Mechanical Properties of Protein-Based Hydrogels Derived from Binary Protein Mixtures—A Feasibility Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Buffer Preparation
2.2. Photoinitiator and Co-Factor
2.3. Elastin-like Protein Production
2.4. Preparation of the Protein Stock Solutions
2.5. Hydrogel Formation
2.6. Oscillatory Rheometry
2.7. Uniaxial Compression Tests
3. Results
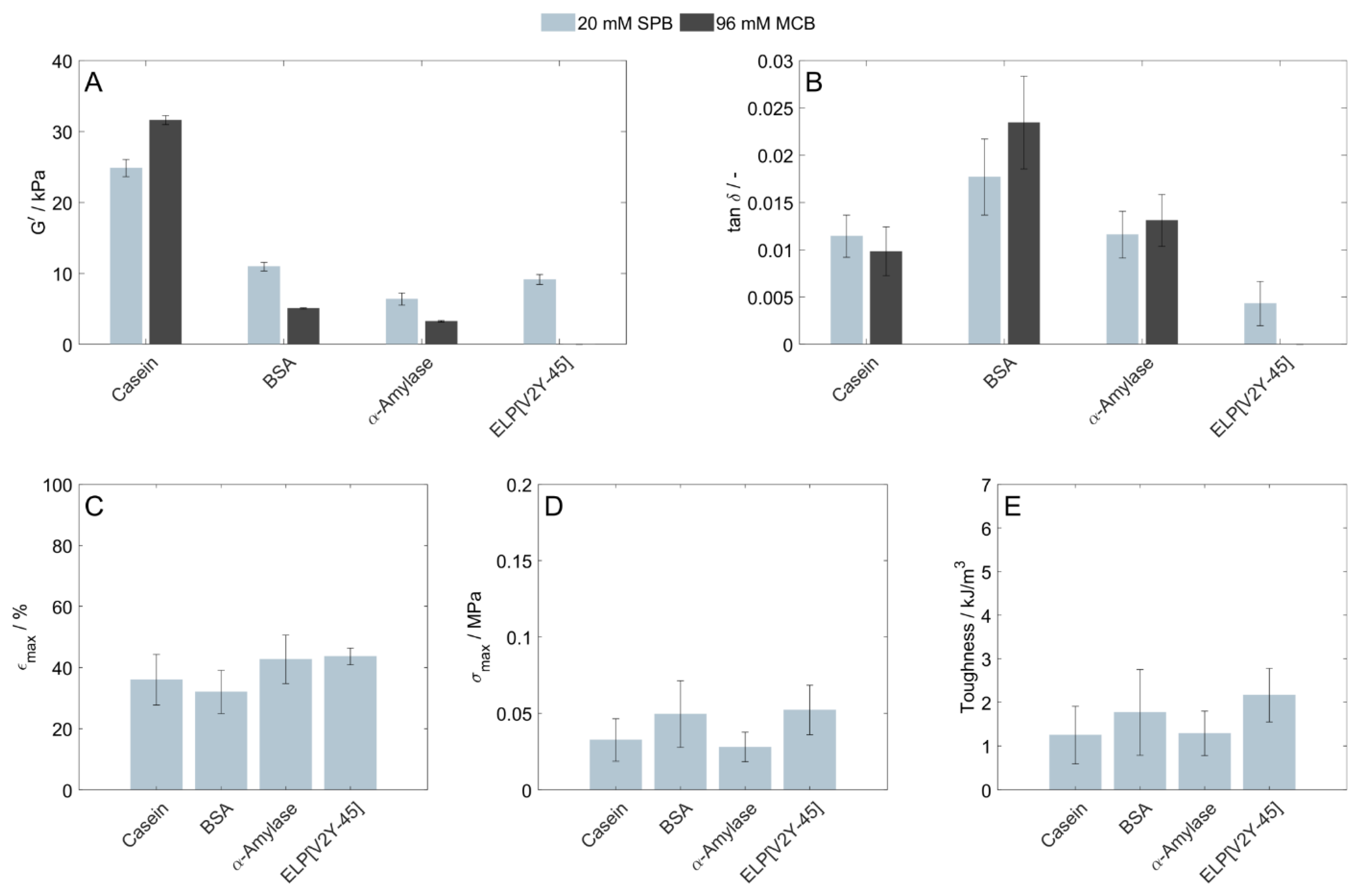
3.1. Homopolymeric Protein-Based Hydrogels
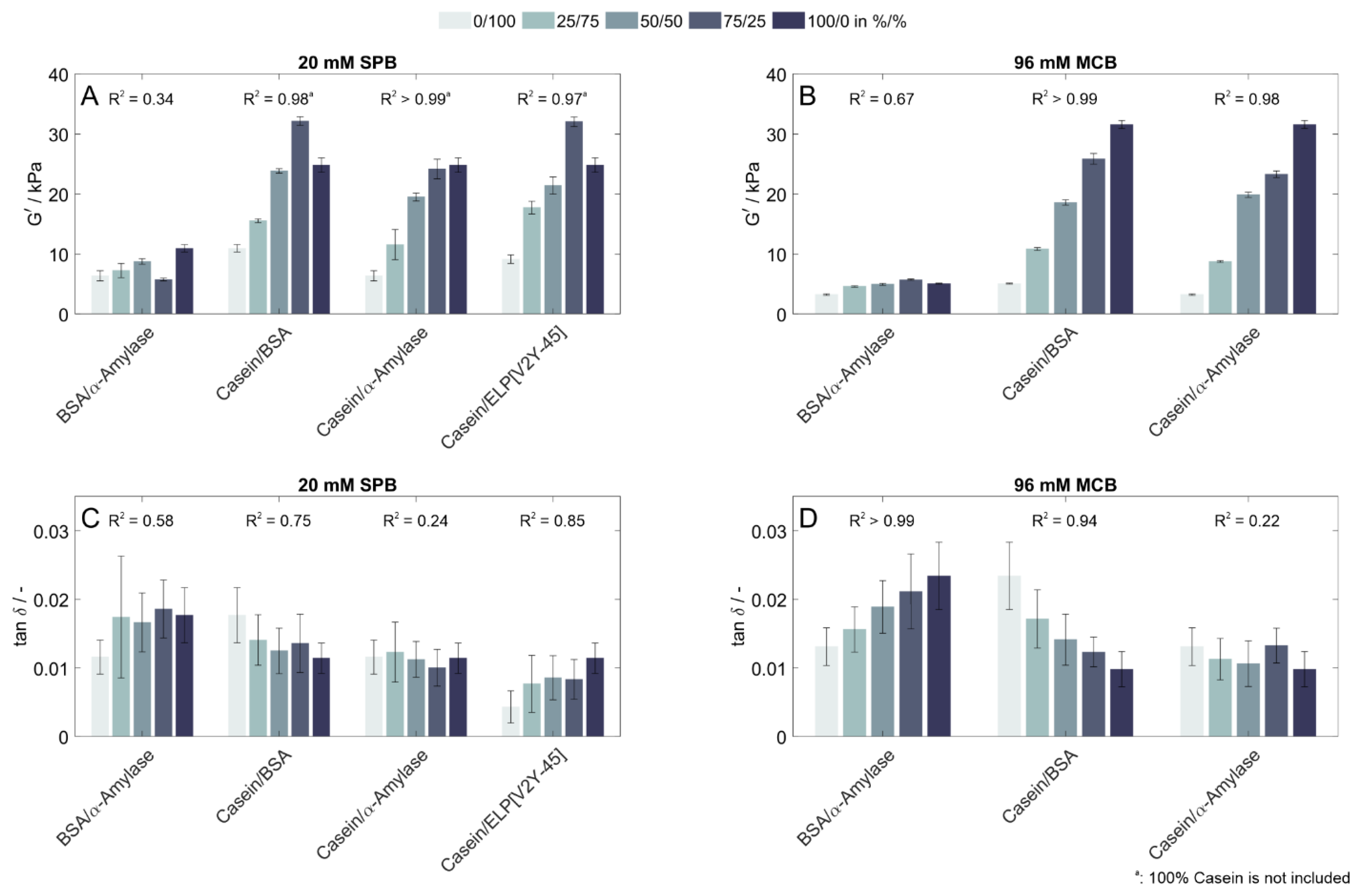
3.2. Rheological Properties of Copolymeric Hydrogels
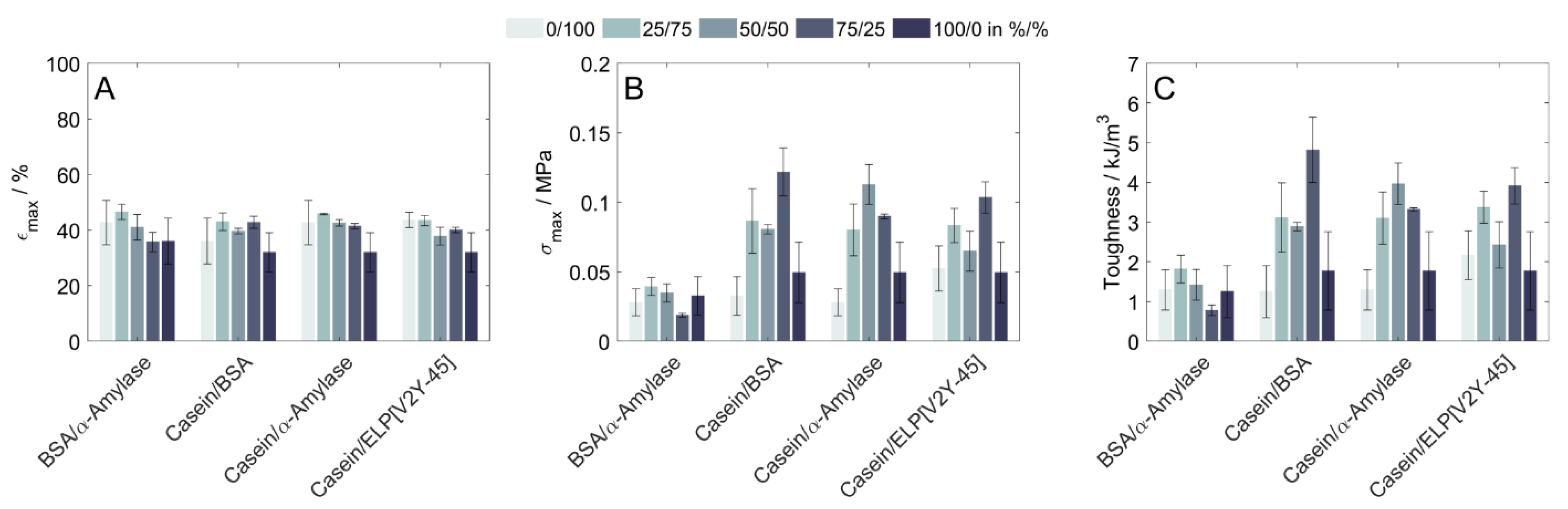
3.3. Uniaxial Compression of Copolymeric Hydrogels
4. Discussion
4.1. Buffer Components
4.2. Influence of Protein Characteristics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | three-dimensional |
| APS | ammonium persulfate |
| BSA | bovine serum albumin |
| DAC | dual asymmetric centrifuge |
| ELP | elastin-like protein |
| ITC | inverse transition cycling |
| LVR | linear viscoelastic region |
| MCB | multi-component buffer |
| pI | isoelectric point |
| TAPS | N-[Tris(hydroxymethyl)methyl]-3-aminopropanesulfonic acid |
| MOPSO | 3-Morpholino-2-hydroxypropanesulfonic acid |
| SPB | sodium phosphate buffer |
| SYMBOLS | |
| Greek letters | |
| tan δ | loss factor |
| εmax | engineered strain at sample fracture in% |
| εi:280 nm | molar extinction coefficient of the protein i at 280 nm |
| σmax | engineered stress at sample fracture in Pa |
| Latin letters | |
| G′ | storage modulus in kPa |
| G″ | loss modulus in kPa |
| n | number of replicates |
| R2 | coefficient of determination |
References
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.P.; Fernández-García, M. Bio-Based Polymers with Antimicrobial Properties towards Sustainable Development. Materials 2019, 24, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonker, A.M.; Löwik, D.W.P.M.; van Hest, J.C.M. Peptide- and Protein-Based Hydrogels. Chem. Mater. 2012, 24, 759–773. [Google Scholar] [CrossRef]
- Frandsen, J.L.; Ghandehari, H. Recombinant Protein-Based Polymers for Advanced Drug Delivery. Chem. Soc. Rev. 2012, 41, 2696–2706. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Katyal, P.; Montclare, J.K. Protein-Engineered Functional Materials. Adv. Healthc. Mater. 2019, 8, 1801374. [Google Scholar] [CrossRef]
