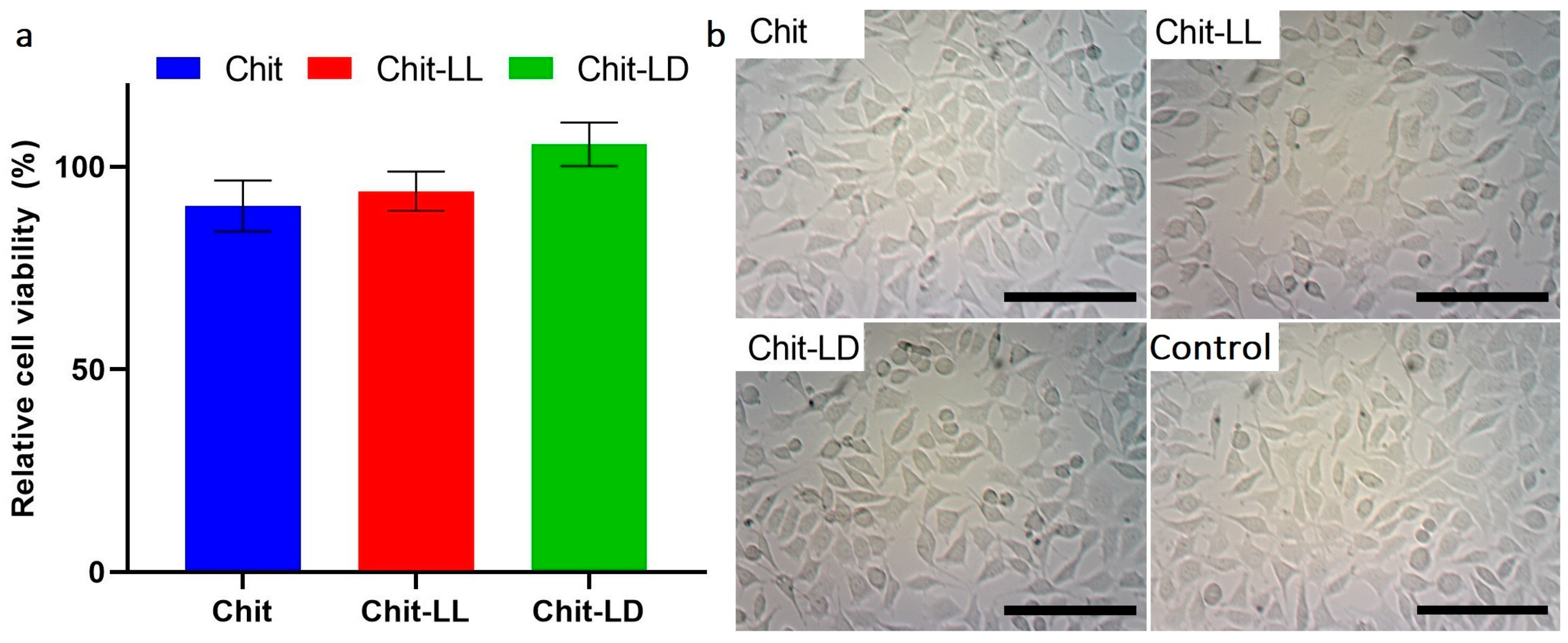
3.4. In Vitro Cytotoxicity Study
3.5. Cell Cultivation on the Films and in Chitosan-Based Macroporous Hydrogels In Vitro
3.5.1. Characterization of MSCs Phenotype
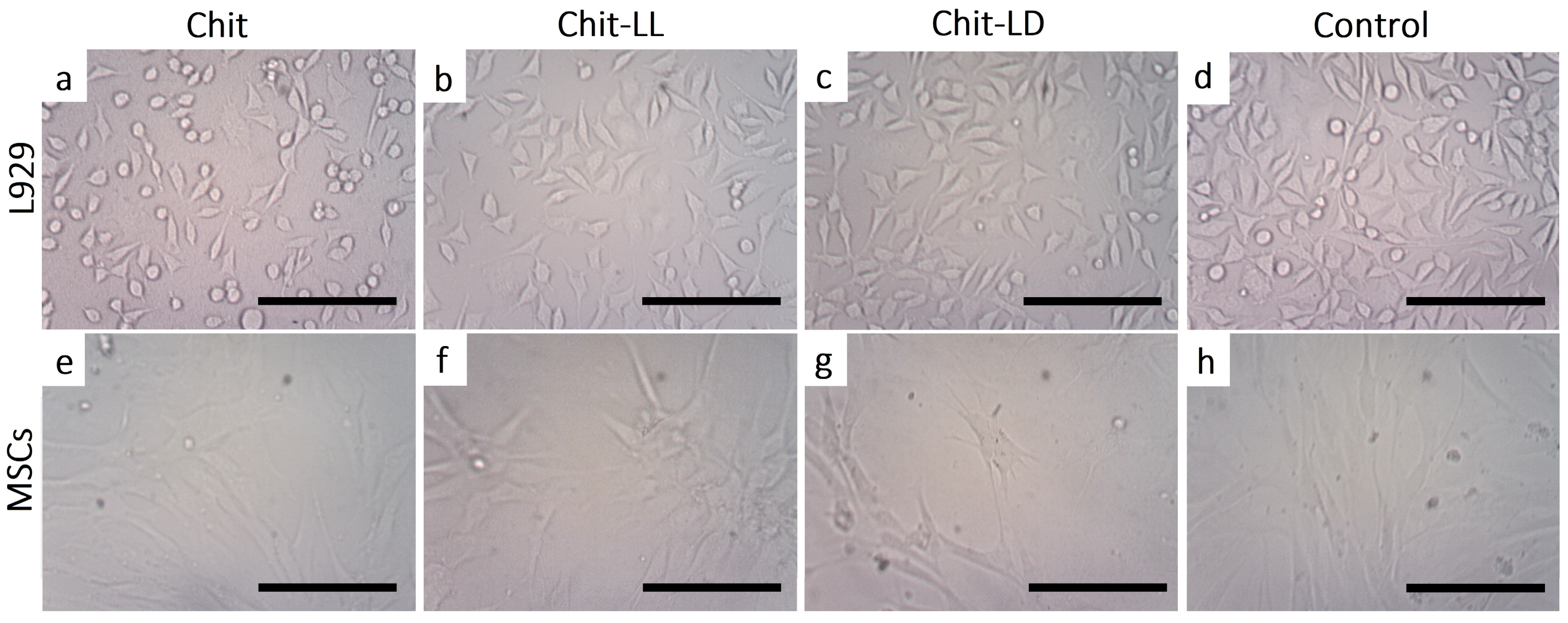
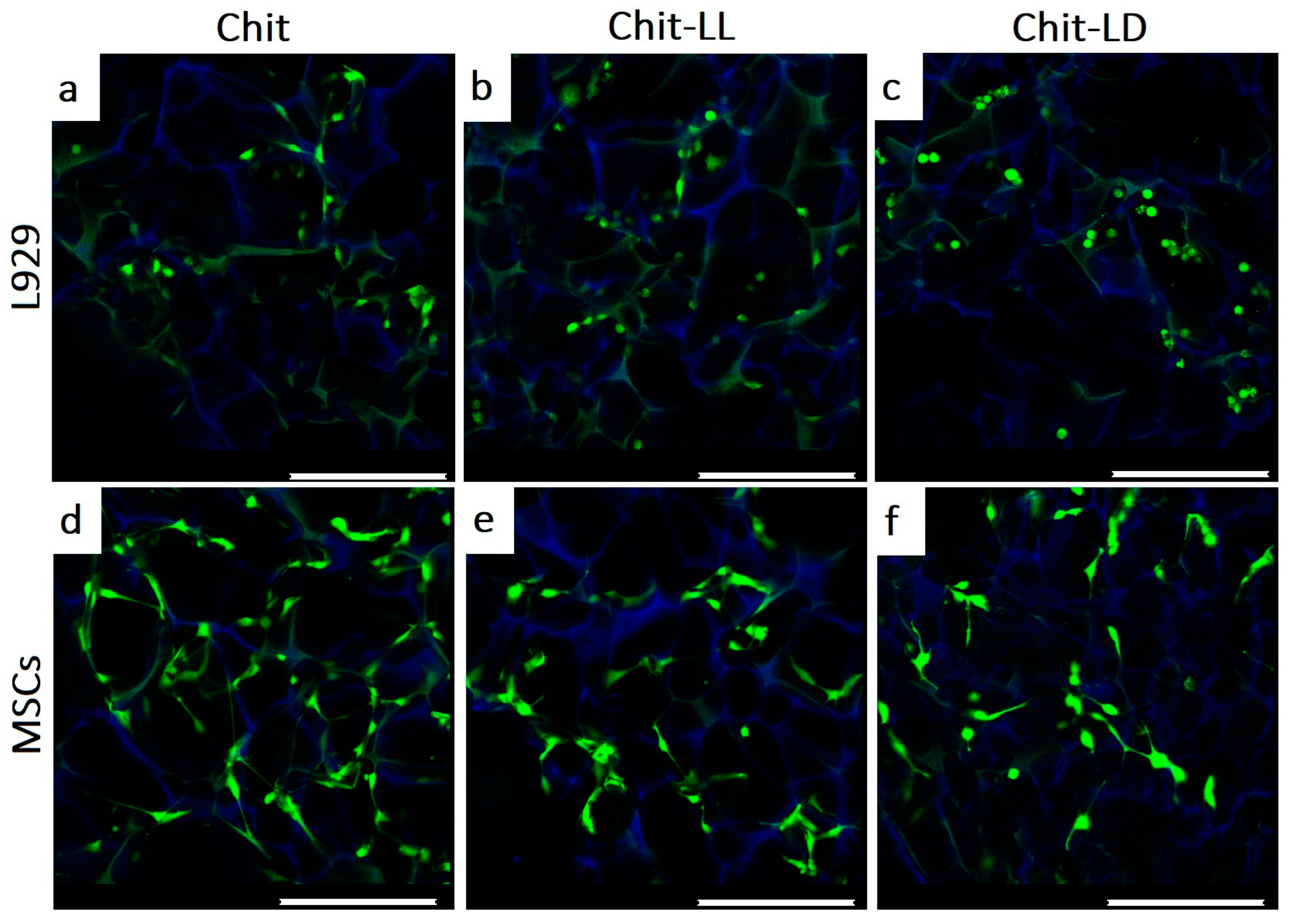
3.5.2. Morphology of Cells Cultured on Chitosan-Based Films
3.5.3. Morphology of Cells Cultivated in Chitosan-Based Macroporous Hydrogels
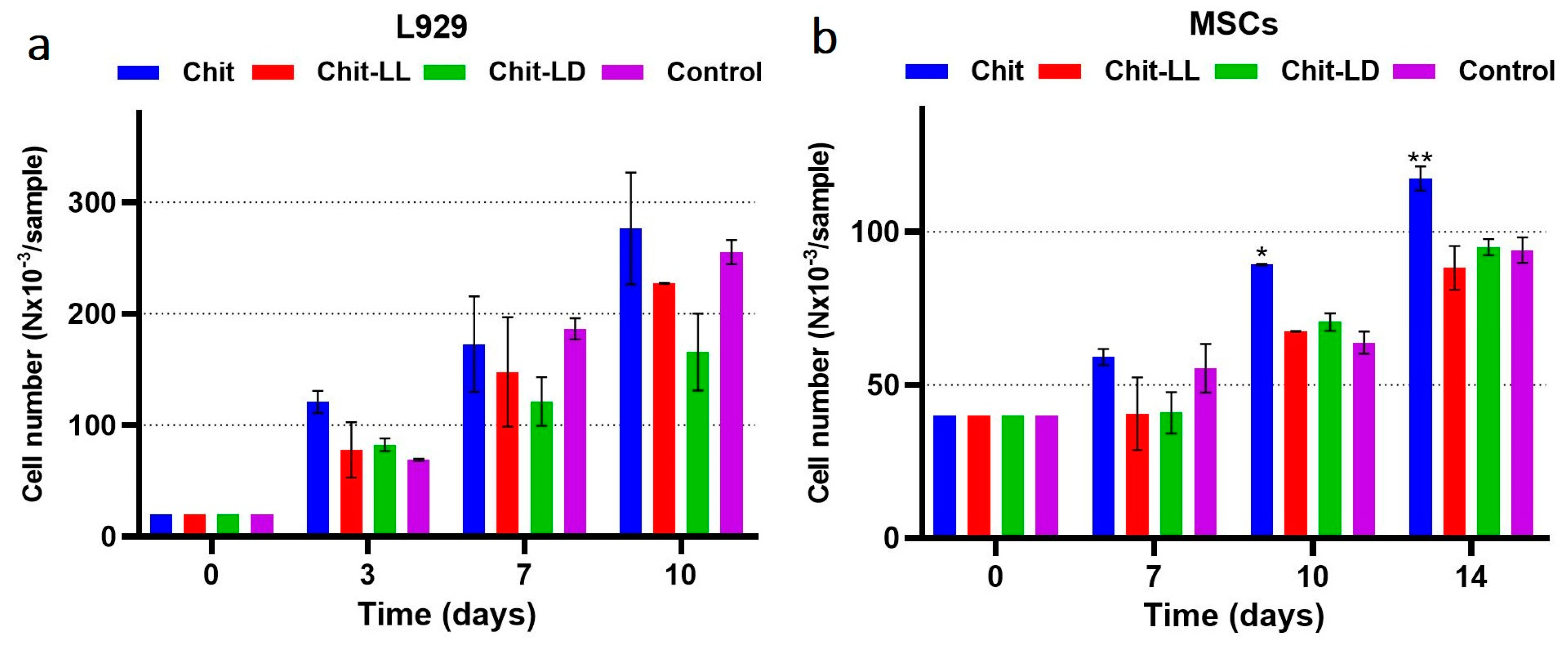
3.5.4. Cell Growth and Proliferation in the Macroporous Hydrogels
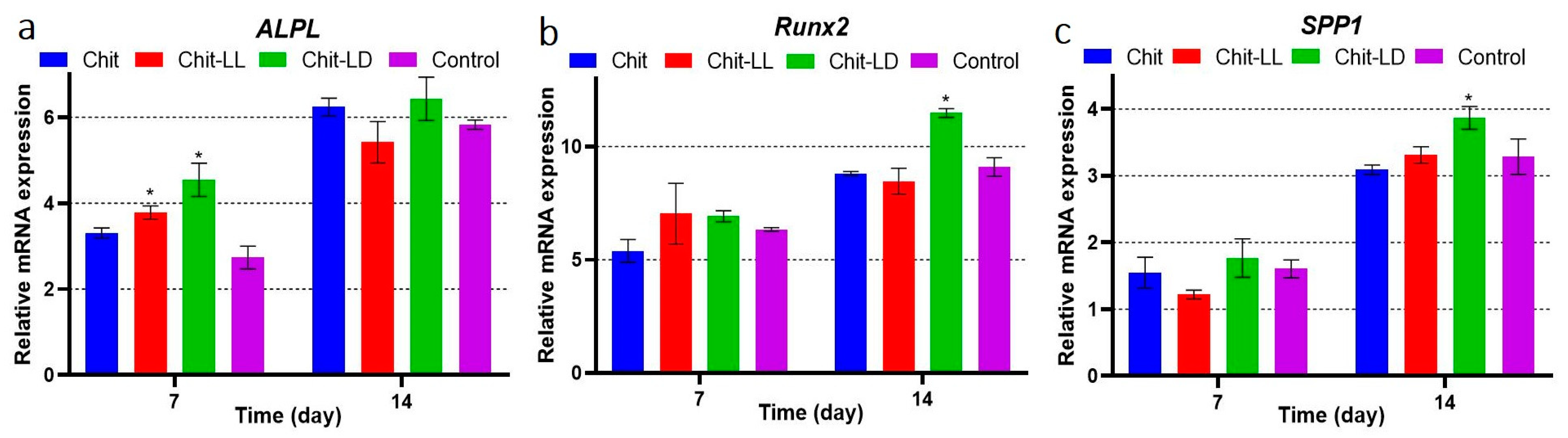
3.6. Cell Differentiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moysidou, C.-M.; Barberio, C.; Owens, R.M. Advances in Engineering Human Tissue Models. Front. Bioeng. Biotechnol. 2021, 8, 620962. [Google Scholar] [CrossRef]
- Haugen, H.J.; Basu, P.; Sukul, M.; Mano, J.F.; Reseland, J.E. Injectable Biomaterials for Dental Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3442. [Google Scholar] [CrossRef]
- Wang, H.; Hu, B.; Li, H.; Feng, G.; Pan, S.; Chen, Z.; Li, B.; Song, J. Biomimetic Mineralized Hydroxyapatite Nanofiber-Incorporated Methacrylated Gelatin Hydrogel with Improved Mechanical and Osteoinductive Performances for Bone Regeneration. Int. J. Nanomed. 2022, 17, 1511–1529. [Google Scholar] [CrossRef]
- Xiang, C.; Zhang, X.; Zhang, J.; Chen, W.; Li, X.; Wei, X.; Li, P. A Porous Hydrogel with High Mechanical Strength and Biocompatibility for Bone Tissue Engineering. J. Funct. Biomater. 2022, 13, 140. [Google Scholar] [CrossRef]
- Celesti, C.; Iannazzo, D.; Espro, C.; Visco, A.; Legnani, L.; Veltri, L.; Visalli, G.; Di Pietro, A.; Bottino, P.; Chiacchio, M.A. Chitosan/POSS Hybrid Hydrogels for Bone Tissue Engineering. Materials 2022, 15, 8208. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Gao, S.; Zhang, Y.-W.; Zhou, R.