Synthesis and Characterization of Solution-Processible Donor-Acceptor Electrochromic Conjugated Copolymers Based on Quinoxalino[2′,3′:9,10]phenanthro[4,5-abc]phenazine as the Acceptor Unit
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Instrumentation
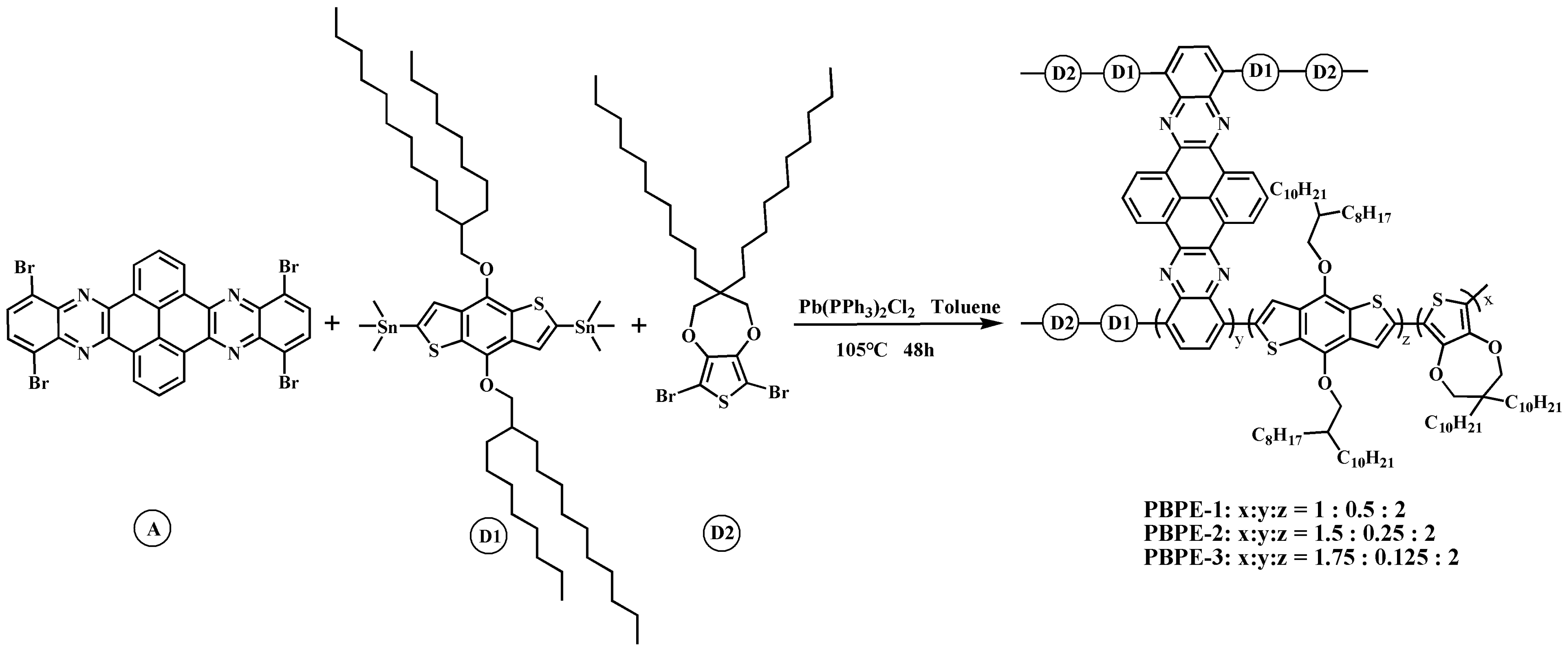
2.3. Synthesis of Monomers and Polymers
2.4. Film Preparation
3. Results and Discussions
3.1. FT-IR Spectra
3.2. XPS Investigation of the Copolymers
3.3. Electrochemical Characterization
3.4. Optical Properties of Polymer Films and Solutions
3.5. Spectroelectrochemical Properties
3.6. Electrochromic Switching Studies
3.7. Colorimetry
3.8. Thermogravimetric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gutiérrez, M.; Duplouy-Armani, L.; Angiolini, L.; Pintado-Sierra, M.; Sánchez, F.; Douhal, A. Femto- to millisecond time-resolved photodynamics of a double-functionalized push-pull organic linker: Potential candidate for optoelectronically active MOFs. Int. J. Mol. Sci. 2020, 21, 4366. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Zhang, G.; Liu, Z. Keep glowing and going: Recent progress in diketopyrrolopyrrole synthesis towards organic optoelectronic materials. Org. Chem. Front. 2021, 8, 4560–4581. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, K.H.; Lim, J.; Lee, J.Y. Interface charge transport of multilayer devices for exact analysis of charge behavior in organic optoelectronic devices. Appl. Phys. Lett. 2021, 118, 203302. [Google Scholar] [CrossRef]
- Park, J.W.; Cho, K.H.; Rhee, Y.M. Mechanism of Ir(ppy)3 guest exciton formation with the exciplex-forming TCTA:TPBI cohost within a phosphorescent organic light-emitting diode environment. Int. J. Mol. Sci. 2022, 23, 5940. [Google Scholar] [CrossRef]
- Cataldo, F. On the optical activity of poly(l-lactic acid) (PLLA) oligomers and polymer: Detection of multiple cotton effect on thin PLLA solid film loaded with two dyes. Int. J. Mol. Sci. 2021, 22, 8. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, L.; Li, Q.; Yin, H.; Shi, Y. The interplay between ESIPT and TADF for the 2,2′-bipyridine-3,3′-diol: A theoretical reconsideration. Int. J. Mol. Sci. 2022, 23, 13969. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.; Boukhili, W.; Wageh, S. Drain-induced barrier lowering effect in organic thin-film transistors based on various organic small molecules: Channel length and drain voltage influences. Synthetic. Met. 2022, 287, 117066. [Google Scholar] [CrossRef]
- Shi, S.; Liao, Q.; Wang, H.; Xiao, G. Narrow bandgap difluorobenzochalcogenadiazole-based polymers for high-performance organic thin-film transistors and polymer solar cells. New J. Chem. 2020, 44, 8032–8043. [Google Scholar] [CrossRef]
