Variation in Abundance Ratio of Isoprene and Dipentene Produced from Wear Particles Composed of Natural Rubber by Pyrolysis Depending on the Particle Size and Thermal Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Samples
2.2. Morphology and Crosslink Density
2.3. Pyrolysis-Gas Chromatography (Py-GC)
3. Results and Discussion
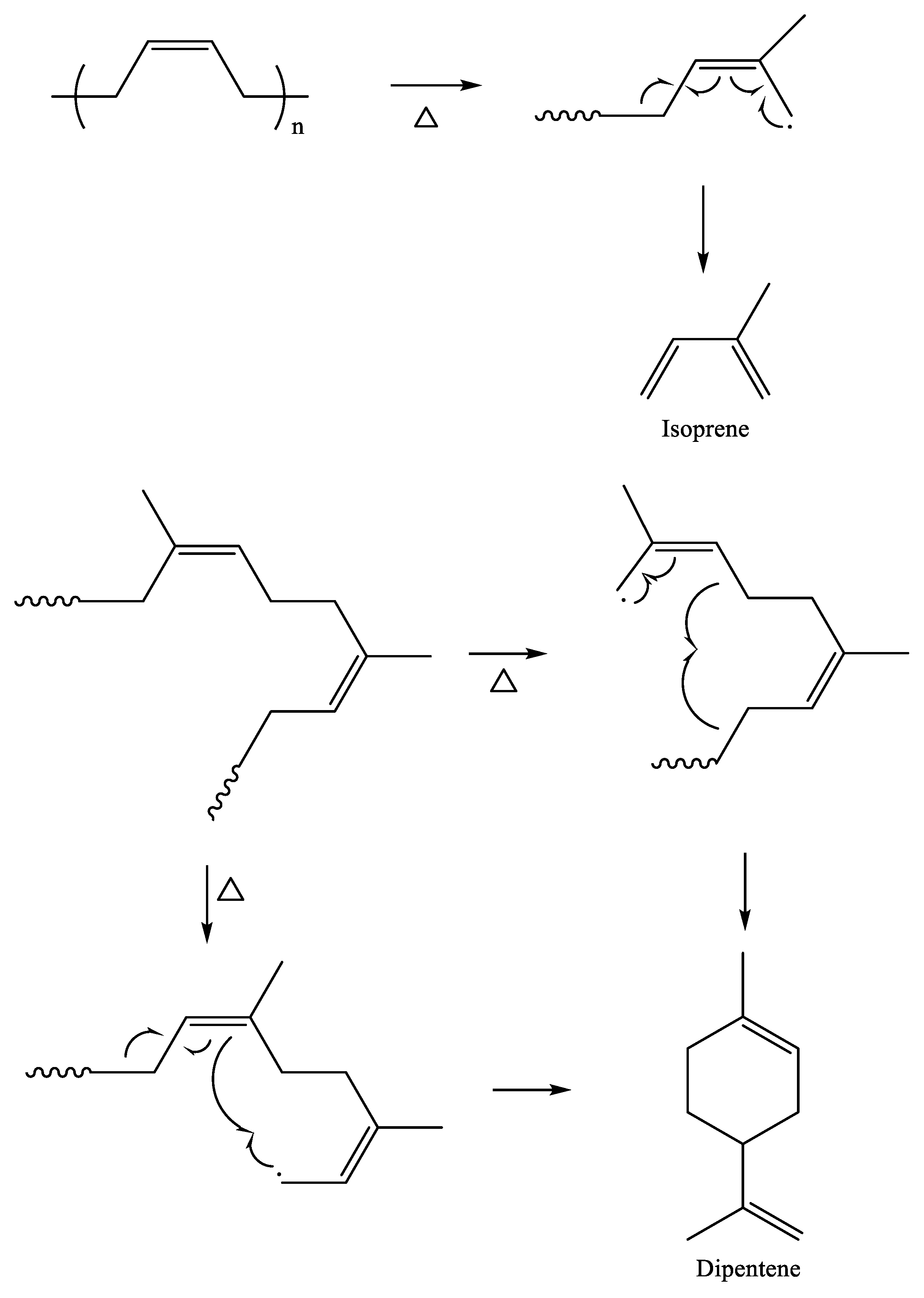
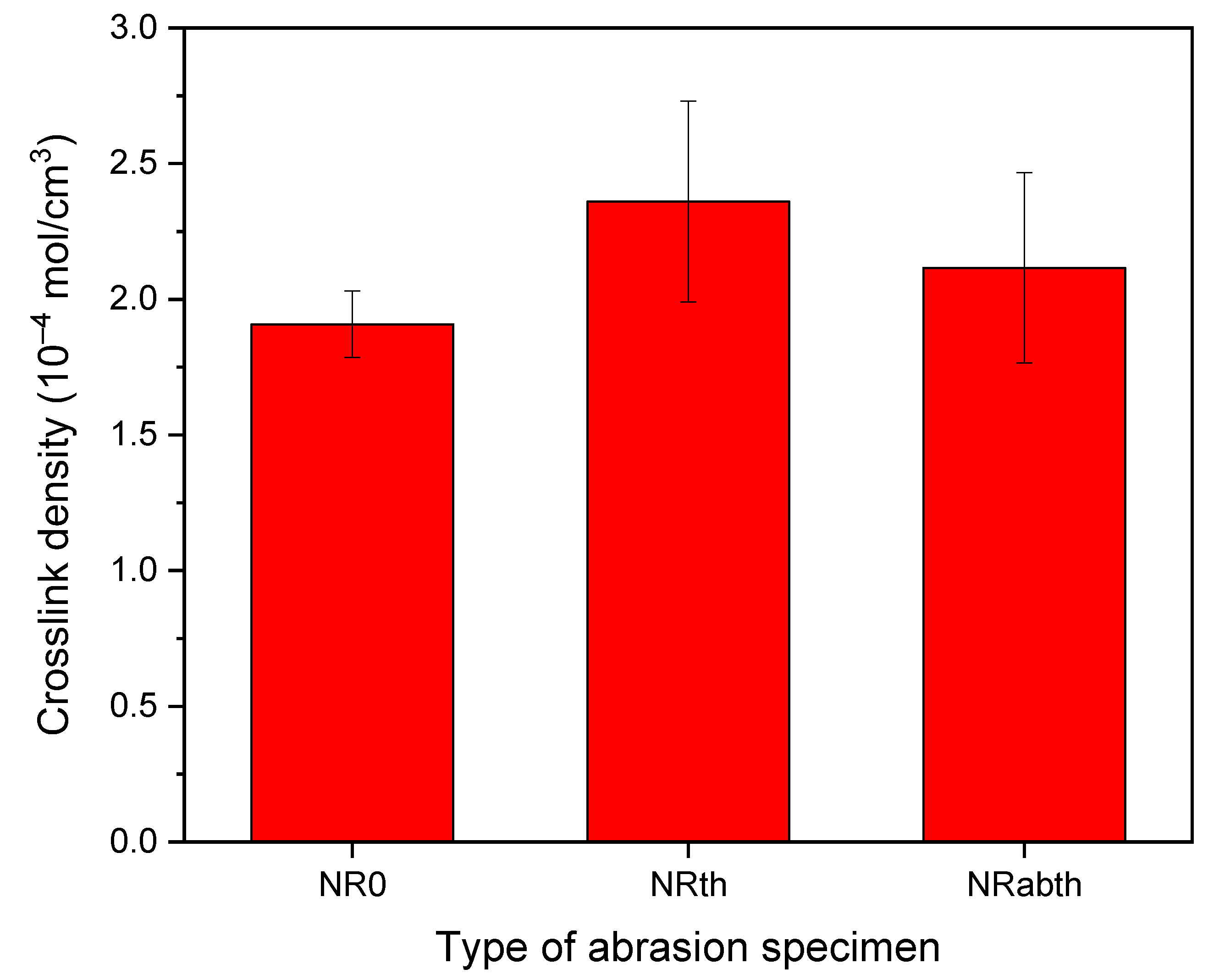
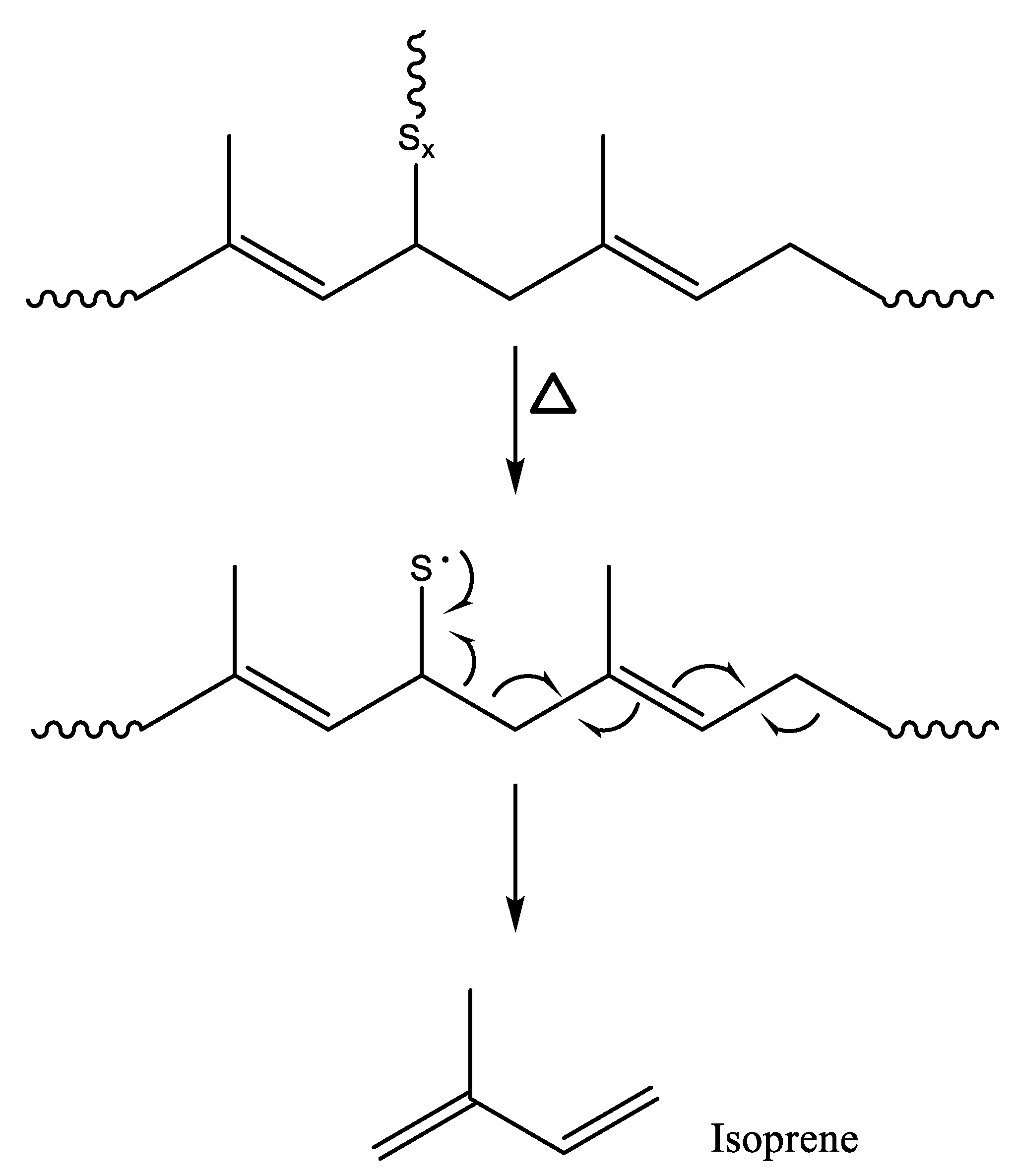
3.1. Wear Particles, Crosslink Density, and Principal Pyrogenic Products
3.2. Variations of the Isoprene/Dipentene Ratios with the Wear Particle Size and Crosslink Density
3.3. Relationship between the Isoprene/Dipentene Ratio and the Wear Particle Size
3.4. Application to PM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kole, P.J.; Löhr, A.J.; Belleghem, F.G.A.J.V.; Ragas, A.M.J. Wear and tear of tyres: A stealthy source of microplastics in the environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Hüffer, T.; Klöckner, P.; Wehrhahn, M.; Hofmann, T.; Reemtsma, T. Tire wear particles in the aquatic environment—A review on generation, analysis, occurrence, fate and effects. Water Resour. Res. 2018, 139, 83–100. [Google Scholar] [CrossRef] [PubMed]
- BaenschN-Baltruschat, B.; Kocher, B.; Stock, F.; Reifferscheid, G. Tyre and road wear particles (TRWP)—A review of generation, properties, emissions, human health risk, ecotoxicity, and fate in the environment. Sci. Total Environ. 2020, 733, 137823. [Google Scholar] [CrossRef] [PubMed]
- Wik, A.; Dave, G. Occurrence and effects of tire wear particles in the environment—A critical review and an initial risk assessment. Environ. Pollut. 2009, 157, 1–11. [Google Scholar] [CrossRef]
- Panko, J.M.; Chu, J.; Kreider, M.L.; Unice, K.M. Measurement of airborne concentrations of tire and road wear particles in urban and rural areas of France, Japan, and the United States. Atmos. Environ. 2013, 72, 192–199. [Google Scholar] [CrossRef]