-B.; Zhou, F. Antibacterial Biomaterials in Bone Tissue Engineering. J. Mater. Chem. B 2021, 9, 2594–2612. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Razak, S.I.A.; Hassan, A.; Qureshi, S.; Stojanović, G.M.; Ihsan-Ul-Haq. Multifunctional Arabinoxylan-Functionalized-Graphene Oxide Based Composite Hydrogel for Skin Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 865059. [Google Scholar] [CrossRef]
- Drozdova, M.G.; Demina, T.S.; Dregval, O.A.; Gaidar, A.I.; Andreeva, E.R.; Zelenetskii, A.N.; Akopova, T.A.; Markvicheva, E. Macroporous Hyaluronic Acid/Chitosan Polyelectrolyte Complex-Based Hydrogels Loaded with Hydroxyapatite Nanoparticles: Preparation, Characterization and in Vitro Evaluation. Polysaccharides 2022, 3, 745–760. [Google Scholar] [CrossRef]
- Washington, K.S.; Bashur, C.A. Delivery of Antioxidant and Anti-Inflammatory Agents for Tissue Engineered Vascular Grafts. Front. Pharmacol. 2017, 8, 659. [Google Scholar] [CrossRef]
- Elviri, L.; Bianchera, A.; Bergonzi, C.; Bettini, R. Controlled Local Drug Delivery Strategies from Chitosan Hydrogels for Wound Healing. Expert Opin. Drug Deliv. 2017, 14, 897–908. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Razak, S.I.A.; Haider, S.; Mannan, H.A.; Hussain, J.; Hasan, A. Sodium Alginate-f-GO Composite Hydrogels for Tissue Regeneration and Antitumor Applications. Int. J. Biol. Macromol. 2022, 208, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Umuhoza, D.; Yang, F.; Long, D.; Hao, Z.; Dai, J.; Zhao, A. Strategies for Tuning the Biodegradation of Silk Fibroin-Based Materials for Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2020, 6, 1290–1310. [Google Scholar] [CrossRef] [PubMed]
- Abdulghani, S.; Mitchell, G. Biomaterials for In Situ Tissue Regeneration: A Review. Biomolecules 2019, 9, 750. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Pharmaceutical Applications of Chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef]