- Song, S.; Liu, Y.; Liu, X.; Ge, J.; Ge, D.; Yang, L. Color brightness modulation of a responsive photonic liquid for multicolored electrochromic displays. J. Mater. Chem. C 2022, 10, 3114–3120. [Google Scholar] [CrossRef]
- Advincula, A.A.; Jones, A.L.; Thorley, K.J.; Österholm, A.M.; Ponder, J.F., Jr.; Reynolds, J.R. Probing comonomer selection effects on dioxythiophene-based aqueous-compatible polymers for redox applications. Chem. Mater. 2022, 34, 4633–4645. [Google Scholar] [CrossRef]
- Dyer, A.L.; Thompson, E.J.; Reynolds, J.R. Completing the color palette with spray-processable polymer electrochromics. ACS Appl. Mater. Inter. 2011, 3, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Gaupp, C.L.; Welsh, D.M.; Reynolds, J.R. Poly(ProDOT-Et2): A high-contrast, high-coloration efficiency electrochromic polymer. Macromol. Rapid. Commun. 2002, 23, 885–889. [Google Scholar] [CrossRef]
- Kim, J.; Rémond, M.; Kim, D.; Jang, H.; Kim, E. Electrochromic conjugated polymers for multifunctional smart windows with integrative functionalities. Adv. Mater. Technol. 2020, 5, 1900890. [Google Scholar] [CrossRef]
- Debnath, S.; Bedi, A.; Zade, S.S. Thienopentathiepine: A sulfur containing fused heterocycle for conjugated systems and their electrochemical polymerization. Polym. Chem. 2015, 6, 7658–7665. [Google Scholar] [CrossRef]
- Xu, Z.; Yue, H.; Wang, B.; Zhao, J.; Wang, M.; Zhang, Y.; Xie, Y. Color tuning for black-to-transmissive conjugated copolymer with excellent electrochromic properties via electrochemical copolymerization of two donor-acceptor type monomers. Mater. Des. 2020, 194, 108903. [Google Scholar] [CrossRef]
- Chua, M.H.; Toh, S.H.G.; Ong, P.J.; Png, Z.M.; Zhu, Q.; Xiong, S.; Xu, J. Towards modulating the colour hues of isoindigo-based electrochromic polymers through variation of thiophene-based donor groups. Polym. Chem. 2022, 13, 967–981. [Google Scholar] [CrossRef]
- Neo, W.T.; Ye, Q.; Chua, S.-J.; Xu, J. Conjugated polymer-based electrochromics: Materials, device fabrication and application prospects. J. Mater. Chem. C 2016, 4, 7364–7376. [Google Scholar] [CrossRef]
- Lv, X.; Li, W.; Ouyang, M.; Zhang, Y.; Wright, D.S.; Zhang, C. Polymeric electrochromic materials with donor-acceptor structures. J. Mater. Chem. C 2017, 5, 12–28. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Recent advances in triphenylamine-based electrochromic derivatives and polymers. Polym. Chem. 2018, 9, 3001–3018. [Google Scholar] [CrossRef]
- Debnath, S.; Singh, S.; Bedi, A.; Krishnamoorthy, k.; Zade, S.S. Synthesis, optoelectronic, and transistor properties of BODIPY- and cyclopenta[c]thiophene-containing π-conjugated copolymers. J. Phys. Chem. C. 2015, 119, 15859–15867. [Google Scholar] [CrossRef]
