A Robust Process to Produce Lignocellulosic Nanofibers from Corn Stover, Reed Canary Grass, and Industrial Hemp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
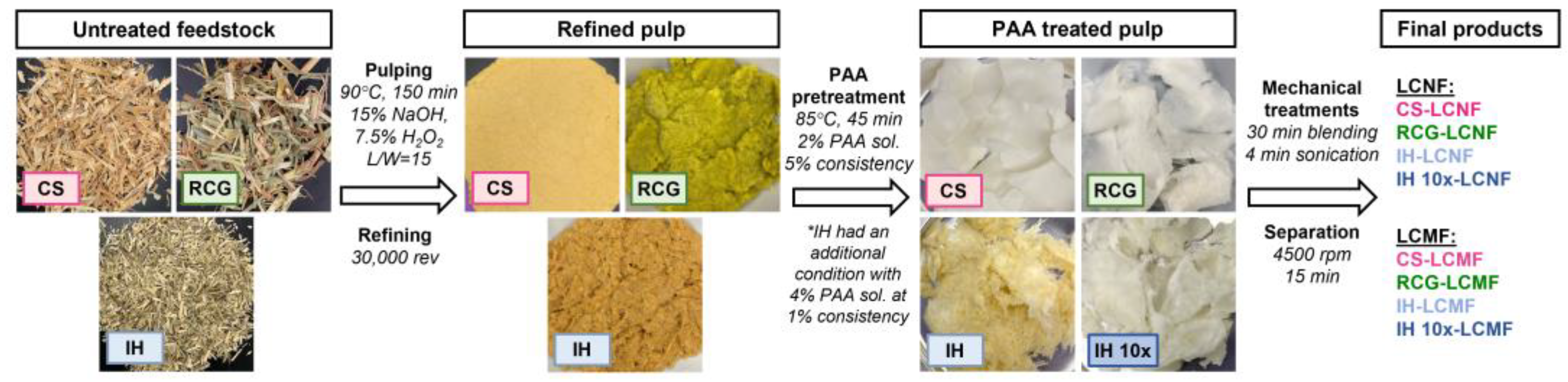
2.2. Alkaline Peroxide Pulping
2.3. Peracetic Acid (PAA) Pretreatment
2.4. Lignocellulosic Nanofibers Production
2.5. Characterization Techniques
2.5.1. Chemical Composition
2.5.2. Mass Yield and Component Recovery after Pulping and Pretreatment
2.5.3. Product Separation Yield
2.5.4. Lignocellulosic Nanofibers Characterization
3. Results and Discussion
3.1. Effect of Chemical Treatments on Mass Yield and Chemical Composition
3.2. Nanofibers Characterization
3.2.1. Suspension Optical Transmittance
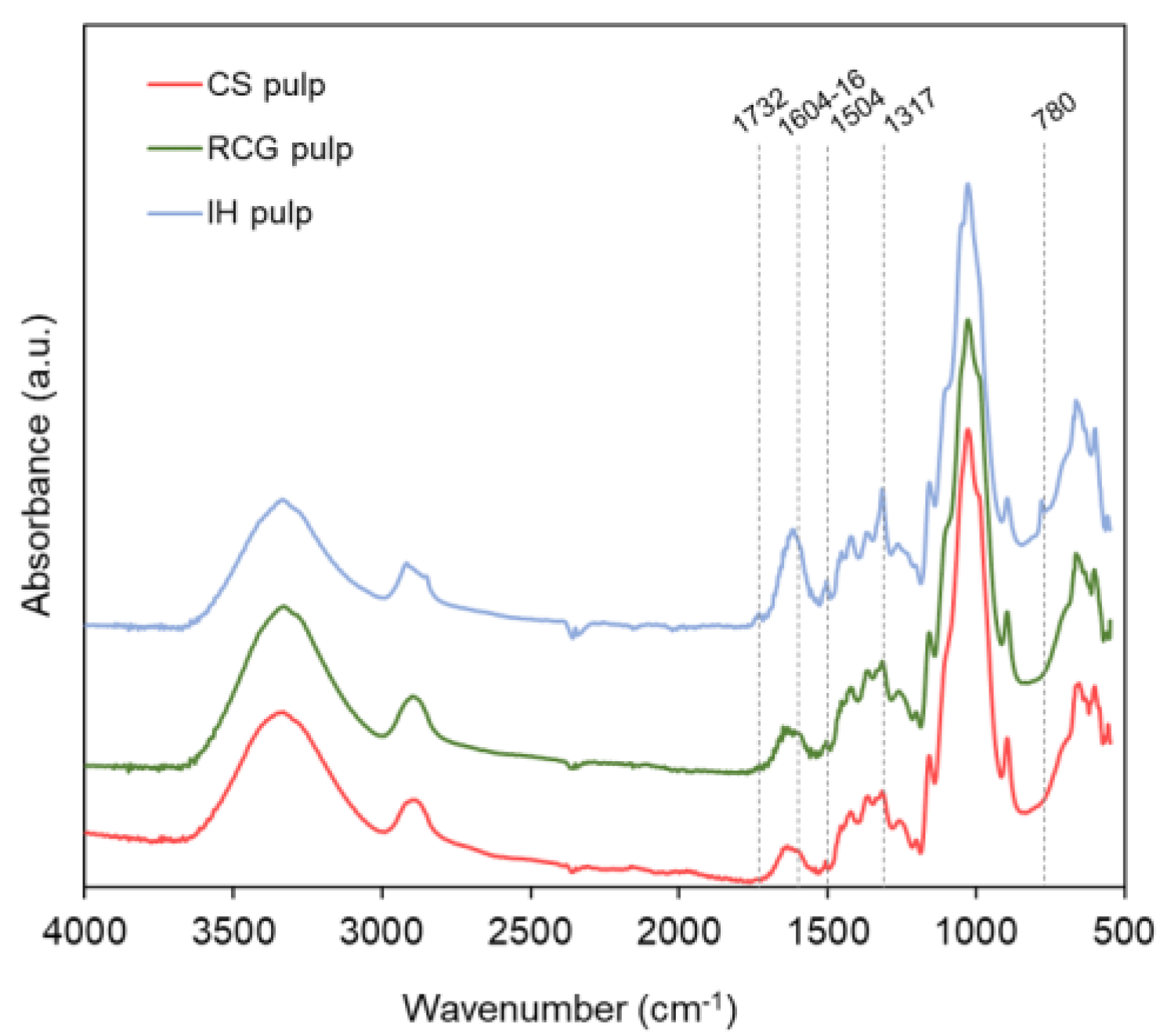
3.2.2. Surface Chemistry
3.2.3. Fiber Structure and Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, S.; Sun, J.; Shi, Y.; Wang, Q.; Wu, J.; Liu, J. Nanocellulose from Various Biomass Wastes: Its Preparation and Potential Usages towards the High Value-Added Products. Environ. Sci. Ecotechnol. 2021, 5, 100077. [Google Scholar] [CrossRef]
- Balea, A.; Fuente, E.; Monte, M.C.; Merayo, N.; Campano, C.; Negro, C.; Blanco, A. Industrial Application of Nanocelluloses in Papermaking: A Review of Challenges, Solutions, and Market Perspectives. Molecules 2020, 25, 526–556. [Google Scholar] [CrossRef]
- Nanografi Cellulose Nanofiber. Available online: https://nanografi.com/popular-products/cellulose-nanofiber-cellulose-nanofibril-nanofibrillated-cellulose-cnfs/ (accessed on 8 November 2021).