- Wahba, M.I. Enhancement of the Mechanical Properties of Chitosan. J. Biomater. Sci. Polym. Ed. 2020, 31, 350–375. [Google Scholar] [CrossRef]
- Wan, Y.; Fang, Y.; Wu, H.; Cao, X. Porous Polylactide/Chitosan Scaffolds for Tissue Engineering. J. Biomed. Mater. Res. A 2007, 80A, 776–789. [Google Scholar] [CrossRef]
- Zhang, H.; Neau, S.H. In Vitro Degradation of Chitosan by a Commercial Enzyme Preparation: Effect of Molecular Weight and Degree of Deacetylation. Biomaterials 2001, 22, 1653–1658. [Google Scholar] [CrossRef]
- Li, S.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. Chitosans for Tissue Repair and Organ Three-Dimensional (3D) Bioprinting. Micromachines 2019, 10, 765. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Sakib, M.N.; Rashid, T.U. Chitosan Based Bioactive Materials in Tissue Engineering Applications-A Review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef] [PubMed]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of Different Factors Affecting Antimicrobial Properties of Chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Huang, M.; Khor, E.; Lim, L.-Y. Uptake and Cytotoxicity of Chitosan Molecules and Nanoparticles: Effects of Molecular Weight and Degree of Deacetylation. Pharm. Res. 2004, 21, 344–353. [Google Scholar] [CrossRef]
- Demina, T.S.; Zaytseva-Zotova, D.S.; Akopova, T.A.; Zelenetskii, A.N.; Markvicheva, E.A. Macroporous Hydrogels Based on Chitosan Derivatives: Preparation, Characterization, and in Vitro Evaluation. J. Appl. Polym. Sci. 2017, 134, 44651. [Google Scholar] [CrossRef]
- Gámiz-González, M.A.; Vidaurre, A.; Gómez Ribelles, J.L. Biodegradable Chitosan-Poly(Ɛ-Caprolactone) Dialdehyde Copolymer Networks for Soft Tissue Engineering. Polym. Degrad. Stab. 2017, 138, 47–54. [Google Scholar] [CrossRef]