- Sommer, F.; Dietze, V.; Baum, A.; Sauer, J.; Gilge, S.; Maschowski, C.; Gieré, R. Tire abrasion as a major source of microplastics in the environment. Aerosol Air Qual. Res. 2018, 18, 2014–2028. [Google Scholar] [CrossRef]
- Järlskog, I.; Strömvall, A.-M.; Magnusson, K.; Gustafsson, M.; Polukarova, M.; Galfi, H.; Aronsson, M.; Andersson-Sköld, Y. Occurrence of tire and bitumen wear microplastics on urban streets and in sweepsand and washwater. Sci. Total Environ. 2020, 729, 138950. [Google Scholar] [CrossRef]
- Singh, V.; Biswal, A.; Kesarkar, A.P.; Mor, S.; Ravindra, K. High resolution vehicular PM10 emissions over megacity Delhi: Relative contributions of exhaust and non-exhaust sources. Sci. Total Environ. 2020, 699, 134273. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, J.; Song, C.; Ma, C.; Men, Z.; Wu, J.; Wu, L.; Wang, T.; Zhang, X.; Tao, S.; et al. Vehicular non-exhaust particulate emissions in Chinese megacities: Source profiles, real-world emission factors, and inventories. Environ. Pollut. 2020, 266, 115268. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, L.; Liu, L.; Wen, S. Investigation of the external conditions and material compositions affecting the formation mechanism and size distribution of tire wear particles. Atmos. Environ. 2021, 244, 118018. [Google Scholar] [CrossRef]
- Goßmann, I.; Halbach, M.; Scholz-Böttcher, B.M. Car and truck tire wear particles in complex environmental samples—A quantitative comparison with “traditional” microplastic polymer mass loads. Sci. Total Environ. 2021, 773, 145667. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Kim, W.; Bae, S.; Kim, J. Influence of loading procedure of liquid butadiene rubber on properties of silica-filled tire tread compounds. Elast. Compos. 2022, 57, 129–137. [Google Scholar]
- Ryu, G.; Kim, D.; Song, S.; Lee, H.H.; Ha, J.U.; Kim, W. Wear particulate matters and physical properties of ENR/BR tread compounds with different ratio of silica and carbon black binary filler systems. Elast. Compos. 2021, 56, 234–242. [Google Scholar]