- de Assis, C.A.; Iglesias, M.C.; Bilodeau, M.; Johnson, D.; Phillips, R.; Peresin, M.S.; Bilek, E.M.; Rojas, O.J.; Venditti, R.A.; Gonzalez, R. Cellulose Micro- and Nanofibrils (CMNF) Manufacturing—Financial and Risk Assessment. Biofuels Bioprod. Biorefining 2018, 12, 251–264. [Google Scholar] [CrossRef]
- Bondancia, T.J.; De Aguiar, J.; Batista, G.; Cruz, A.J.G.; Marconcini, J.M.; Mattoso, L.H.C.; Farinas, C.S. Production of Nanocellulose Using Citric Acid in a Biorefinery Concept: Effect of the Hydrolysis Reaction Time and Techno-Economic Analysis. Ind. Eng. Chem. Res. 2020, 59, 11505–11516. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Mateo, S.; Peinado, S.; Morillas-Gutiérrez, F.; La Rubia, M.D.; Moya, A.J. Nanocellulose from Agricultural Wastes: Products and Applications—A Review. Processes 2021, 9, 1594. [Google Scholar] [CrossRef]
- Solala, I.; Iglesias, M.C.; Peresin, M.S. On the Potential of Lignin-Containing Cellulose Nanofibrils (LCNFs): A Review on Properties and Applications. Cellulose 2020, 27, 1853–1877. [Google Scholar] [CrossRef]
- Liu, K.; Du, H.; Zheng, T.; Liu, W.; Zhang, M.; Liu, H.; Zhang, X.; Si, C. Lignin-Containing Cellulose Nanomaterials: Preparation and Applications. Green Chem. 2021, 23, 9723–9746. [Google Scholar] [CrossRef]
- Chaker, A.; Alila, S.; Mutjé, P.; Vilar, M.R.; Boufi, S. Key Role of the Hemicellulose Content and the Cell Morphology on the Nanofibrillation Effectiveness of Cellulose Pulps. Cellulose 2013, 20, 2863–2875. [Google Scholar] [CrossRef]
- Iwamoto, S.; Abe, K.; Yano, H. The Effect of Hemicelluloses on Wood Pulp Nanofibrillation and Nanofiber Network Characteristics. Biomacromolecules 2008, 9, 1022–1026. [Google Scholar] [CrossRef]
- Rojo, E.; Peresin, M.S.; Sampson, W.W.; Hoeger, I.C.; Vartiainen, J.; Laine, J.; Rojas, O.J. Comprehensive Elucidation of the Effect of Residual Lignin on the Physical, Barrier, Mechanical and Surface Properties of Nanocellulose Films. Green Chem. 2015, 17, 1853–1866. [Google Scholar] [CrossRef]
- Delgado-Aguilar, M.; González, I.; Tarrés, Q.; Pèlach, M.À.; Alcalà, M.; Mutjé, P. The Key Role of Lignin in the Production of Low-Cost Lignocellulosic Nanofibres for Papermaking Applications. Ind. Crops Prod. 2016, 86, 295–300. [Google Scholar] [CrossRef]
- Pennells, J.; Godwin, I.D.; Amiralian, N.; Martin, D.J. Trends in the Production of Cellulose Nanofibers from Non-Wood Sources. Cellulose 2020, 27, 575–593. [Google Scholar] [CrossRef]
- Pascoli, D.U. Nanocellulose Production from Low-Cost Agricultural Wastes: Process Development, Economic Assessment, and Process Robustness. Doctoral Thesis, University of Washington, Seattle, WA, USA, 2022. Unpublished. [Google Scholar]
- Pascoli, D.U.; Dichiara, A.B.; Roumeli, E.; Gustafson, R.; Bura, R. Lignocellulosic Nanomaterials Production from Wheat Straw via Peracetic Acid Pretreatment and Their Application in Plastic Composites. Carbohydr. Polym. 2022, 295, 119857. [Google Scholar] [CrossRef]
- U.S. Department of Energy. US Billion-Ton Update: Biomass Supply for a Bioenergy and Bioproducts Industry. 2011. Available online: https://www.energy.gov/eere/bioenergy/articles/us-billion-ton-update-biomass-supply-bioenergy-and-bioproducts-industry (accessed on 20 December 2022).
- Iowa Corn Promotion Board (ICPB). Sustainable Corn Stover Harvest. 2013. Available online: https://www.iowacorn.org/media/cms/IowaCornResearchBrochure_Final_IFT_F4B608A12ED16.pdf (accessed on 20 December 2022).
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol; National Renewable Energy Lab. 2011. Available online: https://www.nrel.gov/docs/fy11osti/47764.pdf (accessed on 20 December 2022).
