Impact of the Chemical Structure of Photoreactive Urethane (Meth)Acrylates with Various (Meth)Acrylate Groups and Built-In Diels–Alder Reaction Adducts on the UV-Curing Process and Self-Healing Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of UV-Curable UA Resins
2.3. General Analytical Methods to Confirm the Structure
2.4. Characteristics of the Photopolymerization Process and Properties of Cured Coatings
2.5. Preparation of Coating Compositions, Cured Films, and Characteristics of Cured Films
2.6. Characteristics of Self-Healing Properties
3. Results and Discussion
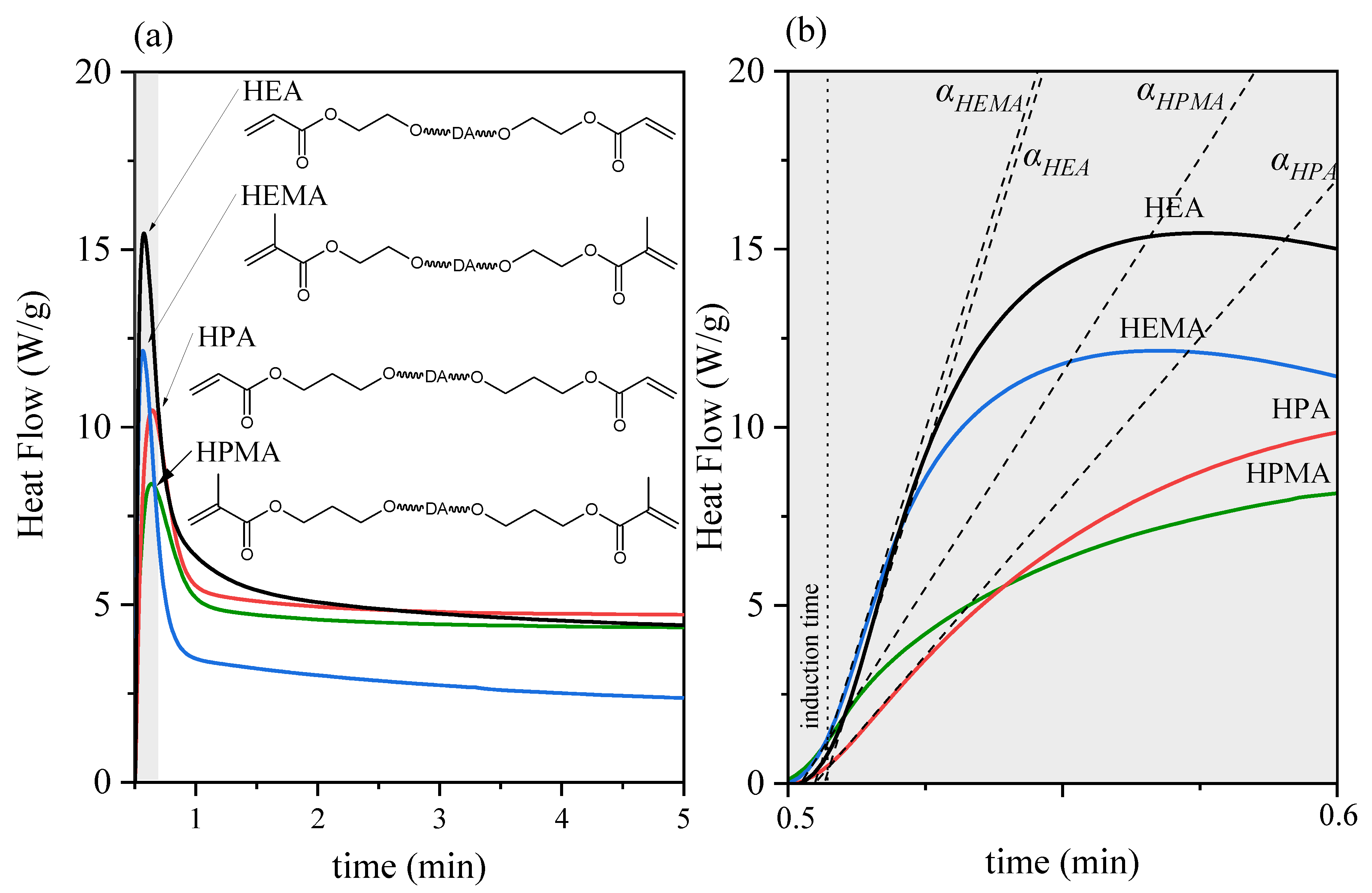
3.1. Synthesis and Characterization of UA-DA Resins
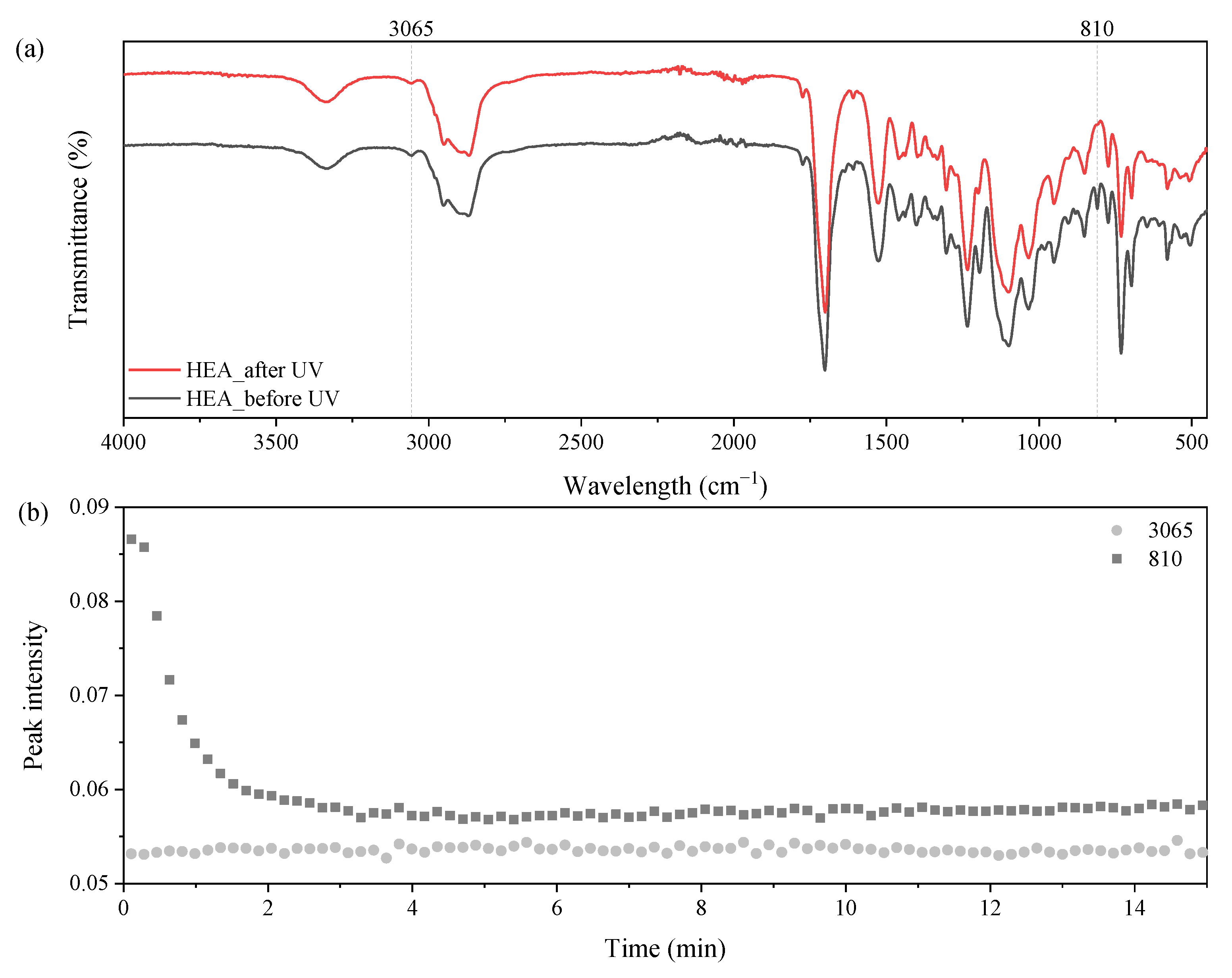
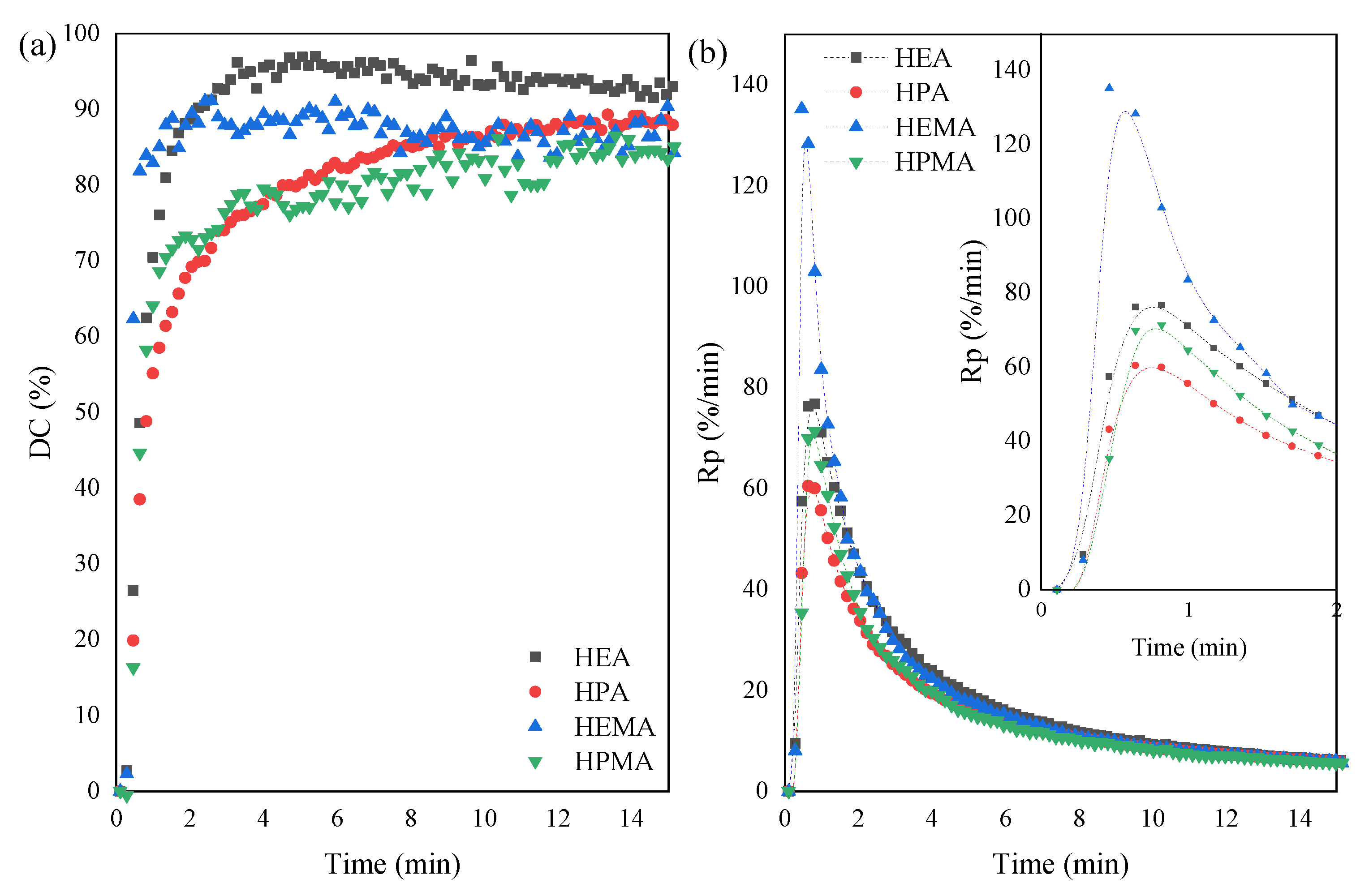
3.2. UV-Curing Mechanism of UA-DA Resins
3.3. Properties of Cured Coatings
3.4. Thermal Properties of UA-DA Film
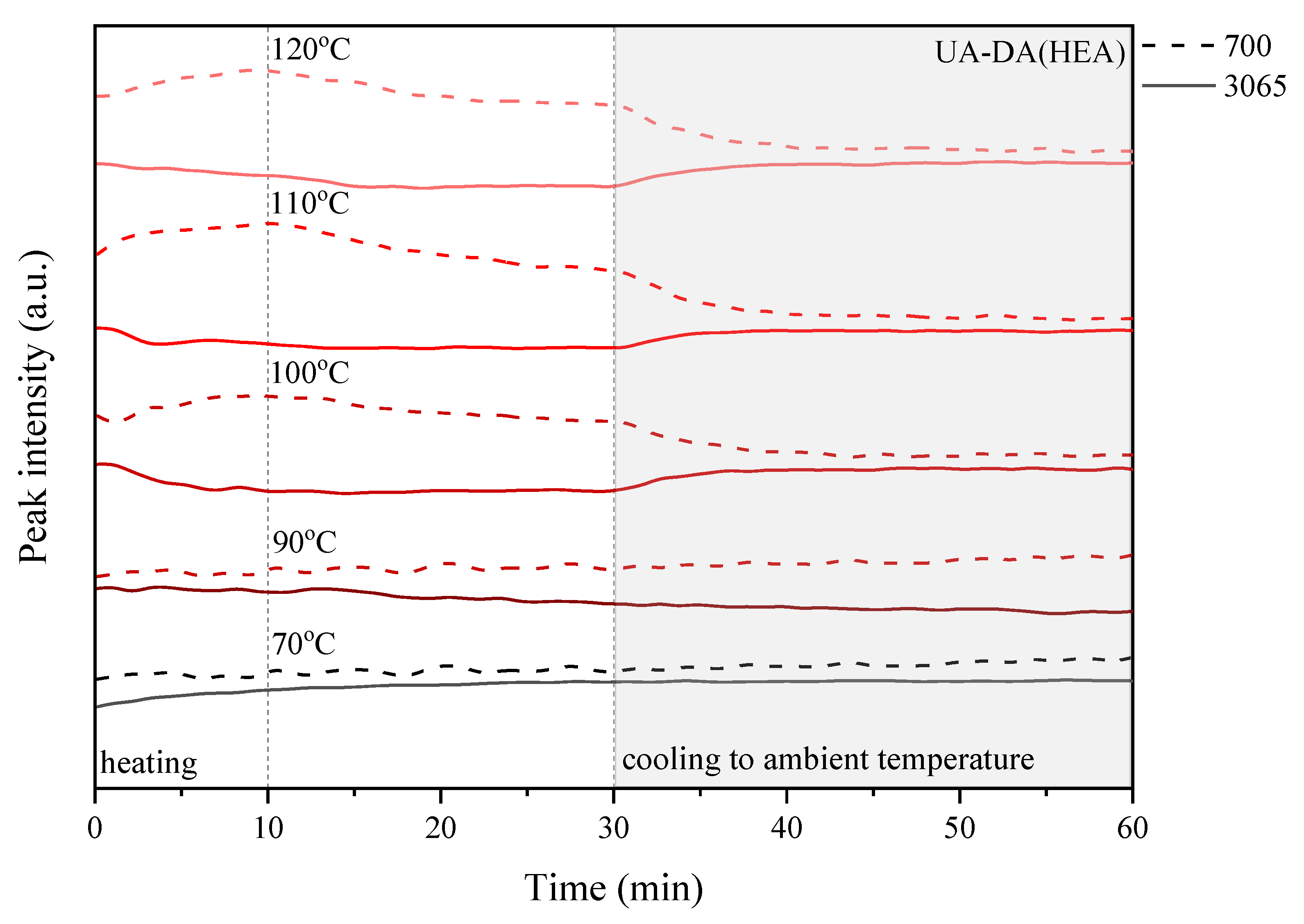
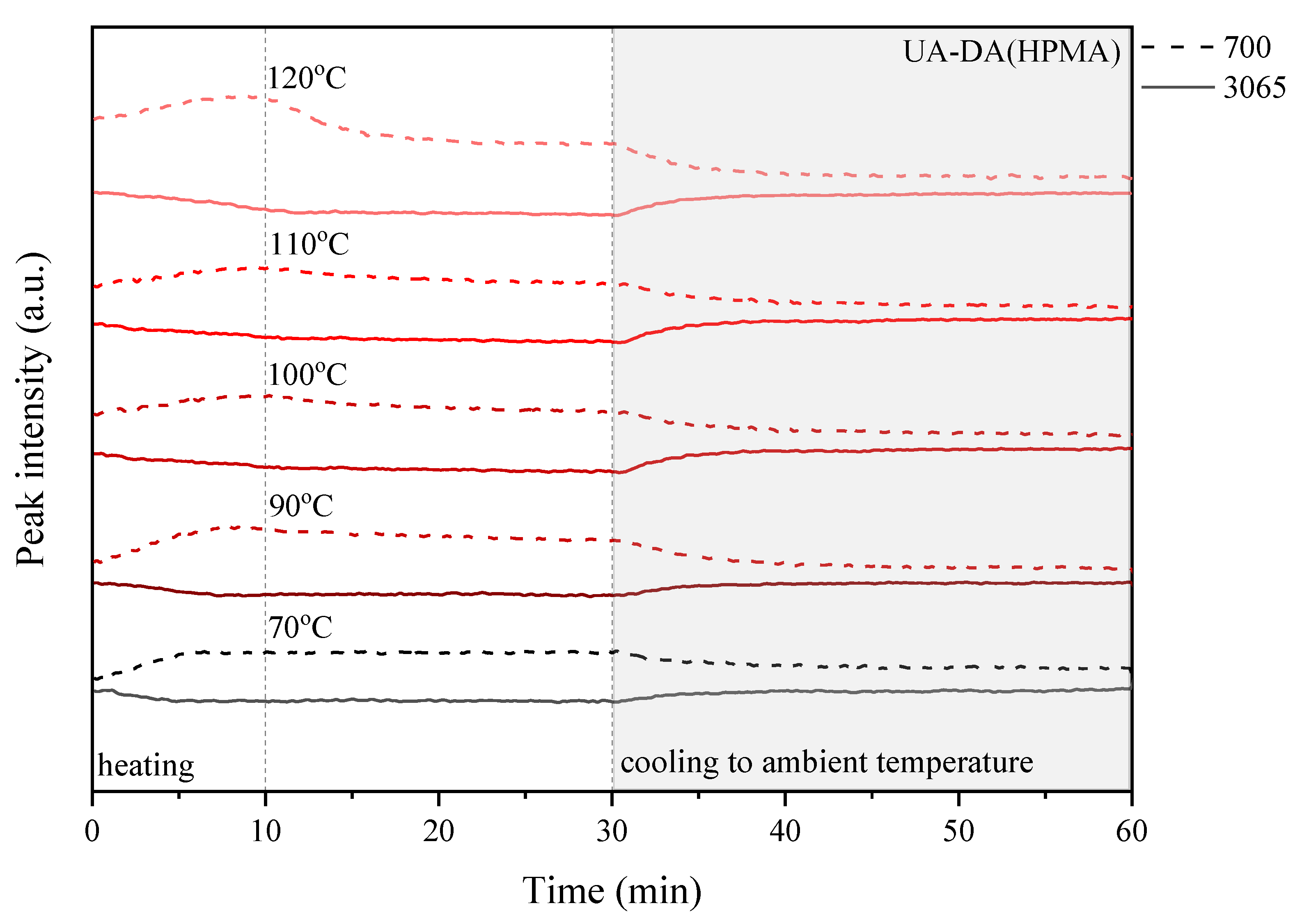
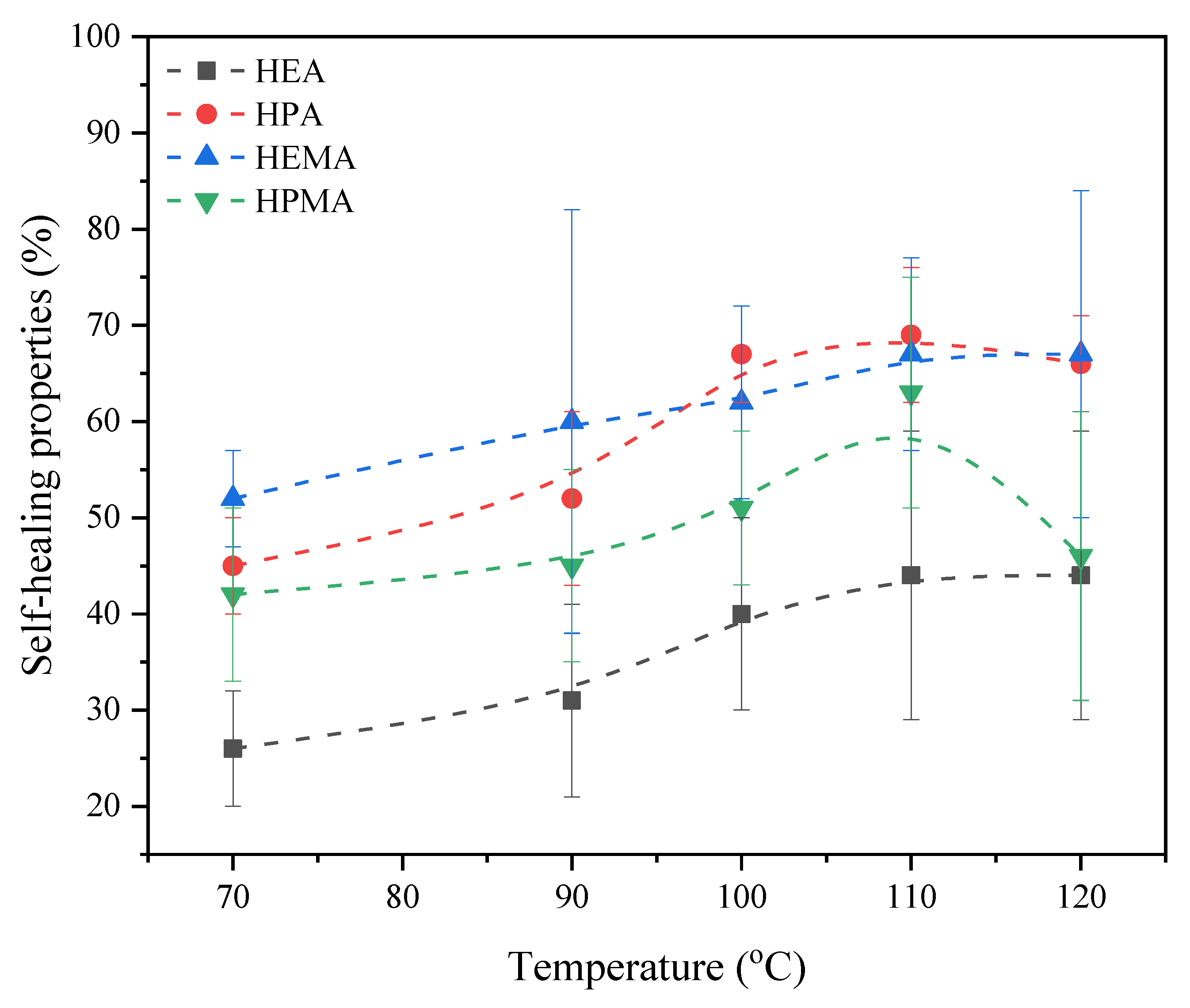
3.5. Self-Healing Ability of UA-DA Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, N.I.; Halder, S.; Gunjan, S.B.; Prasad, T. A Review on Diels-Alder Based Self-Healing Polymer Composites. IOP Conf. Ser. Mater. Sci. Eng. 2018, 377, 012007. [Google Scholar] [CrossRef]