- Kim, D.; Ahn, B.; Ryu, G.; Hwang, K.; Song, S.; Kim, W. Effect of vinyl group content of the functionalized liquid butadiene rubber as a processing aid on the properties of silica filled rubber compounds. Elast. Compos. 2021, 56, 152–163. [Google Scholar]
- Ryu, G.; Kim, D.; Song, S.; Hwang, K.; Kim, W. Effect of molecular weight of epoxidized liquid isoprene rubber as a processing aid on the vulcanizate structure of silica filled NR compounds. Elast. Compos. 2021, 56, 223–233. [Google Scholar]
- Hess, W.M.; Vegvari, P.G.; Swor, R.A. Carbon black in NR/BR blends for truck tires. Rubber Chem. Technol. 1985, 58, 350–382. [Google Scholar] [CrossRef]
- Bisschop, R.; Grunert, F.; Llisch, S.; Stratton, T.; Blume, A. Influence of molecular properties of SSBR and BR types on composite performance. Polym. Test. 2021, 99, 107219. [Google Scholar] [CrossRef]
- Rodgers, B.; Waddell, W. The Science of rubber compounding. Sci. Tech. Rubber 2005, 401–454. [Google Scholar] [CrossRef]
- Coran, A.Y. Vulcanization. part VII. kinetics of sulfur vulcanization of natural rubber in presence of delayed-action accelerators. Rubber Chem. Technol. 1965, 38, 1–14. [Google Scholar] [CrossRef]
- Kulkarni, A.; Pugh, C.; Jana, S.C.; Wims, D.T.; Gawad, A.A. Crosslinking of SBR compounds for tire tread using benzocyclobutene chemictry. Rubber Chem. Technol. 2019, 92, 25–42. [Google Scholar] [CrossRef]
- Han, S.; Ryu, G.; Kim, S.; Kim, J.; Mun, D.; Morita, K.; Kim, W. Effect of silane and sulfur variation on the vulcanizate structure of silica-filled styrene-butadiene rubber compounds. Elast. Compos. 2021, 56, 32–42. [Google Scholar]
- Chakraborty, S.K.; Bhowmick, A.K.; De, S.K. Mixed cross-link systems in elastomers. J. Macromol. Sci. 1981, 21, 313–332. [Google Scholar] [CrossRef]
- Zhao, F.; Bi, W.; Zhao, S. Influence of crosslink density on mechanical properties of natural rubber vulcanizates. J. Macromol. Sci. 2011, 50, 1460–1469. [Google Scholar] [CrossRef]