- Aden, A.; Ruth, M.; Ibsen, K.; Jechura, J.; Neeves, K.; Sheehan, J.; Wallace, B.; Montague, L.; Slayton, A.; Lukas, J. Lignocellulosic Biomass to Ethanol Process Design and Economics Utilizing Co-Current Dilute Acid Prehydrolysis and Enzymatic Hydrolysis for Corn Stover; National Renewable Energy Lab.: Golden, CO, USA, 2002. [Google Scholar]
- Davis, R.; Grundl, N.; Tao, L.; Biddy, M.J.; Tan, E.C.D.; Beckham, G.T.; Humbird, D.; Thompson, D.N.; Roni, M.S. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbon Fuels and Coproducts: 2018 Biochemical Design Case Update: Biochemical Deconstruction and Conversion of Biomass to Fuels and Products via Integrated Biorefinery Path; National Renewable Energy Lab.: Golden, CO, USA, 2018. [Google Scholar]
- Jensen, E.F.; Casler, M.D.; Farrar, K.; Finnan, J.M.; Lord, R.; Palmborg, C.; Valentine, J.; Donnison, I.S. Reed Canary Grass: From Production Fo End Use. In Perennial Grasses for Bioenergy and Bioproducts; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 2, pp. 153–173. ISBN 9780128129005. [Google Scholar]
- Spyreas, G.; Wilm, B.W.; Plocher, A.E.; Ketzner, D.M.; Matthews, J.W.; Ellis, J.L.; Heske, E.J. Biological Consequences of Invasion by Reed Canary Grass (Phalaris arundinacea). Biol. Invasions 2010, 12, 1253–1267. [Google Scholar] [CrossRef]
- Maurer, D.A.; Lindig-Cisneros, R.; Werner, K.J.; Kercher, S.; Miller, R.; Zedler, J.B. The Replacement of Wetland Vegetation by Reed Canarygrass (Phalaris arundinacea). Ecol. Restor. 2003, 21, 116–119. [Google Scholar] [CrossRef]
- Finell, M. The Use of Reed Canary-Grass (Phalaris arundinacea) as a Short Fibre Raw Material for the Pulp and Paper Industry. Doctoral Thesis, Swedish University of Agricultural Sciences, Umeå, Sweden, 2003. [Google Scholar]
- Marrot, L.; Candelier, K.; Valette, J.; Lanvin, C.; Horvat, B.; Legan, L.; DeVallance, D.B. Valorization of Hemp Stalk Waste Through Thermochemical Conversion for Energy and Electrical Applications. Waste Biomass Valoriz. 2022, 13, 2267–2285. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of Hemp in Textiles, Paper Industry, Insulation and Building Materials, Horticulture, Animal Nutrition, Food and Beverages, Nutraceuticals, Cosmetics and Hygiene, Medicine, Agrochemistry, Energy Production and Environment: A Review. Environ. Chem. Lett. 2020, 18, 1451–1476. [Google Scholar] [CrossRef]
- Correia, F.; Roy, D.N.; Goel, K. Chemistry and Delignification Kinetics of Canadian Industrial Hemp. J. Wood Chem. Technol. 2001, 21, 97–111. [Google Scholar] [CrossRef]
- Pascoli, D.U.; Suko, A.; Gustafson, R.; Gough, H.L.; Bura, R. Novel Ethanol Production Using Biomass Preprocessing to Increase Ethanol Yield and Reduce Overall Costs. Biotechnol. Biofuels 2021, 14, 1–18. [Google Scholar] [CrossRef]
- Dou, C.; Marcondes, W.F.; Djaja, J.E.; Bura, R.; Gustafson, R. Can We Use Short Rotation Coppice Poplar for Sugar Based Biorefinery Feedstock? Bioconversion of 2-Year-Old Poplar Grown as Short Rotation Coppice. Biotechnol. Biofuels 2017, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Bura, R.; Ewanick, S.; Morales-Vera, R. Blending Short Rotation Coppice Poplar with Wheat Straw as a Biorefinery Feedstock in the State of Washington. Ind. Crops Prod. 2019, 132, 407–412. [Google Scholar] [CrossRef]
- Hörhammer, H.; Dou, C.; Suko, A.V.; Gustafson, R.; Bura, R. Removal of Non-Structural Components from Poplar Whole-Tree Chips to Enhance Hydrolysis and Fermentation Performance. Biotechnol. Biofuels 2018, 11, 222. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.O.; Scarlata, C.; Sluiter, J.; Templeton, D.; Energy, D.; Dötsch, A.; Severin, J.; Alt, W.; et al. Determination of Ash in Biomass: Laboratory Analytical Procedure (LAP). 2008; TP-510-42622. Available online: https://www.nrel.gov/docs/gen/fy08/42622.pdf (accessed on 20 December 2022).