- Simnick, A.J.; Lim, D.W.; Chow, D.; Chilkoti, A. Biomedical and Biotechnological Applications of Elastin-like Polypeptides. Polym. Rev. 2007, 47, 121–154. [Google Scholar] [CrossRef]
- Varanko, A.K.; Su, J.C.; Chilkoti, A. Elastin-like Polypeptides for Biomedical Applications. Annu. Rev. Biomed. Eng. 2020, 22, 343–369. [Google Scholar] [CrossRef]
- Acosta, S.; Quintanilla-Sierra, L.; Mbundi, L.; Reboto, V.; Rodríguez-Cabello, J.C. Elastin-Like Recombinamers: Deconstructing and Recapitulating the Functionality of Extracellular Matrix Proteins Using Recombinant Protein Polymers. Adv. Funct. Mater. 2020, 30, 1909050. [Google Scholar] [CrossRef]
- Salinas-Fernández, S.; Santos, M.; Alonso, M.; Quintanilla, L.; Rodríguez-Cabello, J.C. Genetically Engineered Elastin-like Recombinamers with Sequence-Based Molecular Stabilization as Advanced Bioinks for 3D Bioprinting. Appl. Mater. Today 2020, 18, 100500. [Google Scholar] [CrossRef]
- Duarte Campos, D.F.; Lindsay, C.D.; Roth, J.G.; LeSavage, B.L.; Seymour, A.J.; Krajina, B.A.; Ribeiro, R.; Costa, P.F.; Blaeser, A.; Heilshorn, S.C. Bioprinting Cell- and Spheroid-Laden Protein-Engineered Hydrogels as Tissue-on-Chip Platforms. Front. Bioeng. Biotechnol. 2020, 8, 374. [Google Scholar] [CrossRef]
- Foster, J.A.; Bruenger, E.; Gray, W.R.; Sandberg, L.B. Isolation and Amino Acid Sequences of Tropoelastin Peptides. J. Biol. Chem. 1973, 248, 2876–2879. [Google Scholar] [CrossRef]
- Urry, D.W. Free Energy Transduction in Polypeptides and Proteins Based on Inverse Temperature Transitions. Prog. Biophys. Mol. Biol. 1992, 57, 23–57. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.E.; Chilkoti, A. Purification of Recombinant Proteins by Fusion with Thermally-Responsive Polypeptides. Nat. Biotechnol. 1999, 17, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-N.; Avery, R.K.; Vallmajo-Martin, Q.; Assmann, A.; Vegh, A.; Memic, A.; Olsen, B.D.; Annabi, N.; Khademhosseini, A. A Highly Elastic and Rapidly Crosslinkable Elastin-Like Polypeptide-Based Hydrogel for Biomedical Applications. Adv. Funct. Mater. 2015, 25, 4814–4826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katyal, P.; Mahmoudinobar, F.; Montclare, J.K. Recent Trends in Peptide and Protein-Based Hydrogels. Curr. Opin. Struct. Biol. 2020, 63, 97–105. [Google Scholar] [CrossRef]