- Hamed, G.R.; Zhao, J. Tensile behavior after oxidative aging of gum and black-filled vulcanizates of SBR and NR. Rubber Chem. Technol. 1999, 72, 721–730. [Google Scholar] [CrossRef]
- Jitkarnka, S.; Chusaksri, B.; Supaphol, P.; Magaraphan, R. Influences of thermal aging on properties and pyrolysis products of tire tread compound. J. Anal. Appl. Pyrolysis 2007, 80, 269–276. [Google Scholar] [CrossRef]
- Rial-Otero, R.; Galesio, M.; Capelo, J.-L.; Simal-Gándara, J. A review of synthetic polymer characterization by pyrolysis–GC–MS. Chromatographia 2009, 70, 339–348. [Google Scholar] [CrossRef]
- Chae, E.; Choi, S.-S. Analytical method for determination of microstructure of SBR and SBR content in blended rubber composites using pyrolytic technique. Elast. Compos. 2022, 57, 188–196. [Google Scholar]
- Bart, J.C.J. Polymer/additive analysis by flash pyrolysis techniques. J. Anal. Appl. Pyrolysis 2001, 58–59, 3–28. [Google Scholar] [CrossRef]
- Blazsó, M. Recent trends in analytical and applied pyrolysis of polymers. J. Anal. Appl. Pyrolysis 1997, 39, 1–25. [Google Scholar] [CrossRef]
- Kumagai, S.; Yoshioka, T. Latest trends in pyrolysis gas chromatography for analytical and applied pyrolysis of plastics. Anal. Sci. 2021, 37, 145–157. [Google Scholar] [CrossRef]
- Groves, S.A.; Lehrle, R.S.; Blazsó, M.; Székely, T. Natural rubber pyrolysis: Study of temperature-and thickness-dependence indicates dimer formation mechanism. J. Anal. Appl. Pyrolysis 1991, 19, 301–309. [Google Scholar] [CrossRef]
- Unice, K.M.; Kreider, M.L.; Panko, J.M. Use of a deuterated internal standard with pyrolysis-GC/MS dimeric marker analysis to quantify tire tread particles in the environment. Int. J. Environ. Res. Public Health 2012, 9, 4033–4055. [Google Scholar] [CrossRef]
- Murillo, J.D.; Ware, E.A.; Biernacki, J.J. Characterization of milling effects on the physical and chemical nature of herbaceous biomass with comparison of fast pyrolysis product distributions using Py-GC/MS. J. Anal. Appl. Pyrolysis 2014, 108, 234–247. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Magdziarz, A.; Dziok, T.; Sieradzka, M.; Nowak, W. Laboratory studies on the influence of biomass particle size on pyrolysis and combustion using TG GC/MS. Fuel 2019, 252, 635–645. [Google Scholar] [CrossRef]