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass: Laboratory Analytical Procedure (LAP). 2008; NREL/TP-510-42619. Available online: https://www.nrel.gov/docs/gen/fy08/42619.pdf (accessed on 20 December 2022).
- Espinosa, E.; Bascón-Villegas, I.; Rosal, A.; Pérez-Rodríguez, F.; Chinga-Carrasco, G.; Rodríguez, A. PVA/(Ligno)Nanocellulose Biocomposite Films. Effect of Residual Lignin Content on Structural, Mechanical, Barrier and Antioxidant Properties. Int. J. Biol. Macromol. 2019, 141, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Besbes, I.; Alila, S.; Boufi, S. Nanofibrillated Cellulose from TEMPO-Oxidized Eucalyptus Fibres: Effect of the Carboxyl Content. Carbohydr. Polym. 2011, 84, 975–983. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal Crystallinity Index Revisited by the Simulation of X-ray Diffraction Patterns of Cotton Cellulose Iβ and Cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef]
- Wawro, A.; Batog, J.; Gieparda, W. Chemical and Enzymatic Treatment of Hemp Biomass for Bioethanol Production. Appl. Sci. 2019, 9, 5348. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Y.; Wang, W.; Griffin, J.; Roozeboom, K.; Wang, D. Bioconversion of Industrial Hemp Biomass for Bioethanol Production: A Review. Fuel 2020, 281, 118725. [Google Scholar] [CrossRef]
- Akpan, E.I. Chemistry and Structure of Lignin. In Sustainable Lignin for Carbon Fibers: Principles, Techniques, and Applications; Springer Cham: Gateway East, Singapore, 2019; pp. 1–50. ISBN 9783030187927. [Google Scholar]
- Joachimiak, K.; Wojech, R.; Wójciak, A. Comparison of Miscanthus Giganteus and Birch Wood NSSC Pulping. Part 1: The Effects of Technological Conditions on Certain Pulp Properties. Wood Res. 2019, 64, 49–58. [Google Scholar]
- Kundu, C.; Samudrala, S.P.; Kibria, M.A.; Bhattacharya, S. One-Step Peracetic Acid Pretreatment of Hardwood and Softwood Biomass for Platform Chemicals Production. Sci. Rep. 2021, 11, 11183. [Google Scholar] [CrossRef]