- Fancy, D.A.; Denison, C.; Kim, K.; Xie, Y.; Holdeman, T.; Amini, F.; Kodadek, T. Scope, Limitations and Mechanistic Aspects of the Photo-Induced Cross-Linking of Proteins by Water-Soluble Metal Complexes. Chem. Biol. 2000, 7, 697–708. [Google Scholar] [CrossRef] [Green Version]
- Fancy, D.A.; Kodadek, T. Chemistry for the Analysis of Protein-Protein Interactions: Rapid and Efficient Cross-Linking Triggered by Long Wavelength Light. Proc. Natl. Acad. Sci. USA 1999, 96, 6020–6024. [Google Scholar] [CrossRef] [Green Version]
- Fuentes-Lemus, E.; Hägglund, P.; López-Alarcón, C.; Davies, M.J. Oxidative Crosslinking of Peptides and Proteins: Mechanisms of Formation, Detection, Characterization and Quantification. Molecules 2021, 27, 15. [Google Scholar] [CrossRef]
- Elvin, C.M.; Vuocolo, T.; Brownlee, A.G.; Sando, L.; Huson, M.G.; Liyou, N.E.; Stockwell, P.R.; Lyons, R.E.; Kim, M.; Edwards, G.A.; et al. A Highly Elastic Tissue Sealant Based on Photopolymerised Gelatin. Biomaterials 2010, 31, 8323–8331. [Google Scholar] [CrossRef]
- Hughes, M.D.G.; Cussons, S.; Mahmoudi, N.; Brockwell, D.J.; Dougan, L. Single Molecule Protein Stabilisation Translates to Macromolecular Mechanics of a Protein Network. Soft Matter 2020, 16, 6389–6399. [Google Scholar] [CrossRef]
- Huber, M.C.; Jonas, U.; Schiller, S.M. An Autonomous Chemically Fueled Artificial Protein Muscle. Adv. Intell. Syst. 2022, 4, 2100189. [Google Scholar] [CrossRef]
- Duan, T.; Li, H. In Situ Phase Transition of Elastin-Like Polypeptide Chains Regulates Thermoresponsive Properties of Elastomeric Protein-Based Hydrogels. Biomacromolecules 2020, 21, 2258–2267. [Google Scholar] [CrossRef] [PubMed]
- Haas, S.; Körner, S.; Zintel, L.; Hubbuch, J. Changing Mechanical Properties of Photopolymerized, Dityrosine-Crosslinked Protein-Based Hydrogels. Front. Bioeng. Biotechnol. 2022, 10, 1716. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.D.G.; Cussons, S.; Mahmoudi, N.; Brockwell, D.J.; Dougan, L. Tuning Protein Hydrogel Mechanics through Modulation of Nanoscale Unfolding and Entanglement in Postgelation Relaxation. ACS Nano 2022, 16, 10667–10678. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.A.; Lenton, S.; Hughes, M.; Brockwell, D.J.; Dougan, L. Assessing the Potential of Folded Globular Polyproteins as Hydrogel Building Blocks. Biomacromolecules 2017, 18, 636–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoury, L.R.; Nowitzke, J.; Shmilovich, K.; Popa, I. Study of Biomechanical Properties of Protein-Based Hydrogels Using Force-Clamp Rheometry. Macromolecules 2018, 51, 1441–1452. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, H.; Sun, B.; Lyu, S. High Water Content Silk Protein-Based Hydrogels with Tunable Elasticity Fabricated via a Ru(II) Mediated Photochemical Cross-Linking Method. Fibers Polym. 2017, 18, 1831–1840. [Google Scholar] [CrossRef]
- Yang, Y.J.; Choi, Y.S.; Cha, H.J. Bioinspired Load-Bearing Hydrogel Based on Engineered Sea Anemone Skin-Derived Collagen-Like Protein. Biotechnol. J. 2018, 13, 1800086. [Google Scholar] [CrossRef]
- Elvin, C.M.; Carr, A.G.; Huson, M.G.; Maxwell, J.M.; Pearson, R.D.; Vuocolo, T.; Liyou, N.E.; Wong, D.C.C.; Merritt, D.J.; Dixon, N.E. Synthesis and Properties of Crosslinked Recombinant Pro-Resilin. Nature 2005, 437, 999–1002. [Google Scholar] [CrossRef]
- Camp, C.P.; Peterson, I.L.; Knoff, D.S.; Melcher, L.G.; Maxwell, C.J.; Cohen, A.T.; Wertheimer, A.M.; Kim, M. Non-Cytotoxic Dityrosine Photocrosslinked Polymeric Materials With Targeted Elastic Moduli. Front. Chem. 2020, 8, 173. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kang, B.; Cui, X.; Lee, S.H.; Lee, K.; Cho, D.W.; Hwang, W.; Woodfield, T.B.F.; Lim, K.S.; Jang, J. Light-Activated Decellularized Extracellular Matrix-Based Bioinks for Volumetric Tissue Analogs at the Centimeter Scale. Adv. Funct. Mater. 2021, 31, 2011252. [Google Scholar] [CrossRef]
- Meyer, D.E.; Trabbic-Carlson, K.; Chilkoti, A. Protein Purification by Fusion with an Environmentally Responsive Elastin-like Polypeptide: Effect of Polypeptide Length on the Purification of Thioredoxin. Biotechnol. Prog. 2001, 17, 720–728. [Google Scholar] [CrossRef]
- Haas, S.; Desombre, M.; Kirschhöfer, F.; Huber, M.C.; Schiller, S.M.; Hubbuch, J. Purification of a Hydrophobic Elastin-Like Protein Toward Scale-Suitable Production of Biomaterials. Front. Bioeng. Biotechnol. 2022, 0, 902. [Google Scholar] [CrossRef]