- Groves, S.; Lehrle, R. Pyrolysis mechanisms of natural rubber deduced from the dependence of product yields on sample size. Eur. Polym. J. 1992, 28, 373–378. [Google Scholar] [CrossRef]
- ISO/TS 20593; Ambient Air—Determination of the Mass Concentration of Tire and Road Wear Particles (TRWP)—Pyrolysis-GC/MS Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- Mun, S.; Chong, H.; Lee, J.; Lim, Y. Characteristics of real-world non-exhaust particulates from vehicles. Energies 2023, 16, 177. [Google Scholar] [CrossRef]
- Choi, S.-S. Correlation of crosslink density with pyrolysis pattern of natural rubber vulcanizates with efficient vulcanizing cure system. J. Anal. Appl. Pyrolysis 1999, 52, 105–112. [Google Scholar] [CrossRef]
- Kreider, M.L.; Panko, J.M.; McAtee, B.L.; Sweet, L.I.; Finley, B.L. Physical and chemical characterization of tire-related particles: Comparison of particles generated using different methodologies. Sci. Total Environ. 2010, 408, 652–659. [Google Scholar] [CrossRef]
- Kovochich, M.; Parker, J.A.; Oh, S.C.; Lee, J.P.; Wagner, S.; Reemtsma, T.; Unice, K.M. Characterization of individual tire and road wear particles in environmental road dust, tunnel dust, and sediment. Environ. Sci. Technol. Lett. 2021, 8, 1057–1064. [Google Scholar] [CrossRef]
- Choi, S.-S.; Kim, J.-C.; Lee, S.G.; Joo, Y.L. Influence of the cure systems on long time thermal aging behaviors of NR composites. Macromol. Res. 2008, 16, 561–566. [Google Scholar] [CrossRef]
- Choi, S.-S.; Ha, S.-H.; Woo, C.-S. Thermal aging behaviors of rubber vulcanizates cured with single and binary cure systems. Bull. Korean Chem. Soc. 2006, 27, 429–431. [Google Scholar]
- Choi, S.-S.; Kim, J.-C.; Woo, C.-S. Accelerated thermal aging behaviors of EPDM and NBR vulcanizates. Bull. Korean Chem. Soc. 2006, 27, 936–938. [Google Scholar]