- Kumar, R.; Hu, F.; Hubbell, C.A.; Ragauskas, A.J.; Wyman, C.E. Comparison of Laboratory Delignification Methods, Their Selectivity, and Impacts on Physiochemical Characteristics of Cellulosic Biomass. Bioresour. Technol. 2013, 130, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Wi, S.G.; Cho, E.J.; Lee, D.S.; Lee, S.J.; Lee, Y.J.; Bae, H.J. Lignocellulose Conversion for Biofuel: A New Pretreatment Greatly Improves Downstream Biocatalytic Hydrolysis of Various Lignocellulosic Materials. Biotechnol. Biofuels 2015, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Carvalheiro, F.; Duarte, L.C.; Gírio, F.M. Hemicellulose Biorefineries: A Review on Biomass Pretreatments. J. Sci. Ind. Res. 2008, 67, 849–864. [Google Scholar]
- Gedik, G.; Avinc, O. Bleaching of Hemp (Cannabis sativa L.) Fibers with Peracetic Acid for Textiles Industry Purposes. Fibers Polym. 2018, 19, 82–93. [Google Scholar] [CrossRef]
- Lyu, Q.; Chen, X.; Zhang, Y.; Yu, H.; Han, L.; Xiao, W. One-Pot Fractionation of Corn Stover with Peracetic Acid and Maleic Acid. Bioresour. Technol. 2021, 320, 124306. [Google Scholar] [CrossRef]
- Espinosa, E.; Domínguez-Robles, J.; Sánchez, R.; Tarrés, Q.; Rodríguez, A. The Effect of Pre-Treatment on the Production of Lignocellulosic Nanofibers and Their Application as a Reinforcing Agent in Paper. Cellulose 2017, 24, 2605–2618. [Google Scholar] [CrossRef]
- Oliaei, E.; Lindén, P.A.; Wu, Q.; Berthold, F.; Berglund, L.; Lindström, T. Microfibrillated Lignocellulose (MFLC) and Nanopaper Films from Unbleached Kraft Softwood Pulp. Cellulose 2020, 27, 2325–2341. [Google Scholar] [CrossRef]
- Zhuang, J.; Li, M.; Pu, Y.; Ragauskas, A.J.; Yoo, C.G. Observation of Potential Contaminants in Processed Biomass Using Fourier Transform Infrared Spectroscopy. Appl. Sci. 2020, 10, 4345. [Google Scholar] [CrossRef]