- Luckachan, G.E.; Pillai, C.K.S. Chitosan/Oligo L-Lactide Graft Copolymers: Effect of Hydrophobic Side Chains on the Physico-Chemical Properties and Biodegradability. Carbohydr. Polym. 2006, 64, 254–266. [Google Scholar] [CrossRef]
- Yan, J.; Li, X.; Liu, L.; Wang, F.; Zhu, T.W.; Zhang, Q. Potential Use of Collagen-Chitosan-Hyaluronan Tri-Copolymer Scaffold for Cartilage Tissue Engineering. Artif. Cells Blood Substit. Biotechnol. 2006, 34, 27–39. [Google Scholar] [CrossRef]
- Shah Mohammadi, M.; Bureau, M.N.; Nazhat, S.N. Polylactic Acid (PLA) Biomedical Foams for Tissue Engineering. In Biomedical Foams for Tissue Engineering Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 313–334. [Google Scholar] [CrossRef]
- Privalova, A.; Markvicheva, E.; Sevrin, C.; Drozdova, M.; Kottgen, C.; Gilbert, B.; Ortiz, M.; Grandfils, C. Biodegradable Polyester-Based Microcarriers with Modified Surface Tailored for Tissue Engineering. J. Biomed. Mater. Res. A 2015, 103, 939–948. [Google Scholar] [CrossRef]
- Sun, Q.; Sheng, J.; Yang, R. Controllable Biodegradation and Drug Release Behavior of Chitosan-Graft-Poly(D, L-Lactic Acid) Synthesized by an Efficient Method. Polym. Degrad. Stab. 2021, 186, 109458. [Google Scholar] [CrossRef]
- Li, L.; Ding, S.; Zhou, C. Preparation and Degradation of PLA/Chitosan Composite Materials. J. Appl. Polym. Sci. 2004, 91, 274–277. [Google Scholar] [CrossRef]
- Li, Z.; Tan, B.H.; Lin, T.; He, C. Recent Advances in Stereocomplexation of Enantiomeric PLA-Based Copolymers and Applications. Prog. Polym. Sci. 2016, 62, 22–72. [Google Scholar] [CrossRef]
- Demina, T.; Bardakova, K.; Minaev, N.; Svidchenko, E.; Istomin, A.; Goncharuk, G.; Vladimirov, L.; Grachev, A.; Zelenetskii, A.; Timashev, P.; et al. Two-Photon-Induced Microstereolithography of Chitosan-g-Oligolactides as a Function of Their Stereochemical Composition. Polymers 2017, 9, 302. [Google Scholar] [CrossRef]