- Moon, B.; Jun, N.; Park, S.; Seok, C.-S.; Hong, U.S. A study on the modified Arrhenius equation using the oxygen permeation block model of crosslink structure. Polymers 2019, 11, 136. [Google Scholar] [CrossRef]
- Woo, C.-S.; Choi, S.-S. Heat-aging effect on the material properties and useful life prediction of rubber materials. Key Eng. Mater. 2007, 353–358, 2640–2643. [Google Scholar] [CrossRef]
- Choi, S.-S.; Kwon, H.-M.; Kim, Y.; Bae, J.W.; Kim, J.-S. Characterization of maleic anhydride-grafted ethylene-propylene-diene terpolymer (MAH-g-EPDM) based thermoplastic elastomers by formation of zinc ionomer. J. Ind. Eng. Chem. 2013, 19, 1990–1995. [Google Scholar] [CrossRef]
- Choi, S.-S.; Kim, J.-C. Lifetime prediction and thermal aging behaviors of SBR and NBR composites using crosslink density changes. J. Ind. Eng. Chem. 2012, 18, 1166–1170. [Google Scholar] [CrossRef]
- Choi, S.-S.; Han, D.-H. Strain effect on recovery behaviors from circular deformation of natural rubber vulcanizate. J. Appl. Polym. Sci. 2009, 114, 935–939. [Google Scholar] [CrossRef]
- Flory, P.J. Statistical mechanics of swelling of network structures. J. Chem. Phys. 1950, 18, 108–111. [Google Scholar] [CrossRef]
- Son, C.E.; Choi, S.-S. Analytical techniques for measurement of crosslink densities of rubber vulcanizates. Elast. Compos. 2019, 54, 209–219. [Google Scholar]
- Moon, B.; Lee, J.; Park, S.; Seok, C.-S. Study on the aging behavior of natural rubber/butadiene rubber (NR/BR) blends using a parallel spring model. Polymers 2018, 10, 658. [Google Scholar] [CrossRef]
- Choi, S.-S. Influence of thermal aging on change of crosslink density and deformation of natural rubber vulcanizates. Bull. Korean Chem. Soc. 2000, 21, 628–634. [Google Scholar]
- Wei, Q.; Yang, D.; Yu, L.; Zhang, L. Grafting of epoxidized natural rubber chains with BN platelets to obtain flexible and thermally conductive papers. Compos. Sci. Technol. 2021, 212, 108881. [Google Scholar] [CrossRef]