- Gevrek, T.N.; Sanyal, A. Furan-Containing Polymeric Materials: Harnessing the Diels-Alder Chemistry for Biomedical Applications. Eur. Polym. J. 2021, 153, 110514. [Google Scholar] [CrossRef]
- Liu, B.; Xia, M.; Ji, X.; Xu, L.; Dong, J. Synthesis and Antiproliferative Effect of Novel Curcumin Analogues. Chem. Pharm. Bull. 2013, 61, 757–763. [Google Scholar] [CrossRef]
- Dello Iacono, S.; Martone, A.; Amendola, E. Diels-Alder Chemistry to Develop Self-Healing Epoxy Resins and Composites Thereof. In Paint and Coatings Industry; Yilmaz, F., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78985-161-8. [Google Scholar]
- Karami, Z.; Zolghadr, M.; Zohuriaan-Mehr, M.J. Self-Healing Diels–Alder Engineered Thermosets. In Self-Healing Polymer-Based Systems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 209–233. ISBN 978-0-12-818450-9. [Google Scholar]
- Wool, R.P. Self-Healing Materials: A Review. Soft Matter 2008, 4, 400. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Urban, M.W. Self-Healing Polymers. Nat. Rev. Mater. 2020, 5, 562–583. [Google Scholar] [CrossRef]
- Ghosh, S.K. (Ed.) Self-Healing Materials: Fundamentals, Design Strategies, and Applications; Wiley-VCH: Weinheim, Germany, 2009; ISBN 978-3-527-31829-2. [Google Scholar]
