Synthesis and Characterization of a pH- and Temperature-Sensitive Fe3O4-SiO2-Poly(NVCL-co-MAA) Nanocomposite for Controlled Delivery of Doxorubicin Anticancer Drug
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Fe3O4 Nanoparticles (MNPsFe3O4)
2.3. Coating of SiO2 onto Fe3O4 Nanoparticles (MNPsFe3O4-SiO2)
2.4. Functionalization of Fe3O4-SiO2 Nanoparticles with VTMS (MNPsFe3O4-SiO2-VTMS)
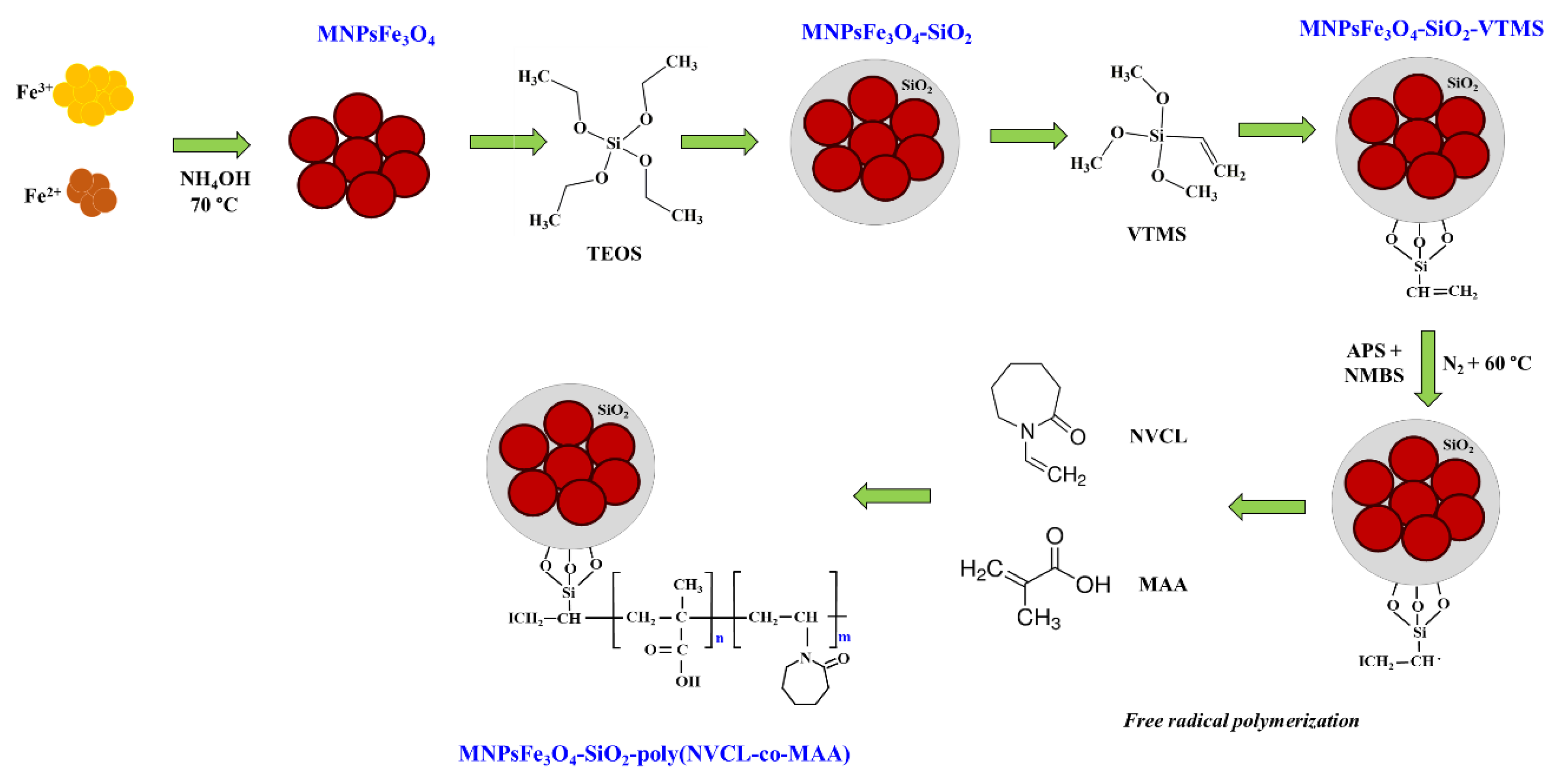
2.5. Synthesis of Fe3O4-SiO2-Poly(NVCL-co-MAA) Nanocomposite
2.6. Characterization
2.7. Loading of DOX onto MNPsFe3O4-SiO2 and MNPsFe3O4-SiO2-Poly(NVCL-co-MAA)
2.8. In Vitro DOX Release Studies
2.9. In Vitro Hemolysis Assay
2.10. Release Kinetic Models
3. Results and Discussion
3.1. Synthesis of MNPsFe3O4-SiO2-g-Poly(NVCL-co-MAA) Nanocomposites
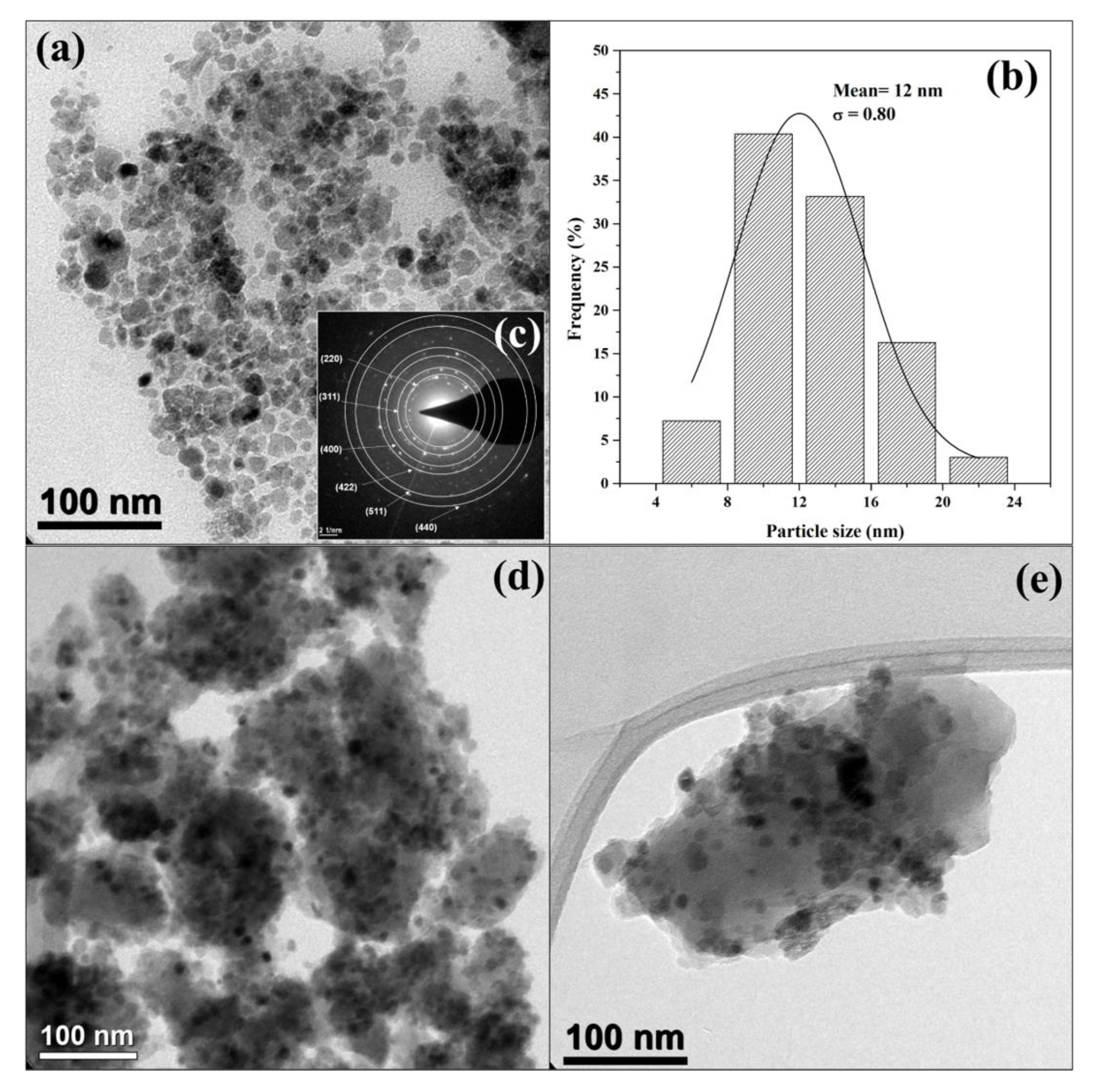
3.2. Characterization
3.3. DOX Loading
3.4. DOX Release
3.5. Hemolysis Assay
3.6. Release Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahimi, M.; Wadajkar, A.; Subramanian, K.; Yousef, M.; Cui, W.; Hsieh, J.T.; Nguyen, K.T. In vitro evaluation of novel polymer-coated magnetic nanoparticles for controlled drug delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 672–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundaresan, V.; Menon, J.U.; Rahimi, M.; Nguyen, K.T.; Wadajkar, A.S. Dual-responsive polymer-coated iron oxide nanoparticles for drug delivery and imaging applications. Int. J. Pharm. 2014, 466, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein Al-Ali, S.H.; El Zowalaty, M.E.; Hussein, M.Z.; Ismail, M.; Webster, T.J. Synthesis, characterization, controlled release, and antibacterial studies of a novel streptomycin chitosan magnetic nanoantibiotic. Int. J. Nanomed. 2014, 9, 549–557. [Google Scholar]
- Fard, S.M.; Farhadian, N.; Bastami, T.R.; Ebrahimi, M.; Karimi, M.; Allahyari, A. Synthesis, characterization and cellular cytotoxicity evaluation of a new magnetic nanoparticle carrier co-functionalized with amine and folic acid. J. Drug Deliv. Sci. Technol. 2017, 38, 116–124. [Google Scholar] [CrossRef]