- Lim, L.P.; Juan, J.C.; Huang, N.M.; Goh, L.K.; Leng, F.P.; Loh, Y.Y. Enhanced tensile strength and thermal conductivity of natural rubber graphene composite properties via rubber-graphene interaction. Mater. Sci. Eng. B 2019, 246, 112–119. [Google Scholar] [CrossRef]
- Choi, S.-S. Variation of pyrolysis pattern of polyisoprene depending on temperature. Bull. Korean Chem. Soc. 1999, 20, 1348–1350. [Google Scholar]
- Choi, S.-S.; Han, D.-H. Pyrolysis paths of polybutadiene depending on pyrolysis temperature. Macromol. Res. 2006, 14, 354–358. [Google Scholar] [CrossRef]
- Choi, S.-S.; Kim, Y.-K. Analysis of 5-ethylidene-2-norbornene in ethylene-propylene-diene terpolymer using pyrolysis-GC/MS. Polym. Test. 2011, 30, 509–514. [Google Scholar] [CrossRef]
- Choi, S.-S.; Kwon, H.-M. Characterization of pyrolysis products formed from styrene-1,2-unit heterosequence of styrene-butadiene copolymer. J. Anal. Appl. Pyrolysis 2013, 99, 1–8. [Google Scholar] [CrossRef]
- Choi, S.-S. Bond dissociation of sulfur crosslinks in IR and BR vulcanizates using semi-empirical calculations. Kor. Polym. J. 1997, 5, 39–43. [Google Scholar]
- Chae, E.; Choi, S.-S. Analysis of polymeric components in particulate matter using pyrolysis-gas chromatography/mass spectrometry. Polymers 2022, 14, 3122. [Google Scholar] [CrossRef]
- Hesse, D.; Feißel, T.; Kunze, M.; Bachmann, E.; Bachmann, T.; Gramstat, S. Comparison of methods for sampling particulate emissions from tires under different test environments. Atmosphere 2022, 13, 1262. [Google Scholar] [CrossRef]
- Foitzik, M.-J.; Unrau, H.-J.; Gauterin, F.; Dornhofer, J.; Koch, T. Investigation of ultra fine particulate matter emission of rubber tires. Wear 2018, 394–395, 87–95. [Google Scholar] [CrossRef]
- Panko, J.M.; Hitchcock, K.M.; Fuller, G.W.; Green, D. Evaluation of tire wear contribution to PM2.5 in urban environments. Atmosphere 2019, 10, 99. [Google Scholar] [CrossRef]





| Sample Code | Pre-Abrasion | Thermal Aging |
|---|---|---|
| NR0 | No | No |
| NRth | No | Yes |