- Popyrina, T.N.; Svidchenko, E.A.; Demina, T.S.; Akopova, T.A.; Zelenetsky, A.N. Effect of the Chemical Structure of Chitosan Copolymers with Oligolactides on the Morphology and Properties of Macroporous Hydrogels Based on Them. Polym. Sci. Ser. B 2021, 63, 536–543. [Google Scholar] [CrossRef]
- Grebenik, E.A.; Surin, A.M.; Bardakova, K.N.; Demina, T.S.; Minaev, N.V.; Veryasova, N.N.; Artyukhova, M.A.; Krasilnikova, I.A.; Bakaeva, Z.V.; Sorokina, E.G.; et al. Chitosan-g-Oligo(L,L-Lactide) Copolymer Hydrogel for Nervous Tissue Regeneration in Glutamate Excitotoxicity: In Vitro Feasibility Evaluation. Biomed. Mater. 2020, 15, 015011. [Google Scholar] [CrossRef] [PubMed]
- Revkova, V.A.; Grebenik, E.A.; Kalsin, V.A.; Demina, T.S.; Bardakova, K.N.; Shavkuta, B.S.; Melnikov, P.A.; Samoilova, E.M.; Konoplyannikov, M.A.; Efremov, Y.M.; et al. Chitosan-g-Oligo(L,L-Lactide) Copolymer Hydrogel Potential for Neural Stem Cell Differentiation. Tissue Eng. Part A 2020, 26, 953–963. [Google Scholar] [CrossRef]
- Akopova, T.A.; Zelenetskii, A.N.; Ozerin, A.N. Solid State Synthesis and Modification of Chitosan. Focus Chitosan Res. 2011, 30, 223–253. [Google Scholar]
- Rogovina, S.Z.; Akopova, T.A.; Vikhoreva, G.A. Investigation of Properties of Chitosan Obtained by Solid-Phase and Suspension Methods. J. Appl. Polym. Sci. 1998, 70, 927–933. [Google Scholar] [CrossRef]
- Buravkova, L.B.; Grinakovskaya, O.S.; Andreeva, E.R.; Zhambalova, A.P.; Kozionova, M.P. Characteristics of Human Lipoaspirate-Isolated Mesenchymal Stromal Cells Cultivated under a Lower Oxygen Tension. Tsitologiya 2009, 51, 5–11. [Google Scholar] [CrossRef]