- Debnath, S.; Singh, S.; Bedi, A.; Krishnamoorthy, k.; Zade, S.S. Site-selective synthesis and characterization of BODIPY-acetylene copolymers and their transistor properties. J. Polym. Sci. Pol. Chem. 2016, 54, 1978–1986. [Google Scholar] [CrossRef]
- Debnath, S.; Boyle, C.J.; Zhou, D.; Wong, B.M.; Kittilstved, K.R.; Venkataraman, D. Persistent radical anion polymers based on naphthalenediimide and a vinylene spacer. RSC. Adv. 2018, 8, 14760–14764. [Google Scholar] [CrossRef] [PubMed]
- Beaujuge, P.M.; Reynolds, J.R. Color control in π-conjugated organic polymers for use in electrochromic devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, C.; Lin, H.; Li, M.; Chang, B.; Hsueh, T.; Tsai, B.; Yang, Y.; Wei, K. Binary alloy of functionalized small-molecule acceptors with the A-DA’D-A structure for ternary-blend photovoltaics displaying high open-circuit voltages and efficiencies. J. Mater. Chem. A. 2022, 10, 23037–23046. [Google Scholar] [CrossRef]
- Ming, S.; Li, Z.; Zhen, S.; Liu, P.; Jiang, F.; Nie, G.; Xu, J. High-performance D-A-D type electrochromic polymer with π spacer applied in supercapacitor. Chem. Eng. J. 2020, 390, 124572. [Google Scholar] [CrossRef]
- He, Z.; Xu, H.; Zhang, Y.; Hou, Y.; Niu, H. Conjugated polymers containing EDOT units as novel materials for electrochromic and resistance memory devices. Polymers 2022, 14, 4965. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Wu, C.; Zhang, G.; Wu, Z.; Tang, S.; Lin, Y.; Li, X.; Jiang, Y.; Lin, H.; Wang, Y.; et al. Toward high-performance electrochromic conjugated polymers: Influence of local chemical environment and side-chain engineering. Molecules 2022, 27, 8424. [Google Scholar] [CrossRef] [PubMed]
- Otep, S.; Lin, Y.; Matsumoto, H.; Mori, T.; Wei, K.; Michinobu, T. Diketopyrrolopyrrole-thiophene-methoxythiophene based random copolymers for organic field effect transistor applications. Org. Electron. 2020, 87, 105986. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, G.; Zhao, X.; Kang, W.; Li, M.; Zhang, X.; Yang, H.; Guo, L.; Lin, B. Solution-processable, hypercrosslinked polymer via post-crosslinking for electrochromic supercapacitor with outstanding electrochemical stability. Sol. Energy Mater. Sol. C 2020, 215, 110661. [Google Scholar] [CrossRef]
- Yao, H.; Ye, L.; Zhang, H.; Li, S.; Zhang, S.; Hou, J. Molecular design of benzodithiophene-based organic photovoltaic materials. Chem. Rev. 2016, 116, 7397–7457. [Google Scholar] [CrossRef]
- Goker, S.; Hizalan, G.; Aktas, E.; Kutkan, S.; Cirpan, A.; Toppare, L. 2,1,3-Benzooxadiazole, thiophene and benzodithiophene based random copolymers for organic photovoltaics: Thiophene versus thieno[3,2-b]thiophene as π-conjugated linkers. New J. Chem. 2016, 40, 10455–10464. [Google Scholar] [CrossRef]