| NRabth | Yes | Yes |
| Sample | Sieve Size (μm) | ||||
|---|---|---|---|---|---|
| 63–106 | 106–212 | 212–500 | 500–1000 | >1000 | |
| NR0 | 2.6–3.3 (2.8) | 5.3 | 30.4 | 206.7 | 268.0 |
| 2.3–2.4 (2.3) | 5.8 | 37.7 | 132.8 | 349.0 | |
| 1.9–2.3 (2.1) | 5.6 | 23.3 | 161.1 | 386.0 | |
| 1.9–2.5 (2.2) | 8.3 | 33.4 | 117.2 | 329.6 | |
| 3.1–4.4 (3.6) | 4.4 | 40.3 | 231.5 | 421.9 | |
| NRth | 1.3–1.6 (1.4) | 2.5 | 17.2 | 95.1 | 224.6 |
| 1.1–1.8 (1.4) | 4.5 | 21.5 | 123.3 | 249.0 | |
| 1.1–1.5 (1.3) | 2.8 | 32.5 | 116.4 | 374.1 | |
| 0.7–0.9 (0.9) | 4.2 | 41.6 | 195.2 | 407.7 | |
| 0.9–1.2 (1.1) | 2.9 | 37.1 | 180.2 | 468.8 | |
| NRabth | 1.1–1.4 (1.2) | 6.1 | 34.6 | 65.2 | 457.3 |
| 1.5–2.0 (1.8) | 4.8 | 32.7 | 166.9 | 529.9 | |
| 1.0–1.3 (1.1) | 4.3 | 49.5 | 92.5 | 490.0 | |
| 1.2–1.7 (1.3) | 3.3 | 37.0 | 153.9 | 346.7 | |
| 1.5–1.8 (1.6) | 3.0 | 24.9 | 111.5 | 243.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, U.; Choi, S.-S. Variation in Abundance Ratio of Isoprene and Dipentene Produced from Wear Particles Composed of Natural Rubber by Pyrolysis Depending on the Particle Size and Thermal Aging. Polymers 2023, 15, 929. https://doi.org/10.3390/polym15040929
Jung U, Choi S-S. Variation in Abundance Ratio of Isoprene and Dipentene Produced from Wear Particles Composed of Natural Rubber by Pyrolysis Depending on the Particle Size and Thermal Aging. Polymers. 2023; 15(4):929. https://doi.org/10.3390/polym15040929
Chicago/Turabian StyleJung, Uiyeong, and Sung-Seen Choi. 2023. "Variation in Abundance Ratio of Isoprene and Dipentene Produced from Wear Particles Composed of Natural Rubber by Pyrolysis Depending on the Particle Size and Thermal Aging" Polymers 15, no. 4: 929. https://doi.org/10.3390/polym15040929
APA StyleJung, U., & Choi, S.-S. (2023). Variation in Abundance Ratio of Isoprene and Dipentene Produced from Wear Particles Composed of Natural Rubber by Pyrolysis Depending on the Particle Size and Thermal Aging. Polymers, 15(4), 929. https://doi.org/10.3390/polym15040929