- Oh, Y.; Lee, N.; Kang, H.W.; Oh, J. In vitro study on apoptotic cell death by effective magnetic hyperthermia with chitosan-coated MnFe2O4. Nanotechnology 2016, 27, 115101. [Google Scholar] [CrossRef]
- Obaidat, I.; Issa, B.; Haik, Y. Magnetic properties of magnetic nanoparticles for efficient hyperthermia. Nanomaterials 2015, 5, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonvico, F.; Mornet, S.; Vasseur, S.; Dubernet, C.; Jaillard, D.; Degrouard, J.; Hoebeke, J.; Duguet, E.; Colombo, P.; Couvreur, P. Folate-conjugated iron oxide nanoparticles for solid tumor targeting as potential specific magnetic hyperthermia mediators: Synthesis, physicochemical characterization, and in vitro experiments. Bioconjug. Chem. 2005, 16, 1181–1188. [Google Scholar] [CrossRef]
- Kumar, S.; Daverey, A.; Khalilzad-Sharghi, V.; Sahu, N.K.; Kidambi, S.; Othman, S.F.; Bahadur, D. Theranostic fluorescent silica encapsulated magnetic nanoassemblies for in vitro MRI imaging and hyperthermia. RSC Adv. 2015, 5, 53180–53188. [Google Scholar] [CrossRef]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef]
- Lee, N.; Hyeon, T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2012, 41, 2575–2589. [Google Scholar] [CrossRef]
- Shukla, S.; Jadaun, A.; Arora, V.; Sinha, R.K.; Biyani, N.; Jain, V.K. In vitro toxicity assessment of chitosan oligosaccharide coated iron oxide nanoparticles. Toxicol. Rep. 2015, 2, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lan, C.Q.; Post, M.; Simard, B.; Deslandes, Y.; Hsieh, T.H. Design of Nanoparticles as Drug Carriers for Cancer Therapy. Cancer Genom. Proteome 2006, 3, 147–157. [Google Scholar]
- Wang, X.; Li, J.; Wang, Y.; Cho, K.J.; Kim, G.; Gjyrezi, A.; Koenig, L.; Giannakakou, P.; Shin, H.J.C.; Tighiouart, M.; et al. HFT-T, a Targeting Nanoparticle, Enhances Specific Delivery of Paclitaxel to Folate Receptor-Positive Tumors. ACS Nano 2009, 3, 3165–3174. [Google Scholar] [CrossRef] [PubMed]
- Keizer, H.G.; Pinedo, H.M.; Schuurhuis, G.J.; Joenje, H. Doxorubicin (adriamycin): A critical review of free radical-dependent mechanisms of cytotoxicity. Pharmacol. Ther. 1990, 47, 219–231. [Google Scholar] [CrossRef]