- Hager, M.D.; Zechel, S. Self-Healing Polymers: From General Basics to Mechanistic Aspects. In Self-Healing Polymer-Based Systems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 75–94. ISBN 978-0-12-818450-9. [Google Scholar]
- Padhan, A.K.; Mandal, D. Types of Chemistries Involved in Self-Healing Polymeric Systems. In Self-Healing Polymer-Based Systems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–73. ISBN 978-0-12-818450-9. [Google Scholar]
- Vilela, C.; Cruciani, L.; Silvestre, A.J.D.; Gandini, A. A Double Click Strategy Applied to the Reversible Polymerization of Furan/Vegetable Oil Monomers: A Double Click Strategy Applied to the Reversible Polymerization of Furan/Vegetable Oil Monomers. Macromol. Rapid Commun. 2011, 32, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ye, G.; Yang, J. Synthesis and Properties of UV-Curable Acrylate Functionalized Tung Oil Based Resins via Diels–Alder Reaction. Prog. Org. Coat. 2015, 78, 28–34. [Google Scholar] [CrossRef]
- Fang, Y.; Du, X.; Jiang, Y.; Du, Z.; Pan, P.; Cheng, X.; Wang, H. Thermal-Driven Self-Healing and Recyclable Waterborne Polyurethane Films Based on Reversible Covalent Interaction. ACS Sustain. Chem. Eng. 2018, 6, 14490–14500. [Google Scholar] [CrossRef]
- Heo, Y.; Sodano, H.A. Self-Healing Polyurethanes with Shape Recovery. Adv. Funct. Mater. 2014, 24, 5261–5268. [Google Scholar] [CrossRef]