- Wakana, S.; Nishiyama, H.; Inagi, S.; Tomita, I. Synthesis of π-conjugated polymer thin films by electropolymerization of benzodithiophene derivatives. Macromol. Chem. Phys. 2017, 218, 1700123. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Li, M.; Tu, L.; Wang, K.; Xiao, D.; Guo, Q.; Zhou, M.; Wei, X.; Shi, Y.; et al. A new dibenzoquinoxalineimide-based wide-bandgap polymer donor for polymer solar cells. Polymers 2022, 14, 3590. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Huang, Y.; Wang, X.; Liang, Z.; Li, J.; Tong, J. Fluorination effect for highly conjugated alternating copolymers involving thienylenevinylene-thiophene-flanked benzodithiophene and benzothiadiazole subunits in photovoltaic application. Polymers 2020, 12, 504. [Google Scholar] [CrossRef]
- Wang, Q.; Zhai, Y.; Chao, D.; Chen, Z.; Jiang, Z. Preparation and electrochromic properties of benzodithiophene-isoindigo conjugated polymers with oligoethylene glycol side chains. Materials 2023, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Durmus, A.; Gunbas, G.E.; Toppare, L. New, highly stable electrochromic polymers from 3,4-ethylenedioxythiophene-bis-substituted quinoxalines toward green polymeric materials. Chem. Mater. 2007, 19, 6247–6251. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, D.; Wang, M.; Kong, L.; Zhao, J. Donor-acceptor type polymers containing the 2,3-bis(2-pyridyl)-5,8-dibromoquinoxalineacceptor and different thiophene donors: Electrochemical, spectroelectrochemistry and electrochromic properties. New J. Chem. 2016, 40, 2178–2188. [Google Scholar] [CrossRef]
- Chu, T.; Ju, X.; Han, X.; Du, H.; Zhang, Y.; Zhao, J.; Zhang, J. Synthesis and electrochromic properties of cross-linked and soluble conjugated polymers based on 5, 8, 14, 17-tetrabromoquinoxaline[2', 3':9, 10]phenanthro[4,5-abc]phenazine as the multifunctionalized acceptor unit. Org. Electron. 2019, 73, 43–54. [Google Scholar] [CrossRef]
- Reenes, B.D.; Grenier, C.R.; Argun, A.A.; Cirpan, A.; Mccarley, T.D.; Reynolds, J.R. Spray coatable electrochromic dioxythiophene polymers with high coloration efficiencies. Macromolecules 2004, 37, 7559–7569. [Google Scholar]
- Xing, X.; Wang, C.; Liu, X.; Qin, L.; Wang, E.; Zhang, F. The trade-off between electrochromic stability and contrast of a thiophene-quinoxaline copolymer. Electrochim. Acta. 2017, 253, 530–535. [Google Scholar] [CrossRef]
- Neo, W.T.; Shi, Z.; Cho, C.M.; Chua, S.-J.; Xu, J. Effects of chemical composition, film thickness, and morphology on the electrochromic properties of donor-acceptor conjugated copolymers based on diketopyrrolopyrrole. ChemPlusChem 2015, 80, 1298–1305. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, B.; Kong, L.; Zhao, J.; Zhang, Y. Color-tuning neutrality for soluble black-to-transmissive electrochromics via solution co-processing. Eur. Polym. J. 2022, 179, 111586. [Google Scholar] [CrossRef]