- Salmon, S.E.; Grogan, T.M.; Miller, T.; Scheper, R.; Dalton, W.S. Prediction of Doxorubicin Resistance In Vitro in Myeloma, Lymphoma, and Breast Cancer by P-Glycoprotein Staining. J. Natl. Cancer. Inst. 1989, 81, 696–701. [Google Scholar] [CrossRef]
- Passemard, S.; Staedler, D.; Učňová, L.; Schneiter, G.S.; Kong, P.; Bonacina, L.; Juillerat-Jeanneret, L.; Gerber-Lemaire, S. Convenient synthesis of heterobifunctional poly(ethylene glycol) suitable for the functionalization of iron oxide nanoparticles for biomedical applications. Bioorg. Med. Chem. Lett. 2013, 23, 5006–5010. [Google Scholar] [CrossRef] [Green Version]
- Aghazadeh, M.; Karimzadeh, I.; Ganjali, M.R. Ethylenediaminetetraacetic acid capped superparamagnetic iron oxide (Fe3O4) nanoparticles: A novel preparation method and characterization. J. Magn. Magn. Mater. 2017, 439, 312–319. [Google Scholar] [CrossRef]
- Karimzadeh, I.; Aghazadeh, M.; Ganjali, M.R.; Doroudi, T.; Kolivand, P.H. Preparation and characterization of iron oxide (Fe3O4) nanoparticles coated with polyvinylpyrrolidone/polyethylenimine through a facile one-pot deposition route. J. Magn. Magn. Mater. 2017, 433, 148–154. [Google Scholar] [CrossRef]
- Enache, D.F.; Vasile, E.; Simonescu, C.M.; Răzvan, A.; Nicolescu, A.; Nechifor, A.C.; Oprea, O.; Pătescu, R.E.; Onose, C.; Dumitru, F. Cysteine-functionalized silica-coated magnetite nanoparticles as potential nanoadsorbents. J. Solid State Chem. 2017, 253, 318–328. [Google Scholar] [CrossRef]
- Allard-Vannier, E.; Hervé-Aubert, K.; Kaaki, K.; Blondy, T.; Shebanova, A.; Shaitan, K.V.; Ignatova, A.A.; Saboungi, M.L.; Feofanov, A.V.; Chourpa, I. Folic acid-capped PEGylated magnetic nanoparticles enter cancer cells mostly via clathrin-dependent endocytosis. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1578–1586. [Google Scholar] [CrossRef]
- Sheng, W.; Wei, W.; Li, J.; Qi, X.; Zuo, G.; Chen, Q.; Pan, X.; Dong, W. Amine-functionalized magnetic mesoporous silica nanoparticles for DNA separation. Appl. Surf. Sci. 2016, 387, 1116–1124. [Google Scholar] [CrossRef]