- Kazemi-Lari, M.A.; Malakooti, M.H.; Sodano, H.A. Active Photo-Thermal Self-Healing of Shape Memory Polyurethanes. Smart Mater. Struct. 2017, 26, 055003. [Google Scholar] [CrossRef]
- Aizpurua, J.; Martin, L.; Formoso, E.; González, A.; Irusta, L. One Pot Stimuli-Responsive Linear Waterborne Polyurethanes via Diels-Alder Reaction. Prog. Org. Coat. 2019, 130, 31–43. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, H.; Yang, H.; Xiong, L.; Zhou, J.; Huang, S.; Zhao, C.; Zhong, J.; Fan, X. UV-Curable Self-Healing Polyurethane Coating Based on Thiol-Ene and Diels-Alder Double Click Reactions. Prog. Org. Coat. 2019, 137, 105282. [Google Scholar] [CrossRef]
- Maassen, E.E.L.; Anastasio, R.; Breemen, L.C.A.; Sijbesma, R.P.; Heuts, J.P.A. Thermally Reversible Diels–Alder Bond-Containing Acrylate Networks Showing Improved Lifetime. Macromol. Chem. Phys. 2020, 221, 2000208. [Google Scholar] [CrossRef]
- Irusta, L.; Fernández-Berridi, M.J.; Aizpurua, J. Polyurethanes Based on Isophorone Diisocyanate Trimer and Polypropylene Glycol Crosslinked by Thermal Reversible Diels Alder Reactions. J. Appl. Polym. Sci. 2017, 134, 44543. [Google Scholar] [CrossRef]
- Fortunato, G.; Tatsi, E.; Rigatelli, B.; Turri, S.; Griffini, G. Highly Transparent and Colorless Self-Healing Polyacrylate Coatings Based on Diels–Alder Chemistry. Macromol. Mater. Eng. 2020, 305, 1900652. [Google Scholar] [CrossRef]