- Gandolfi, S.; Ottolina, G.; Riva, S.; Fantoni, G.P.; Patel, I. Complete Chemical Analysis of Carmagnola Hemp Hurds and Structural Features of Its Components. BioResources 2013, 8, 2641–2656. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.; Xu, J.; Xu, N.; He, Y. Quantitative Visualization of Lignocellulose Components in Transverse Sections of Moso Bamboo Based on Ftir Macro- and Micro-Spectroscopy Coupled with Chemometrics. Biotechnol. Biofuels 2018, 11, 1–16. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Almeida, G.; Perré, P. TGA-FTIR Analysis of Torrefaction of Lignocellulosic Components (Cellulose, Xylan, Lignin) in Isothermal Conditions over a Wide Range of Time Durations. BioResources 2015, 10, 4239–4251. [Google Scholar] [CrossRef] [Green Version]
- Putnina, A.; Kukle, S.; Gravitis, J. STEX Treated and Untreated Hemp Fiber Comparative Structural Analysis. Sci. J. Riga Tech. Univ. Mater. Sci. Text. Cloth. Technol. 2011, 6, 36–42. [Google Scholar]
- Zhao, J.; Xu, Y.; Wang, W.; Griffin, J.; Wang, D. Conversion of Liquid Hot Water, Acid and Alkali Pretreated Industrial Hemp Biomasses to Bioethanol. Bioresour. Technol. 2020, 309, 123383. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Fan, M. Characteristic and Performance of Elementary Hemp Fibre. Mater. Sci. Appl. 2010, 1, 336–342. [Google Scholar] [CrossRef]
- Szymanska-Chargot, M.; Zdunek, A. Use of FT-IR Spectra and PCA to the Bulk Characterization of Cell Wall Residues of Fruits and Vegetables Along a Fraction Process. Food Biophys. 2013, 8, 29–42. [Google Scholar] [CrossRef]
- Bag, R.; Beaugrand, J.; Dole, P.; Kurek, B. Treatment of Chenevotte, a Co-Product of Industrial Hemp Fiber, by Water or Hydrochloric Acid: Impact on Polymer Mobility in the Lignified Cell Walls. J. Wood Sci. 2012, 58, 493–504. [Google Scholar] [CrossRef]