- Lu, Y.; Yin, Y.; Mayers, B.T.; Xia, Y. Modifying the surface properties of Superparamagnetic Iron Oxide Nanoparticles through A Sol−Gel Approach. Nano Lett. 2002, 2, 183–186. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Monga, Y.; Zboril, R.; Sharma, R.K. Silica-decorated magnetic nanocomposites for catalytic applications. Coord. Chem. Rev. 2015, 288, 118–143. [Google Scholar] [CrossRef]
- Tian, Z.; Yu, X.; Ruan, Z.; Zhu, M.; Zhu, Y.; Hanagata, N. Magnetic mesoporous silica nanoparticles coated with thermo-responsive copolymer for potential chemo- and magnetic hyperthermia therapy. Micropor. Mesopor. Mat. 2018, 256, 1–9. [Google Scholar] [CrossRef]
- Bolla, P.K.; Rodriguez, V.A.; Kalhapure, R.S.; Kolli, C.S.; Andrews, S.; Renukuntla, J. A review on pH and temperature responsive gels and other less explored drug delivery systems. J. Drug. Deliv. Sci. Technol. 2018, 46, 416–435. [Google Scholar] [CrossRef]
- Song, F.; Wang, X.L.; Wang, Y.Z. Poly (N-isopropylacrylamide)/poly (ethylene oxide) blend nanofibrous scaffolds: Thermo-responsive carrier for controlled drug release. Colloids Surf. B. 2011, 88, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Nastyshyn, S.; Stetsyshyn, Y.; Raczkowska, J.; Nastishin, Y.; Melnyk, Y.; Panchenko, Y.; Budkowski, A. Temperature-Responsive Polymer Brush Coatings for Advanced Biomedical Applications. Polymers 2022, 14, 4245. [Google Scholar] [CrossRef]
- Stetsyshyn, Y.; Raczkowska, J.; Harhay, K.; Gajos, K.; Melnyk, Y.; Dąbczyński, P.; Shevtsova, T.; Budkowski, A. Temperature-responsive and multi-responsive grafted polymer brushes with transitions based on critical solution temperature: Synthesis, properties, and applications. Colloid Polym Sci. 2021, 299, 363–383. [Google Scholar] [CrossRef]
- Inoue, T.; Chen, G.; Nakamae, K.; Hoffman, A.S. Temperature sensitivity of a hydrogel network containing different LCST oligomers grafted to the hydrogel backbone. Polym. Gels Netw. 1998, 5, 561–575. [Google Scholar] [CrossRef]
- Prabaharan, M.; Grailer, J.J.; Steeber, D.A.; Gong, S. Stimuli-Responsive Chitosan-graft-Poly(N-vinylcaprolactam) as a Promising Material for Controlled Hydrophobic Drug Delivery. Macromol. Biosci. 2008, 8, 843–851. [Google Scholar] [CrossRef]