- Debnath, S.; Masilamani, G.; Agrawal, A.; Kumar, N.R.; Kumar, C.; Zade, S.S.; Bedi, A. Cyclopenta[c]thiophene- and diketopyrrolopyrrole-based red-green-blue electrochromic polymers. Org. Mater. 2022, 4, 268–276. [Google Scholar] [CrossRef]
- Xu, Z.; Kong, L.; Wang, Y.; Wang, B.; Zhao, J. Tuning band gap, color switching, optical contrast, and redox stability in solution-processable BDT-based electrochromic materials. Org. Electron. 2018, 54, 94–103. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, M.; Zhao, J.; Cui, C.; Fan, W.; Liu, J. Donor-acceptor type neutral green polymers containing 2,3-di(5-methylfuran-2-yl) quinoxaline acceptor and different thiophene donors. Electrochim. Acta 2014, 125, 241–249. [Google Scholar] [CrossRef]









| Polymer Film | λmax (nm) | λonset (nm) | Eonset,ox (V) | Eonset,red (V) | HOMO (eV) a | LUMO (eV) b | Eg,ec (eV) c | Eg,op (eV) d |
|---|---|---|---|---|---|---|---|---|
| PBPE-1 | 492 | 624 | 1.07 | −0.98 | −5.32 | −3.27 | 2.05 | 1.99 |
| PBPE-2 | 508 | 615 | 0.86 | −1.00 | −5.11 | −3.25 | 1.86 | 2.02 |
| PBPE-3 | 503 | 610 | 1.03 | −0.96 | −5.28 | −3.29 | 1.99 | 2.03 |
| Polymer | Optical Contrast (∆T %) | CE (cm2·C−1) | Response Time (s) | |
|---|---|---|---|---|
| tb (Time for Oxidation) | tc (Time for Reduction) | |||
| PBPE-1 | 11.66% (491 nm) | 145 | 2.89 | 1.52 |
| 25.34% (1300 nm) | 268 | 1.22 | 3.21 | |
| PBPE-2 | 11.12% (508 nm) | 229 | 3.43 | 1.33 |
| 21.77% (1500 nm) | 274 | 2.36 | 3.38 | |
| PBPE-3 | 17.80% (504 nm) | 513 | 3.00 | 1.32 |
| 27.22% (1500 nm) | 475 | 2.92 | 3.41 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Wang, B.; Kong, L.; Zhao, J.; Du, Y. Synthesis and Characterization of Solution-Processible Donor-Acceptor Electrochromic Conjugated Copolymers Based on Quinoxalino[2′,3′:9,10]phenanthro[4,5-abc]phenazine as the Acceptor Unit. Polymers 2023, 15, 940. https://doi.org/10.3390/polym15040940
Xu Z, Wang B, Kong L, Zhao J, Du Y. Synthesis and Characterization of Solution-Processible Donor-Acceptor Electrochromic Conjugated Copolymers Based on Quinoxalino[2′,3′:9,10]phenanthro[4,5-abc]phenazine as the Acceptor Unit. Polymers. 2023; 15(4):940. https://doi.org/10.3390/polym15040940
Chicago/Turabian StyleXu, Zhen, Bozhen Wang, Lingqian Kong, Jinsheng Zhao, and Yuchang Du. 2023. "Synthesis and Characterization of Solution-Processible Donor-Acceptor Electrochromic Conjugated Copolymers Based on Quinoxalino[2′,3′:9,10]phenanthro[4,5-abc]phenazine as the Acceptor Unit" Polymers 15, no. 4: 940. https://doi.org/10.3390/polym15040940