- Ke, X.; Liang, H.; Xiong, L.; Huang, S.; Zhu, M. Synthesis, Curing Process and Thermal Reversible Mechanism of UV Curable Polyurethane Based on Diels-Alder Structure. Prog. Org. Coat. 2016, 100, 63–69. [Google Scholar] [CrossRef]
- Lu, X.; Fei, G.; Xia, H.; Zhao, Y. Ultrasound Healable Shape Memory Dynamic Polymers. J. Mater. Chem. A 2014, 2, 16051–16060. [Google Scholar] [CrossRef]
- Syrett, J.A.; Mantovani, G.; Barton, W.R.S.; Price, D.; Haddleton, D.M. Self-Healing Polymers Prepared via Living Radical Polymerisation. Polym. Chem. 2010, 1, 102. [Google Scholar] [CrossRef]
- Heath, W.H.; Palmieri, F.; Adams, J.R.; Long, B.K.; Chute, J.; Holcombe, T.W.; Zieren, S.; Truitt, M.J.; White, J.L.; Willson, C.G. Degradable Cross-Linkers and Strippable Imaging Materials for Step-and-Flash Imprint Lithography. Macromolecules 2008, 41, 719–726. [Google Scholar] [CrossRef]
- Duran, H.; Meng, S.; Kim, N.; Hu, J.; Kyu, T.; Natarajan, L.V.; Tondiglia, V.P.; Bunning, T.J. Kinetics of Photopolymerization-Induced Phase Separation and Morphology Development in Mixtures of a Nematic Liquid Crystal and Multifunctional Acrylate. Polymer 2008, 49, 534–545. [Google Scholar] [CrossRef]