- Lau, A.C.W.; Wu, C. Thermally Sensitive and Biocompatible Poly(N-vinylcaprolactam): Synthesis and Characterization of High Molar Mass Linear Chains. Macromolecules 1999, 32, 581–584. [Google Scholar] [CrossRef]
- Mark, H.F.; Bikales, N.M.; Overberger, C.G.; Menges, G. Encyclopedia of Polymer Science and Engineering, 2nd ed.; Wiley-Interscience: New York, NY, USA, 1985. [Google Scholar]
- Sánchez-Orozco, J.L.; Puente-Urbina, B.; Mercado-Silva, J.A.; Meléndez-Ortiz, H.I. β-Cyclodextrin-functionalized mesocellular silica foams as nanocarriers of doxorubicin. J. Solid State Chem. 2020, 292, 121728. [Google Scholar] [CrossRef]
- Mdlovu, N.V.; Lin, K.S.; Weng, M.T.; Lin, Y.S.; Liu, S.Y. Preparation and in-vitro/in-vivo evaluation of doxorubicin-loaded magnetic SBA-15 nanocomposites from rice husk for enhancing therapeutic efficacy. Colloids Surf. B. 2022, 220, 112923. [Google Scholar] [CrossRef]
- Mdlovu, N.V.; Lin, K.S.; Weng, M.T.; Lin, Y.S. Design of doxorubicin encapsulated pH-/thermo-responsive and cationic shell-crosslinked magnetic drug delivery system. Colloids Surf. B. 2022, 209, 112168. [Google Scholar] [CrossRef]
- Mandal, P.; Panja, S.; Banerjee, S.L.; Ghorai, S.K.; Maji, S.; Maiti, T.K.; Chattopadhyay, S. Magnetic particle anchored reduction and pH responsive nanogel for enhanced intracellular drug delivery. Eur. Polym. J. 2020, 129, 109638. [Google Scholar] [CrossRef]
- Meléndez-Ortiz, H.I.; Saucedo-Zuñiga, N.; Puente-Urbina, B.; Castruita-de León, G.; Mercado-Silva, J.A.; Saucedo-Salazar, E. Polymer-grafted mesocellular silica foams: Influence of reaction conditions on the mesostructure and polymer content. Mater. Chem. Phys. 2018, 203, 333–339. [Google Scholar] [CrossRef]
- Meléndez-Ortiz, H.I.; Puente-Urbina, B.; Castruita-de León, G.; Saucedo-Salazar, E.; Mercado-Silva, J.A.; García-Cerda, L.A. Polyamine-decorated mesocellular silica foam nanocomposites: Effect of the reaction parameters on the grafted polymer content and silica mesostructure. J. Solgel Sci. Technol. 2020, 94, 118–126. [Google Scholar] [CrossRef]
- ASTM F756-13; Standard Practice for Assessment of Hemolytic Properties of Materials. ASTM International: West Conshohocken, PA, USA, 2013.