- Amrhein, S.; Bauer, K.C.; Galm, L.; Hubbuch, J. Non-Invasive High Throughput Approach for Protein Hydrophobicity Determination Based on Surface Tension. Biotechnol. Bioeng. 2015, 112, 2485–2494. [Google Scholar] [CrossRef] [PubMed]
- Nazmi, A.R.; Reinisch, T.; Hinz, H.J. Ca-Binding to Bacillus Licheniformis α-Amylase (BLA). Arch. Biochem. Biophys. 2006, 453, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.Y.; Dar, T.A.; Singh, L.R. Casein Proteins: Structural and Functional Aspects. In Milk Proteins—From Structure to Biological Properties and Health Aspects; InTech: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Swaisgood, H.E. Chemistry of the Caseins. In Advanced Dairy Chemistry—1 Proteins: Part A/Part B; Fox, P.F., McSweeney, P.L.H., Eds.; Springer: Boston, MA, USA, 2003; pp. 139–201. [Google Scholar] [CrossRef]
- Fox, P.F. Milk Proteins: General and Historical Aspects. In Advanced Dairy Chemistry—1 Proteins: Part A/Part B; Fox, P.F., McSweeney, P.L.H., Eds.; Springer: Boston, MA, USA, 2003; pp. 1–48. [Google Scholar] [CrossRef]
- Brown, P.R.; Grushka, E.; Lunte, S. Advances in Chromatography; CRC Press: Boca Raton, FL, USA, 2004; Volume 43. [Google Scholar] [CrossRef]
- Josuran, R. Available online: https://www.protpi.ch/Calculator/ProteinTool (accessed on 10 December 2022).
- Gekko, K.; Hasegawa, Y. Compressibility-Structure Relationship of Globular Proteins. Biochemistry 1986, 25, 6563–6571. [Google Scholar] [CrossRef] [PubMed]
- De Kruif, C.G.; Holt, C. Casein Micelle Structure, Functions and Interactions. In Advanced Dairy Chemistry—1 Proteins: Part A/Part B; Fox, P.F., McSweeney, P.L.H., Eds.; Springer: Boston, MA, USA, 2003; pp. 233–276. [Google Scholar] [CrossRef]
- Taha, M.; Gupta, B.S.; Khoiroh, I.; Lee, M.J. Interactions of Biological Buffers with Macromolecules: The Ubiquitous “Smart” Polymer PNIPAM and the Biological Buffers MES, MOPS, and MOPSO. Macromolecules 2011, 44, 8575–8589. [Google Scholar] [CrossRef]
- Gupta, B.S.; Taha, M.; Lee, M.J. Interactions of Bovine Serum Albumin with Biological Buffers, TES, TAPS, and TAPSO in Aqueous Solutions. Process Biochem. 2013, 48, 1686–1696. [Google Scholar] [CrossRef]