- Liao, Y.-J.; Tang, P.-C.; Chen, L.-R.; Yang, J.-R. A Protocol for Differential Staining of Cartilages and Ossified Bones in Fetal and Adult Mouse Skeletons Using Alcian Blue and Alizarin Red S. J. Histotechnol. 2020, 43, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Kraus, N.A.; Ehebauer, F.; Zapp, B.; Rudolphi, B.; Kraus, B.J.; Kraus, D. Quantitative Assessment of Adipocyte Differentiation in Cell Culture. Adipocyte 2016, 5, 351–358. [Google Scholar] [CrossRef]
- Bergholt, N.L.; Lysdahl, H.; Lind, M.; Foldager, C.B. A Standardized Method of Applying Toluidine Blue Metachromatic Staining for Assessment of Chondrogenesis. Cartilage 2019, 10, 370–374. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and Cells for Tissue Regeneration: Different Scaffold Pore Sizes—Different Cell Effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N.; et al. Application of Chitosan in Bone and Dental Engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef] [PubMed]
- Mahumane, G.D.; Kumar, P.; du Toit, L.C.; Choonara, Y.E.; Pillay, V. 3D Scaffolds for Brain Tissue Regeneration: Architectural Challenges. Biomater. Sci. 2018, 6, 2812–2837. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Das, A.; Mukherjee, S.; Rajput, M.; Gope, A.; Chaudhary, A.; Choudhury, K.; Barui, A.; Chatterjee, J.; Mukherjee, R. Improved Mesenchymal Stem Cell Proliferation, Differentiation, Epithelial Transition, and Restrained Senescence on Hierarchically Patterned Porous Honey Silk Fibroin Scaffolds. ACS Appl. Bio Mater. 2021, 4, 4328–4344. [Google Scholar] [CrossRef]
- Qi, W.; Cai, P.; Yuan, W.; Wang, H. Tunable Swelling of Polyelectrolyte Multilayers in Cell Culture Media for Modulating NIH-3T3 Cells Adhesion. J. Biomed. Mater. Res. A 2014, 102, 4071–4077. [Google Scholar] [CrossRef] [PubMed]
- Gorochovceva, N.; Makuška, R. Synthesis and Study of Water-Soluble Chitosan-O-Poly(Ethylene Glycol) Graft Copolymers. Eur. Polym. J. 2004, 4, 685–691. [Google Scholar] [CrossRef]