- Son, G.H.; Lee, B.J.; Cho, C.W. Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles. J. Pharm. Investig. 2017, 47, 287–296. [Google Scholar] [CrossRef]
- Brey Gil, V.S.; Brey Gil, C.S.; Goulart, G.A.C.; Oréfice, R.L. Multi-drug hybrid delivery systems with distinct release profiles based on gelatin/collagen containing vesicles derived from block copolymers. Int. J. Biol. Macromol. 2019, 139, 967–974. [Google Scholar] [CrossRef]
- Qureshi, D.; Nayak, S.K.; Maji, S.; Anis, A.; Kim, D.; Pal, K. Environment sensitive hydrogels for drug delivery applications. Eur. Polym. J. 2019, 120, 109220. [Google Scholar] [CrossRef]
- Morfin-Gutierrez, A.; Sánchez-Orozco, J.L.; García-Cerda, L.A.; Puente-Urbina, B.; Meléndez-Ortiz, H.I. Preparation and characterization of nanocomposites based on poly(N-vinycaprolactam) and magnetic nanoparticles for using as drug delivery system. J. Drug. Deliv. Sci. Technol. 2020, 60, 102028. [Google Scholar] [CrossRef]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Langer, R. Nanomedicine: Developing smarter therapeutic and diagnostic modalities. Adv. Drug. Deliv. Rev. 2006, 58, 1456–1459. [Google Scholar] [CrossRef]
- Okhapkin, I.M.; Nasimova, I.R.; Makhaeva, E.E.; Khokhlov, A.R. Effect of Complexation of Monomer Units on pH- and Temperature-Sensitive Properties of Poly(N-vinylcaprolactam-co-methacrylic acid). Macromolecules 2003, 36, 8130–8138. [Google Scholar] [CrossRef]
- Chang, B.; Chen, D.; Wang, Y.; Chen, Y.; Jiao, Y.; Sha, X.; Yang, W. Bioresponsive Controlled Drug Release Based on Mesoporous Silica Nanoparticles Coated with Reductively Sheddable Polymer Shell. Chem. Mater. 2013, 25, 574–585. [Google Scholar] [CrossRef]
- Adam, F.; Hello, K.M.; Osman, H. Synthesis of Mesoporous Silica Immobilized with 3-[(Mercapto or amino)propyl]trialkoxysilane by a Simple One-pot Reaction. Chin. J. Chem. 2010, 28, 2383–2388. [Google Scholar] [CrossRef]
- Vázquez-González, B.; Meléndez-Ortiz, H.I.; Díaz-Gómez, L.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Silicone Rubber Modified with Methacrylic Acid to Host Antiseptic Drugs. Macromol. Mater. Eng. 2014, 299, 1240–1250. [Google Scholar] [CrossRef]
- Digigow, R.G.; Dechézelles, J.F.; Dietsch, H.; Geissbühler, I.; Vanhecke, D.; Geers, C.; Hirt, A.M.; Rothen-Rutishauser, B.; Petri-Fink, A. Preparation and characterization of functional silica hybrid magnetic nanoparticles. J. Magn. Magn. Mater. 2014, 362, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous Materials for Drug Delivery. Angew. Chem. Int. Ed. 2007, 4, 7548–7558. [Google Scholar] [CrossRef]
- Esquivel, R.; Canale, I.; Ramirez, M.; Hernández, P.; Zavala-Rivera, P.; Álvarez-Ramos, E.; Lucero-Acuña, A. Poly(N-isopropylacrylamide)-coated gold nanorods mediated by thiolated chitosan layer: Thermo-pH responsiveness and optical properties. e-Polymers 2018, 18, 163–174. [Google Scholar] [CrossRef]
- Chen, C.; Ng, D.Y.W.; Weil, T. Polymer-grafted gold nanoflowers with temperature-controlled catalytic features by in situ particle growth and polymerization. Mater. Chem. Front. 2019, 3, 1449–1453. [Google Scholar] [CrossRef]