- Udabage, P.; McKinnon, I.R.; Augustin, M.A. Mineral and Casein Equilibria in Milk: Effects of Added Salts and Calcium-Chelating Agents. J. Dairy Res. 2000, 67, 361–370. [Google Scholar] [CrossRef]
- Haller, H.S.; Pallansch, M.J. The Solubility in Aqueous Urea Solutions of the Micellar Caseinates of Milk and Milk Products Subjected to Various Sterilizing Heat Treatments. J. Dairy Sci. 1960, 43, 1407–1413. [Google Scholar] [CrossRef]
- Chang, C.; He, M.; Zhou, J.; Zhang, L. Swelling Behaviors of PH- and Salt-Responsive Cellulose-Based Hydrogels. Macromolecules 2011, 44, 1642–1648. [Google Scholar] [CrossRef]
- Moinpour, M.; Barker, N.K.; Guzman, L.E.; Jewett, J.C.; Langlais, P.R.; Schwartz, J.C. Discriminating Changes in Protein Structure Using Tyrosine Conjugation. Protein Sci. 2020, 29, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Li, L.; Xue, B.; Jin, J.; Cao, Y.; Jiang, Q.; Li, H. Converting Muscle-Mimetic Biomaterials to Cartilage-like Materials. BioRxiv 2021, 1–35. [Google Scholar] [CrossRef]
- Zhang, Z.; Dmitrieva, N.I.; Park, J.H.; Levine, R.L.; Burg, M.B. High Urea and NaCl Carbonylate in Renal Cells in Culture and in Vivo, and High Urea Causes 8-Oxoguanine Lesions in Their DNA. Proc. Natl. Acad. Sci. USA 2004, 101, 9491–9496. [Google Scholar] [CrossRef] [Green Version]
- Michea, L.; Ferguson, D.R.; Peters, E.M.; Andrews, P.M.; Kirby, M.R.; Burg, M.B. Cell Cycle Delay and Apoptosis Are Induced by High Salt and Urea in Renal Medullary Cells. Am. J. Physiol.-Ren. Physiol. 2000, 278, F209–F218. [Google Scholar] [CrossRef] [Green Version]
- Elvin, C.M.; Brownlee, A.G.; Huson, M.G.; Tebb, T.A.; Kim, M.; Lyons, R.E.; Vuocolo, T.; Liyou, N.E.; Hughes, T.C.; Ramshaw, J.A.M.; et al. The Development of Photochemically Crosslinked Native Fibrinogen as a Rapidly Formed and Mechanically Strong Surgical Tissue Sealant. Biomaterials 2009, 30, 2059–2065. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Dudek, D.M.; Cao, Y.; Balamurali, M.M.; Gosline, J.; Li, H. Designed Biomaterials to Mimic the Mechanical Properties of Muscles. Nature 2010, 465, 69–73. [Google Scholar] [CrossRef]
- Lu, S.; Zhu, L.; Wang, Q.; Liu, Z.; Tang, C.; Sun, H.; Yang, J.; Qin, G.; Sun, G.; Chen, Q. High-Strength Albumin Hydrogels With Hybrid Cross-Linking. Front. Chem. 2020, 8, 106. [Google Scholar] [CrossRef]