- Yin, B.; Zhang, J. A Novel Photocurable Modified Epoxy Resin for High Heat Resistance Coatings. Colloid. Polym. Sci. 2020, 298, 1303–1312. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Irska, I.; Gziut, K.; Ossowicz-Rupniewska, P. Novel Multifunctional Epoxy (Meth)Acrylate Resins and Coatings Preparation via Cationic and Free-Radical Photopolymerization. Polymers 2021, 13, 1718. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, P.; Irska, I.; Gziut, K.; Ossowicz-Rupniewska, P. Synthesis of Epoxy Methacrylate Resin and Coatings Preparation by Cationic and Radical Photocrosslinking. Molecules 2021, 26, 7663. [Google Scholar] [CrossRef] [PubMed]
- Boutelle, R.C.; Northrop, B.H. Substituent Effects on the Reversibility of Furan–Maleimide Cycloadditions. J. Org. Chem. 2011, 76, 7994–8002. [Google Scholar] [CrossRef] [PubMed]
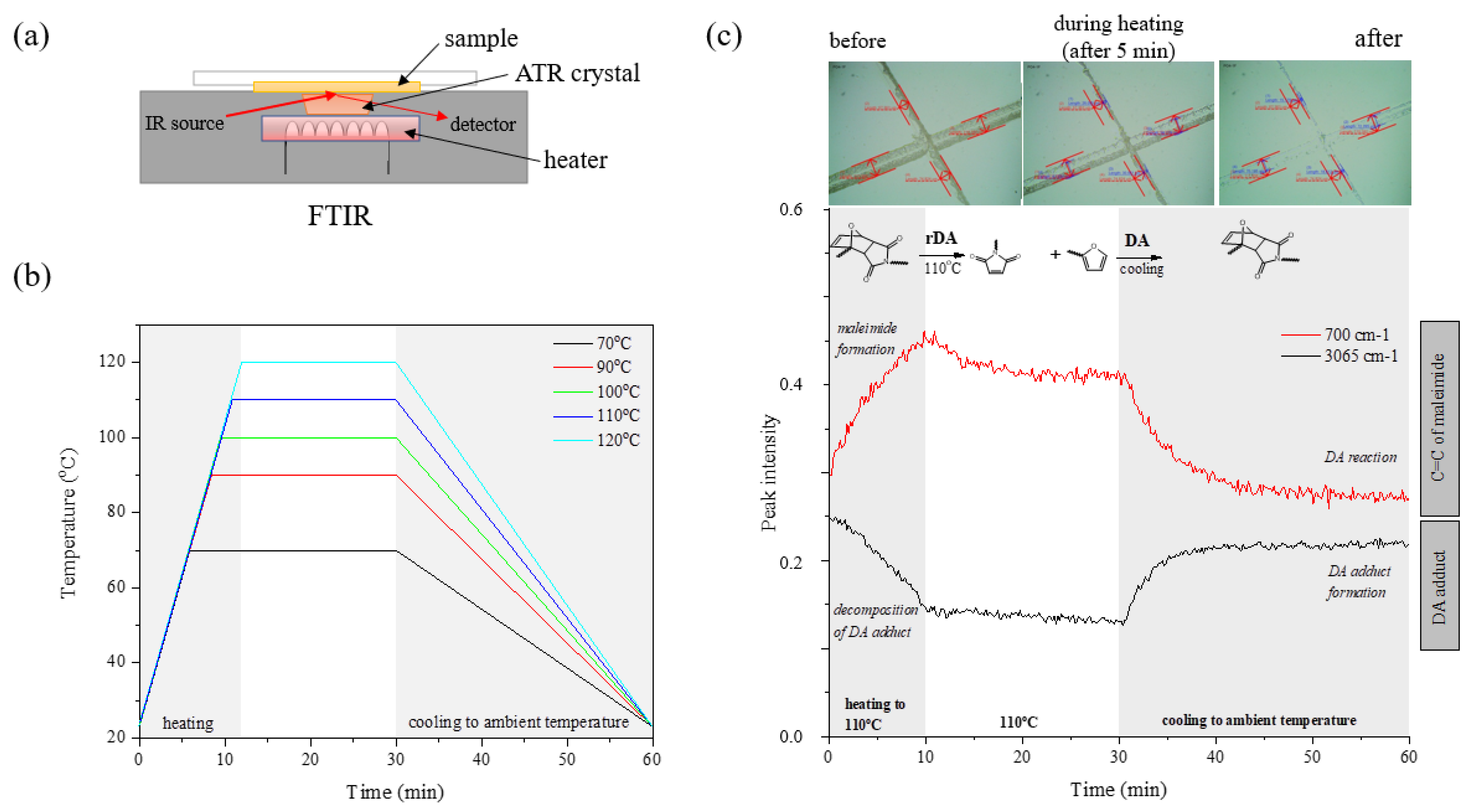
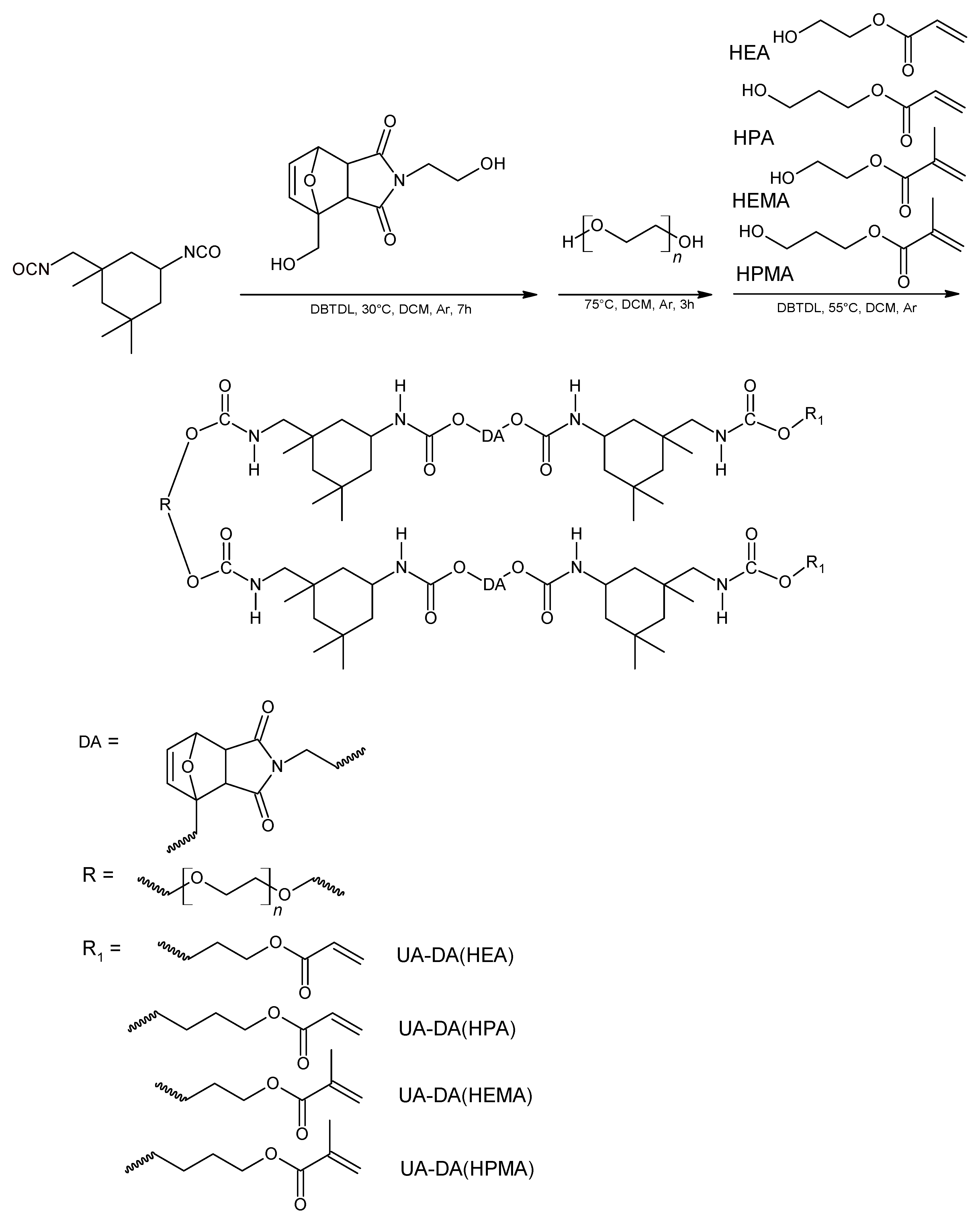
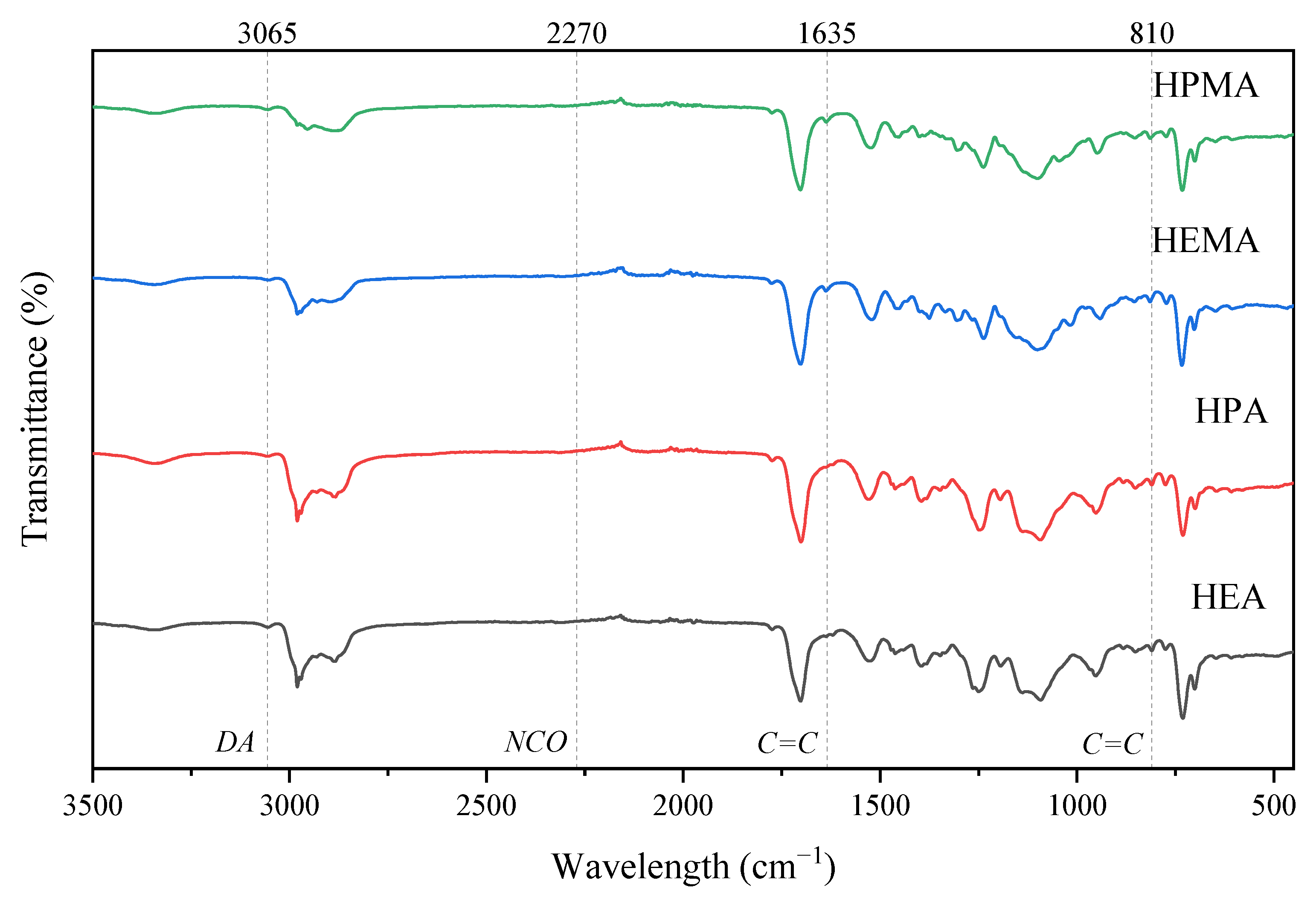
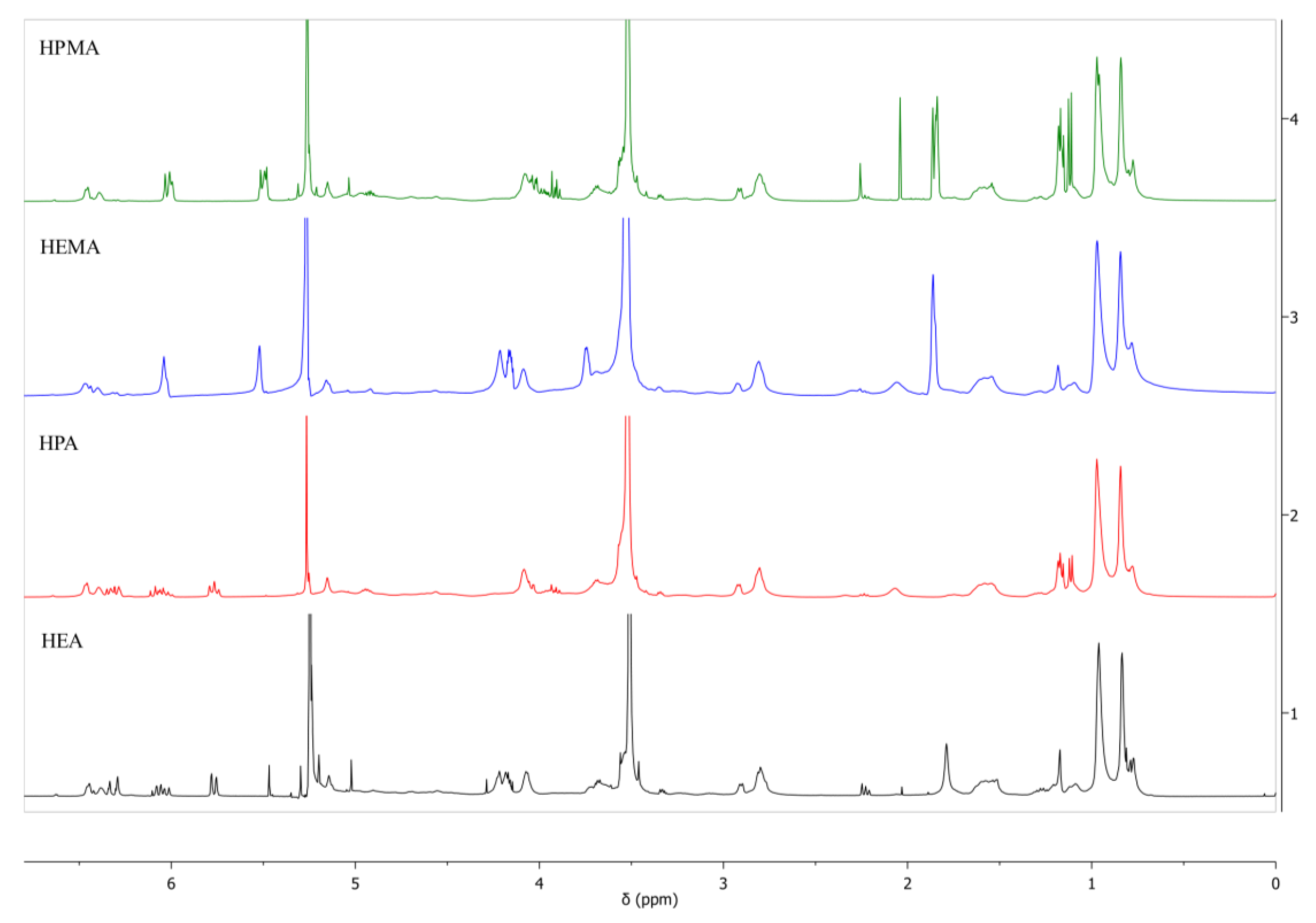




| Sample Code | mHODA [g] | mDD [g] | mIPDI [g] | MPEG | mPEG [g] | mDD [g] | HR(M)A | mHR(M)A | mDD [g] |
|---|---|---|---|---|---|---|---|---|---|
| UA-DA(HEA) | 9.40 | 0.43 | 17.54 | 1000 | 19.73 | 0.28 | HEA | 4.58 | 0.29 |
| UA-DA(HPA) | 7.93 | 0.25 | 14.75 | 1000 | 20.02 | 0.14 | HPA | 5.20 | 0.17 |
| UA-DA(HEMA) | 6.51 | 0.11 | 12.19 | 1000 | 13.70 | 0.13 | HEMA | 3.58 | 0.13 |