- Sedlacek, O.; Janouskova, O.; Verbraeken, B.; Hoogenboom, R. Straightforward Route to Superhydrophilic Poly(2-oxazoline)s via Acylation of Well-Defined Polyethylenimine. Biomacromolecules 2019, 20, 222–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Hao, X.; Chen, S.; Yang, Z.; Wang, C.; Yan, R.; Zhang, X.; Liu, H.; Shao, Q.; Guo, Z. pH-responsive Capsaicin@chitosan nanocapsules for antibiofouling in marine applications. Polymers 2018, 158, 223–230. [Google Scholar] [CrossRef]
- Torres-Ávalos, J.A.; Cajero-Zul, L.R.; Vázquez-Lepe, M.; López-Dellamary, F.A.; Martínez-Richa, A.; Barrera-Rivera, K.A.; López-Serrano, F.; Nuño-Donlucas, S.M. Synthesis of Poly(methacrylic acid-co-butyl acrylate) Grafted onto Functionalized Carbon Nanotube Nanocomposites for Drug Delivery. Polymers 2021, 13, 533. [Google Scholar] [CrossRef]
- Clara-Rahola, J.; Moscoso, A.; Belén Ruiz-Muelle, A.; Laurenti, M.; Formanek, P.; Lopez-Romero, J.M.; Fernández, I.; Diaz, J.F.; Rubio-Retama, J.; Fery, A.; et al. Au@p4VP core@shell pH-sensitive nanocomposites suitable for drug entrapment. J. Colloid Interface Sci. 2018, 514, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.F.; Wu, K.Y.; Tang, J.; Li, D.; Wei, C.; Guo, J.; Wang, S.L.; Wang, C.C. Magnetic drug carrier with a smart pH-responsive polymer network shell for controlled delivery of doxorubicin. J. Mater. Chem. 2012, 22, 15206–15214. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive polymers with lower critical solution temperature: From fundamental aspects and measuring techniques to recommended turbidimetry conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Ab Wab, H.A.; Abdul Razak, K.; Zakaria, N.D. Properties of amorphous silica nanoparticles colloid drug delivery system synthesized using the micelle formation method. J. Nanopart. Res. 2014, 16, 2256. [Google Scholar] [CrossRef]
- Chang, B.; Sha, X.; Guo, J.; Jiao, Y.; Wang, C.; Yang, W. Thermo and pH dual responsive, polymer shell coated, magnetic mesoporous silica nanoparticles for controlled drug release. J. Mater. Chem. 2011, 21, 9239–9247. [Google Scholar] [CrossRef]
- Asoh, T.A.; Kaneko, T.; Matsusaki, M.; Akashi, M. Rapid deswelling of semi-IPNs with nanosized tracts in response to pH and temperature. J. Control Release 2006, 110, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Pon-On, W.; Tithito, T.; Maneeprakorn, W.; Phenrat, T.; Tang, I.M. Investigation of magnetic silica with thermoresponsive chitosan coating for drug controlled release and magnetic hyperthermia application. Mater. Sci. Eng. C 2019, 97, 23–30. [Google Scholar] [CrossRef]
- Roy Chowdhury, S.K.; Mishra, A.; Pradhan, B.; Saha, D. Wear characteristic and biocompatibility of some polymer composite acetabular cups. Wear 2004, 256, 1026–1036. [Google Scholar] [CrossRef]