- Oveissi, F.; Fletcher, D.F.; Dehghani, F.; Naficy, S. Tough Hydrogels for Soft Artificial Muscles. Mater. Des. 2021, 203, 109609. [Google Scholar] [CrossRef]
| Protein | Casein | BSA | α-Amylase | ELP[V2Y-45] |
|---|---|---|---|---|
| Type | Heteroprotein (consisting of mainly 4 subunits, ≈ 38% αs1-, ≈10% αs2-, ≈36% β-, ≈13% κ-casein) (a) | Globular protein | Enzyme | Modified protein |
| Origin | Bovine milk | Bovine blood serum | Bacillus species | Recombinant production in Escherichia coli |
| Structural arrangement | No well-defined secondary and tertiary structure of subunits which are forming micelles (a) | Ordered tertiary structure | Ordered tertiary structure | Intrinsically disordered protein |
| Size/kDa | Subunits: ≈19–25 (b) Micelles: 250–500 (c) | 66.0 | 52.9 (d) | 21.6 |
| Tyrosines/% | ≈3.9–4.2 (b), (c) | 3.6 (e) | 4.2 (d) | 6.1 |
| pI (native structure)/- | ≈4.6 (c) | ≈ 5.0–5.2 (f) | unknown | unknown |
| Theoretical pI (g)/- | 5.0 (h) 5.3 (i) | 6.2 (e) | 5.3 (d) | 6.8 |
| Theoretical charge at pH 8 (g)/- | −12.6 (h) −8.4 (i) | −20.9 (e) | −15.1 (d) | −2.0 |
| Adiabatic compressibility (native structure) (j)/Pa−1 | 5.68 × 10−11 (k) | 10.5 × 10−11 | 5.12 × 10−11 | unknown |
| Disulfide bonds/- | Rare, inter-rather than intra-molecular cystine bridges (l) | 15 (e) | 0 (d) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haas, S.; Hubbuch, J. Mechanical Properties of Protein-Based Hydrogels Derived from Binary Protein Mixtures—A Feasibility Study. Polymers 2023, 15, 964. https://doi.org/10.3390/polym15040964
Haas S, Hubbuch J. Mechanical Properties of Protein-Based Hydrogels Derived from Binary Protein Mixtures—A Feasibility Study. Polymers. 2023; 15(4):964. https://doi.org/10.3390/polym15040964
Chicago/Turabian StyleHaas, Sandra, and Jürgen Hubbuch. 2023. "Mechanical Properties of Protein-Based Hydrogels Derived from Binary Protein Mixtures—A Feasibility Study" Polymers 15, no. 4: 964. https://doi.org/10.3390/polym15040964
APA StyleHaas, S., & Hubbuch, J. (2023). Mechanical Properties of Protein-Based Hydrogels Derived from Binary Protein Mixtures—A Feasibility Study. Polymers, 15(4), 964. https://doi.org/10.3390/polym15040964