| UA-DA(HPMA) | 9.07 | 0.21 | 16.93 | 1000 | 19.03 | 0.22 | HPMA | 5.49 | 0.27 |
| Sample Code | ΔHtotal (J/g) | tmax (s) | α | DCmax (%) | Rpmax (%/min) |
|---|---|---|---|---|---|
| UA-DA(HEA) | 466 | 4.2 | 67 | 97 | 78 |
| UA-DA(HPA) | 139 | 8.4 | 42 | 89 | 61 |
| UA-DA(HEMA) | 278 | 4.2 | 68 | 91 | 135 |
| UA-DA(HPMA) | 204 | 8.4 | 50 | 86 | 71 |
| Sample Code | Tack-Free Time (s) | Hardness | Adhesion | Gloss (GU) | Yellowness Index |
|---|---|---|---|---|---|
| UA-DA(HEA) | 3 | 86 | 2.0 | 215 | 4.5 |
| UA-DA(HPA) | 6 | 58 | 1.0 | 166 | 5.5 |
| UA-DA(HEMA) | 6 | 190 | 1.5 | 140 | 7.5 |
| UA-DA(HPMA) | 6 | 111 | 1.5 | 151 | 3.6 |
| Sample Code | TIDT [°C] | TMDT [°C] |
|---|---|---|
| UA-DA(HEA) | 274.7 | 303.9 |
| UA-DA(HPA) | 307.5 | 332.3 |
| UA-DA(HEMA) | 294.1 | 326.2 |
| UA-DA(HPMA) | 304.7 | 326.9 |
| Sample Code | 70 °C | 90 °C | 100 °C | 110 °C | 120 °C | |
|---|---|---|---|---|---|---|
| UA-DA (HEA) | before |   | ||||
| after | ||||||
| UA-DA (HPA) | before |   | ||||
| after | ||||||
| UA-DA (HEMA) | before |   | ||||
| after | ||||||
| UA-DA (HPA) | before |   | ||||
| after | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarczyk, P.; Mozelewska, K.; Klebeko, J.; Rokicka, J.; Ossowicz-Rupniewska, P. Impact of the Chemical Structure of Photoreactive Urethane (Meth)Acrylates with Various (Meth)Acrylate Groups and Built-In Diels–Alder Reaction Adducts on the UV-Curing Process and Self-Healing Properties. Polymers 2023, 15, 924. https://doi.org/10.3390/polym15040924