- de Jonge, N.; Foolen, J.; Brugmans, M.C.P.; Söntjens, S.H.M.; Baaijens, F.P.T.; Bouten, C.V.C. Degree of Scaffold Degradation Influences Collagen (Re)Orientation in Engineered Tissues. Tissue Eng. Part A 2014, 20, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.; Liang, H.; Pandit, S.; Parikh, Z.; Schwartz, J.A.; Goldstein, T.; Lavelle, L.P.; Datta, A.; Grande, D.A. Optimization of Degradation Profile for New Scaffold in Cartilage Repair. Cartilage 2018, 9, 438–449. [Google Scholar] [CrossRef]
- Kim, S.; Cui, Z.-K.; Koo, B.; Zheng, J.; Aghaloo, T.; Lee, M. Chitosan-Lysozyme Conjugates for Enzyme-Triggered Hydrogel Degradation in Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2018, 10, 41138–41145. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-S.; Dai, L.-G.; Yen, B.L.; Hsu, S. Spheroid Formation of Mesenchymal Stem Cells on Chitosan and Chitosan-Hyaluronan Membranes. Biomaterials 2011, 32, 6929–6945. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.-C.; Wang, S.; Young, T.-H. The Influence of Spheroid Formation of Human Adipose-Derived Stem Cells on Chitosan Films on Stemness and Differentiation Capabilities. Biomaterials 2012, 33, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Kanawa, M.; Igarashi, A.; Fujimoto, K.; Saskianti, T.; Nakashima, A.; Higashi, Y.; Kurihara, H.; Kato, Y.; Kawamoto, T. The Identification of Marker Genes for Predicting the Osteogenic Differentiation Potential of Mesenchymal Stromal Cells. Curr. Issues Mol. Biol. 2021, 43, 2157–2166. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P.; Zhang, L.; Xu, C.; Zheng, C.; Han, Y.; Li, W.; Huang, Y.; Zhang, X.; Shao, C.; et al. An Osteopontin-Integrin Interaction Plays a Critical Role in Directing Adipogenesis and Osteogenesis by Mesenchymal Stem Cells. Stem Cells 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, J.; Song, W.-X.; Tang, N.; Luo, J.; Deng, Z.-L.; Sharff, K.A.; He, G.; Bi, Y.; He, B.