- Petit, J.; Gulisano, A.; Dechesne, A.; Trindade, L.M. Phenotypic Variation of Cell Wall Composition and Stem Morphology in Hemp (Cannabis sativa L.): Optimization of Methods. Front. Plant Sci. 2019, 10, 959. [Google Scholar] [CrossRef]



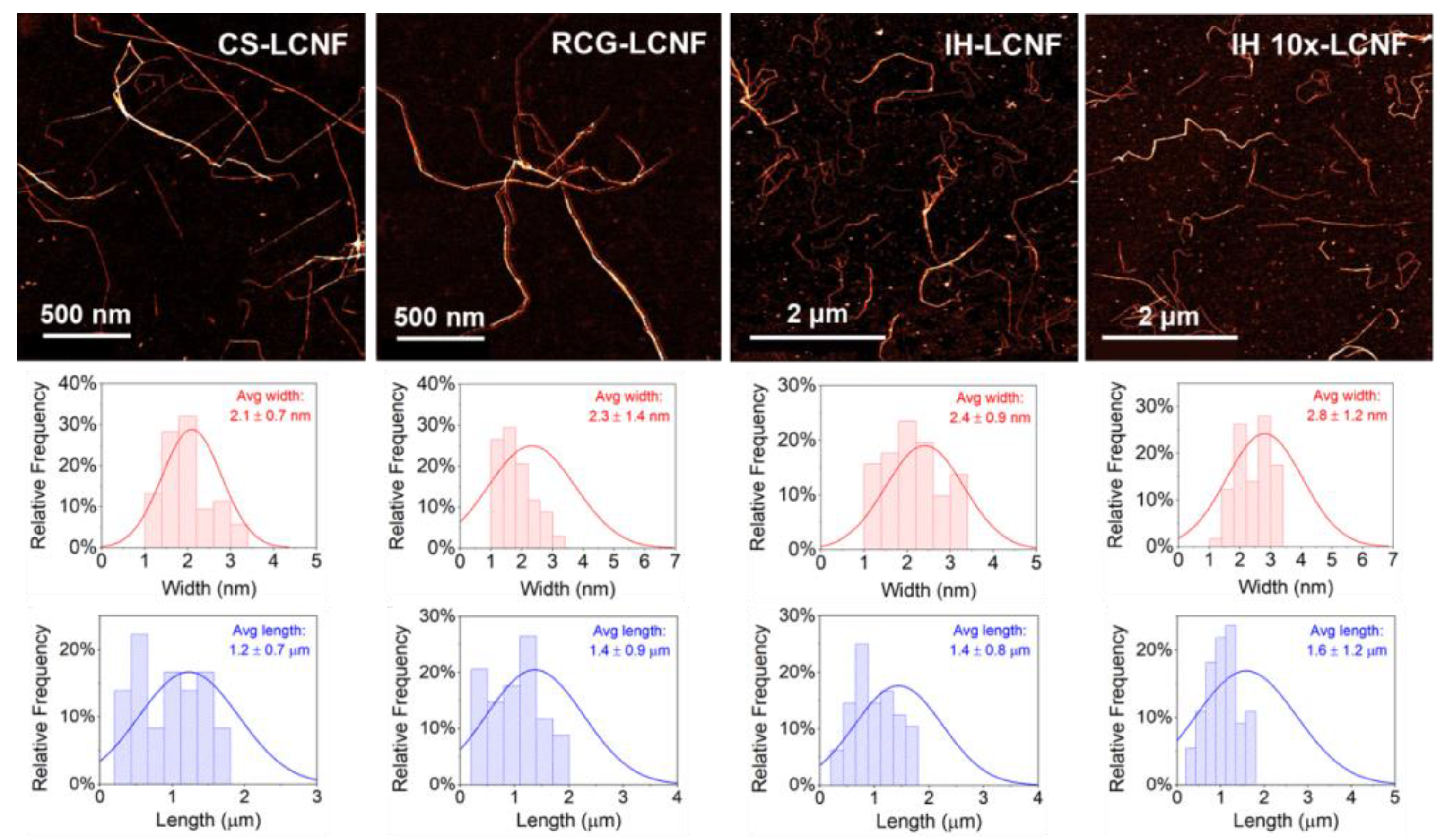
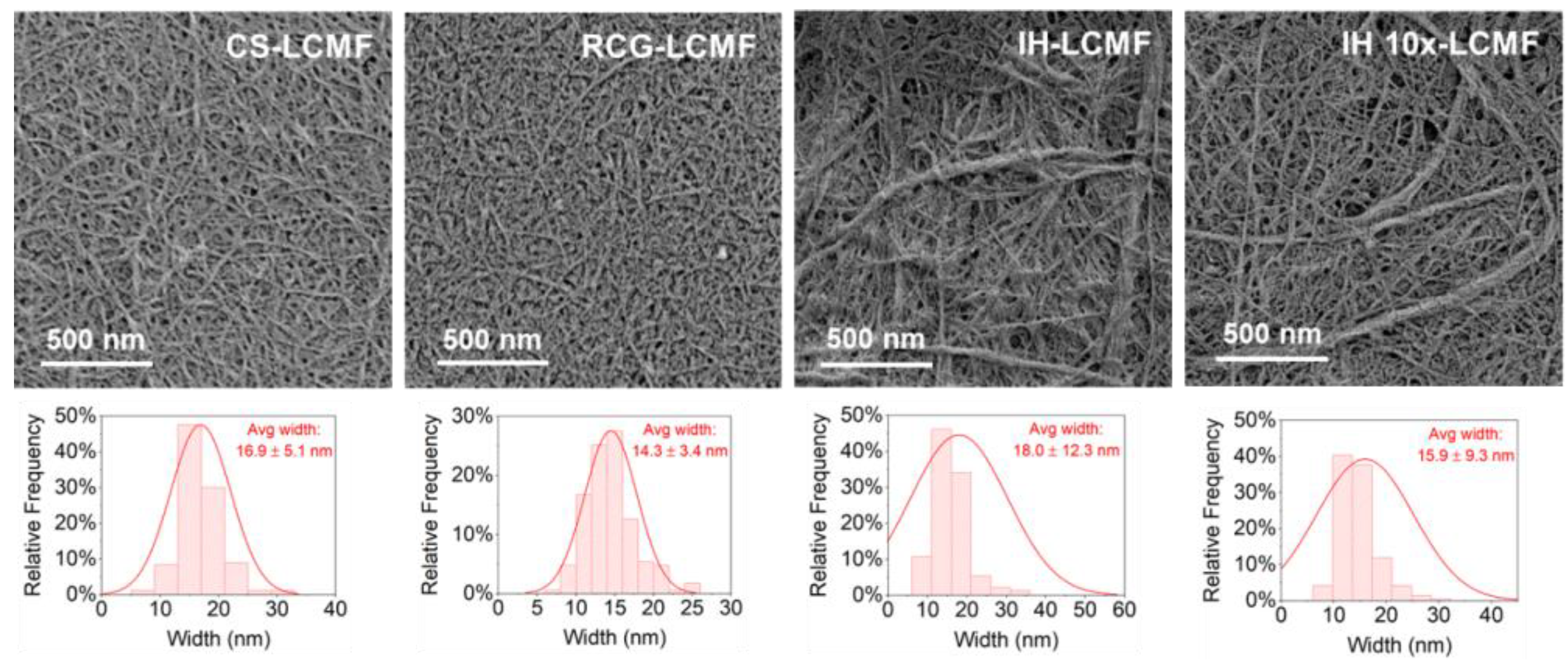
| Separation Yield (%) | CI (%) | Fibril Width (nm) | Fibril Length (μm) | Fibril Aspect Ratio | ||
|---|---|---|---|---|---|---|
| LCNF | CS | 30 | 71 | 2.1 ± 0.7 | 1.2 ± 0.7 | 585 |
| RCG | 34 | 66 | 2.3 ± 1.4 | 1.4 ± 0.9 | 591 | |
| IH | 27 | 64 | 2.4 ± 0.9 | 1.4 ± 0.8 | 599 | |
| IH 10× | 25 | 66 | 2.8 ± 1.2 | 1.6 ± 1.2 | 565 | |
| LCMF | CS | 70 | 72 | 16.9 ± 5.1 | - | - |
| RCG | 66 | 69 | 14.3 ± 3.4 | - | - | |
| IH | 73 | 72 | 18.0 ± 12.3 | - | - | |
| IH 10× | 75 | 75 | 15.9 ± 9.3 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascoli, D.U.; Dichiara, A.; Gustafson, R.; Bura, R. A Robust Process to Produce Lignocellulosic Nanofibers from Corn Stover, Reed Canary Grass, and Industrial Hemp. Polymers 2023, 15, 937. https://doi.org/10.3390/polym15040937
Pascoli DU, Dichiara A, Gustafson R, Bura R. A Robust Process to Produce Lignocellulosic Nanofibers from Corn Stover, Reed Canary Grass, and Industrial Hemp. Polymers. 2023; 15(4):937. https://doi.org/10.3390/polym15040937
Chicago/Turabian StylePascoli, Danielle Uchimura, Anthony Dichiara, Rick Gustafson, and Renata Bura. 2023. "A Robust Process to Produce Lignocellulosic Nanofibers from Corn Stover, Reed Canary Grass, and Industrial Hemp" Polymers 15, no. 4: 937. https://doi.org/10.3390/polym15040937
APA StylePascoli, D. U., Dichiara, A., Gustafson, R., & Bura, R. (2023). A Robust Process to Produce Lignocellulosic Nanofibers from Corn Stover, Reed Canary Grass, and Industrial Hemp. Polymers, 15(4), 937. https://doi.org/10.3390/polym15040937