Bednarczyk P, Mozelewska K, Klebeko J, Rokicka J, Ossowicz-Rupniewska P. Impact of the Chemical Structure of Photoreactive Urethane (Meth)Acrylates with Various (Meth)Acrylate Groups and Built-In Diels–Alder Reaction Adducts on the UV-Curing Process and Self-Healing Properties. Polymers. 2023; 15(4):924. https://doi.org/10.3390/polym15040924
Chicago/Turabian StyleBednarczyk, Paulina, Karolina Mozelewska, Joanna Klebeko, Joanna Rokicka, and Paula Ossowicz-Rupniewska. 2023. "Impact of the Chemical Structure of Photoreactive Urethane (Meth)Acrylates with Various (Meth)Acrylate Groups and Built-In Diels–Alder Reaction Adducts on the UV-Curing Process and Self-Healing Properties" Polymers 15, no. 4: 924. https://doi.org/10.3390/polym15040924
APA StyleBednarczyk, P., Mozelewska, K., Klebeko, J., Rokicka, J., & Ossowicz-Rupniewska, P. (2023). Impact of the Chemical Structure of Photoreactive Urethane (Meth)Acrylates with Various (Meth)Acrylate Groups and Built-In Diels–Alder Reaction Adducts on the UV-Curing Process and Self-Healing Properties. Polymers, 15(4), 924. https://doi.org/10.3390/polym15040924