-C.; et al. Osteogenic BMPs Promote Tumor Growth of Human Osteosarcomas That Harbor Differentiation Defects. Lab. Investig. J. Tech. Methods Pathol. 2008, 88, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Runx2, an Inducer of Osteoblast and Chondrocyte Differentiation. Histochem. Cell Biol. 2018, 149, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Grzesiak, J.; Śmieszek, A.; Marycz, K. Ultrastructural Changes during Osteogenic Differentiation in Mesenchymal Stromal Cells Cultured in Alginate Hydrogel. Cell Biosci. 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolstova, T.; Drozdova, M.; Popyrina, T.; Matveeva, D.; Demina, T.; Akopova, T.; Andreeva, E.; Markvicheva, E. Preparation and In Vitro Evaluation of Chitosan-g-Oligolactide Based Films and Macroporous Hydrogels for Tissue Engineering. Polymers 2023, 15, 907. https://doi.org/10.3390/polym15040907
Tolstova T, Drozdova M, Popyrina T, Matveeva D, Demina T, Akopova T, Andreeva E, Markvicheva E. Preparation and In Vitro Evaluation of Chitosan-g-Oligolactide Based Films and Macroporous Hydrogels for Tissue Engineering. Polymers. 2023; 15(4):907. https://doi.org/10.3390/polym15040907
Chicago/Turabian StyleTolstova, Tatiana, Maria Drozdova, Tatiana Popyrina, Diana Matveeva, Tatiana Demina, Tatiana Akopova, Elena Andreeva, and Elena Markvicheva. 2023. "Preparation and In Vitro Evaluation of Chitosan-g-Oligolactide Based Films and Macroporous Hydrogels for Tissue Engineering" Polymers 15, no. 4: 907. https://doi.org/10.3390/polym15040907
APA StyleTolstova, T., Drozdova, M., Popyrina, T., Matveeva, D., Demina, T., Akopova, T., Andreeva, E., & Markvicheva, E. (2023). Preparation and In Vitro Evaluation of Chitosan-g-Oligolactide Based Films and Macroporous Hydrogels for Tissue Engineering. Polymers, 15(4), 907. https://doi.org/10.3390/polym15040907