- Martínez-Martínez, M.; Rodríguez-Berna, G.; Bermejo, M.; Gonzalez-Alvarez, I.; Gonzalez-Alvarez, M.; Merino, V. Covalently crosslinked organophosphorous derivatives-chitosan hydrogel as a drug delivery system for oral administration of camptothecin. Eur. J. Pharm. Biopharm. 2019, 136, 174–183. [Google Scholar] [CrossRef] [PubMed]









| Research Group | Hybrid Magnetic Nanocomposites | DOX Loading (mg DOX/mg NC) | DOX Release at 37 °C | |
|---|---|---|---|---|
| pH | (%) | |||
| This work | MNPsFe3O4-SiO2-poly(NVCL-co-MAA)33% | 1.29 × 10−2 | 7.4 | 55 |
| 5.8 | 98 | |||
| MNPs-Fe3O4-SiO2-poly(NVCL-co-MAA)45% | 1.2 × 10−2 | 7.4 | 60 | |
| 5.8 | 88 | |||
| Ma et al. [59] | MNPsFe3O4-SiO2-PAA | 1.6 × 10−2 | 7.4 | 11 |
| 5 | 69 | |||
| Chang et al. [62]. | MNPsFe3O4-MSN-poly(NIPAAm-co-MAA) | 6.3 × 10−2 | 7.4 | 7.2 |
| 6.5 | 37 | |||
| 5 | 80 | |||
| Pon et al. [64] | MNPsFe3O4-SiO2-(CS-PNIPAAm) | 7.4 × 10−2 | 7.4 | 26 |
| 4 | 57 | |||
| Kinetic Model | Zero-Order | First-Order | Higuchi | Korsmeyer-Peppas | |||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | K0 | R2 | K1 | R2 | KH | R2 | KK | η | R2 |
| pH 7.4, 25 °C | 0.6171 | 0.9730 | 0.0097 | 0.9122 | 6.2459 | 0.9974 | 0.8998 | 0.4403 | 0.9971 |
| pH 7.4, 37 °C | 0.7390 | 0.9707 | 0.0095 | 0.8963 | 5.9874 | 0.9854 | 1.2129 | 0.2901 | 0.9775 |
| pH 5.8, 25 °C | 1.7640 | 0.9218 | 0.0174 | 0.8016 | 11.903 | 0.9768 | 1.2594 | 0.4194 | 0.9744 |
| pH 5.8, 37 °C | 8.3970 | 0.8619 | 0.0608 | 0.7514 | 33.854 | 0.9594 | 1.5212 | 0.5357 | 0.9587 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Orozco, J.L.; Meléndez-Ortiz, H.I.; Puente-Urbina, B.A.; Rodríguez-Fernández, O.S.; Martínez-Luévanos, A.; García-Cerda, L.A. Synthesis and Characterization of a pH- and Temperature-Sensitive Fe3O4-SiO2-Poly(NVCL-co-MAA) Nanocomposite for Controlled Delivery of Doxorubicin Anticancer Drug. Polymers 2023, 15, 968. https://doi.org/10.3390/polym15040968
Sánchez-Orozco JL, Meléndez-Ortiz HI, Puente-Urbina BA, Rodríguez-Fernández OS, Martínez-Luévanos A, García-Cerda LA. Synthesis and Characterization of a pH- and Temperature-Sensitive Fe3O4-SiO2-Poly(NVCL-co-MAA) Nanocomposite for Controlled Delivery of Doxorubicin Anticancer Drug. Polymers. 2023; 15(4):968. https://doi.org/10.3390/polym15040968
Chicago/Turabian StyleSánchez-Orozco, Jorge Luis, Héctor Iván Meléndez-Ortiz, Bertha Alicia Puente-Urbina, Oliverio Santiago Rodríguez-Fernández, Antonia Martínez-Luévanos, and Luis Alfonso García-Cerda. 2023. "Synthesis and Characterization of a pH- and Temperature-Sensitive Fe3O4-SiO2-Poly(NVCL-co-MAA) Nanocomposite for Controlled Delivery of Doxorubicin Anticancer Drug" Polymers 15, no. 4: 968. https://doi.org/10.3390/polym15040968
APA StyleSánchez-Orozco, J. L., Meléndez-Ortiz, H. I., Puente-Urbina, B. A., Rodríguez-Fernández, O. S., Martínez-Luévanos, A., & García-Cerda, L. A. (2023). Synthesis and Characterization of a pH- and Temperature-Sensitive Fe3O4-SiO2-Poly(NVCL-co-MAA) Nanocomposite for Controlled Delivery of Doxorubicin Anticancer Drug. Polymers, 15(4), 968. https://doi.org/10.3390/polym15040968










