Lignin Nanoparticles for Enhancing Physicochemical and Antimicrobial Properties of Polybutylene Succinate/Thymol Composite Film for Active Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SLNPs
2.3. Preparation of PBS Composite Films
2.4. Physicochemical Characterization of PBS Composite Films
2.4.1. Morphology
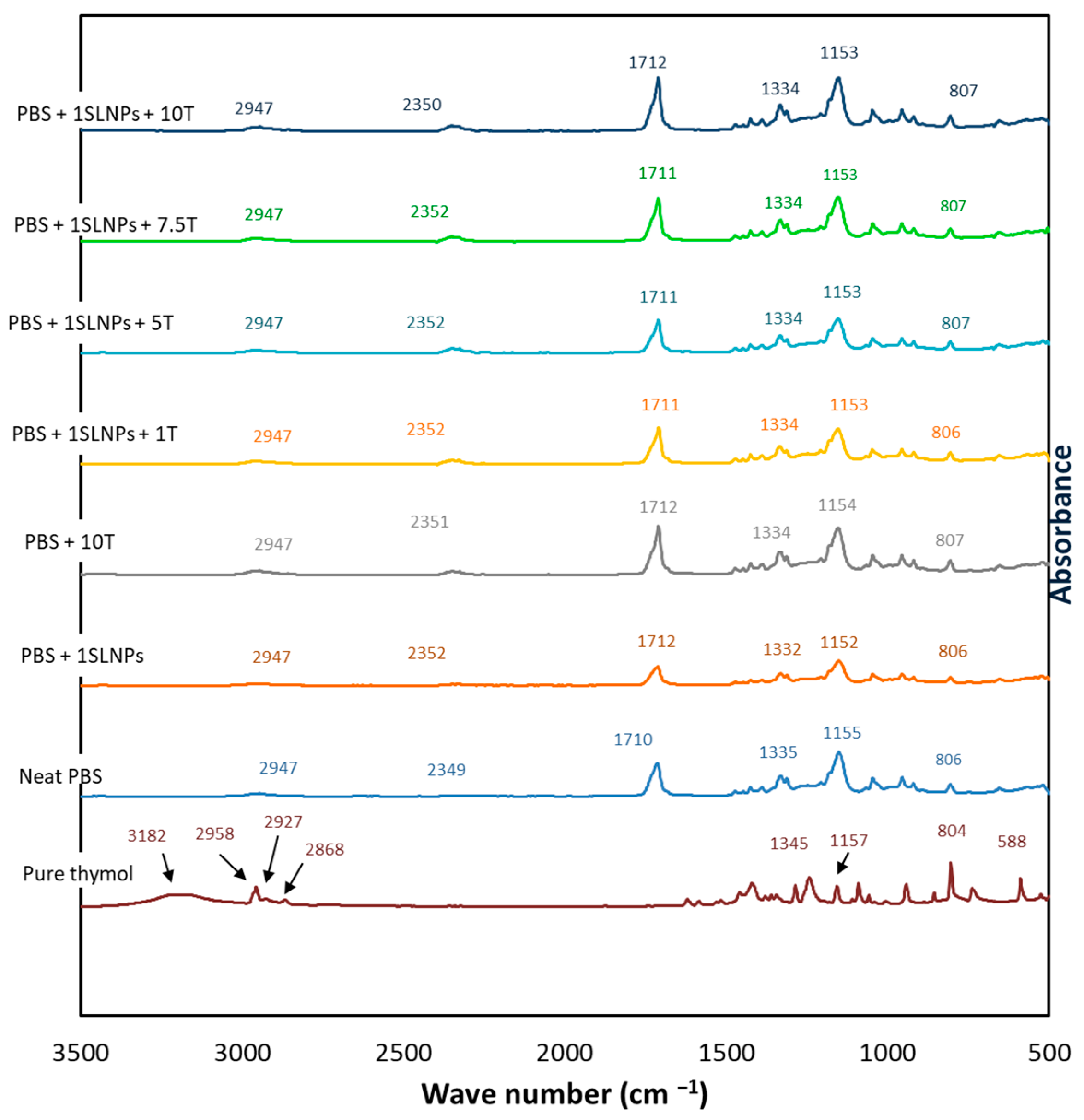
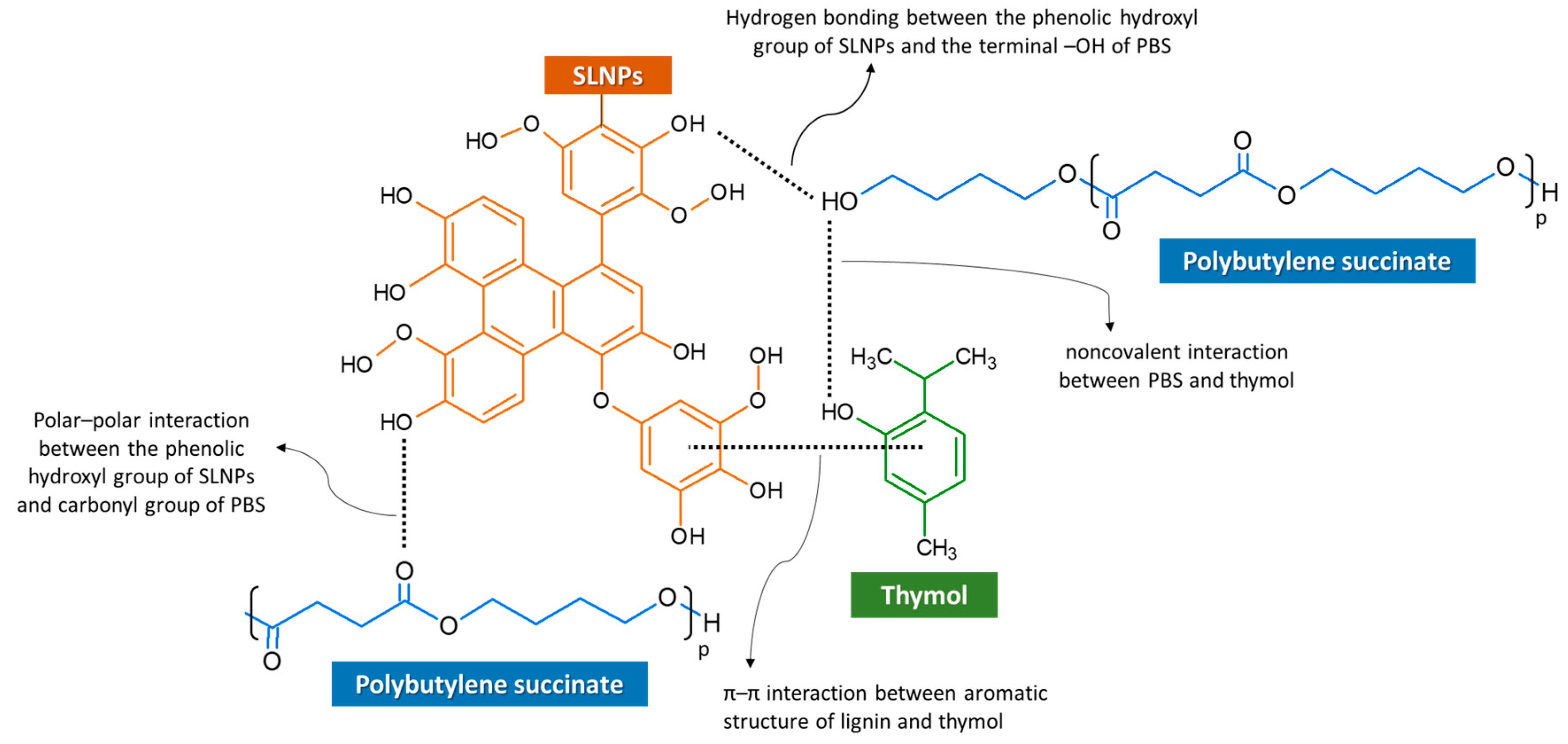
2.4.2. FTIR Analysis
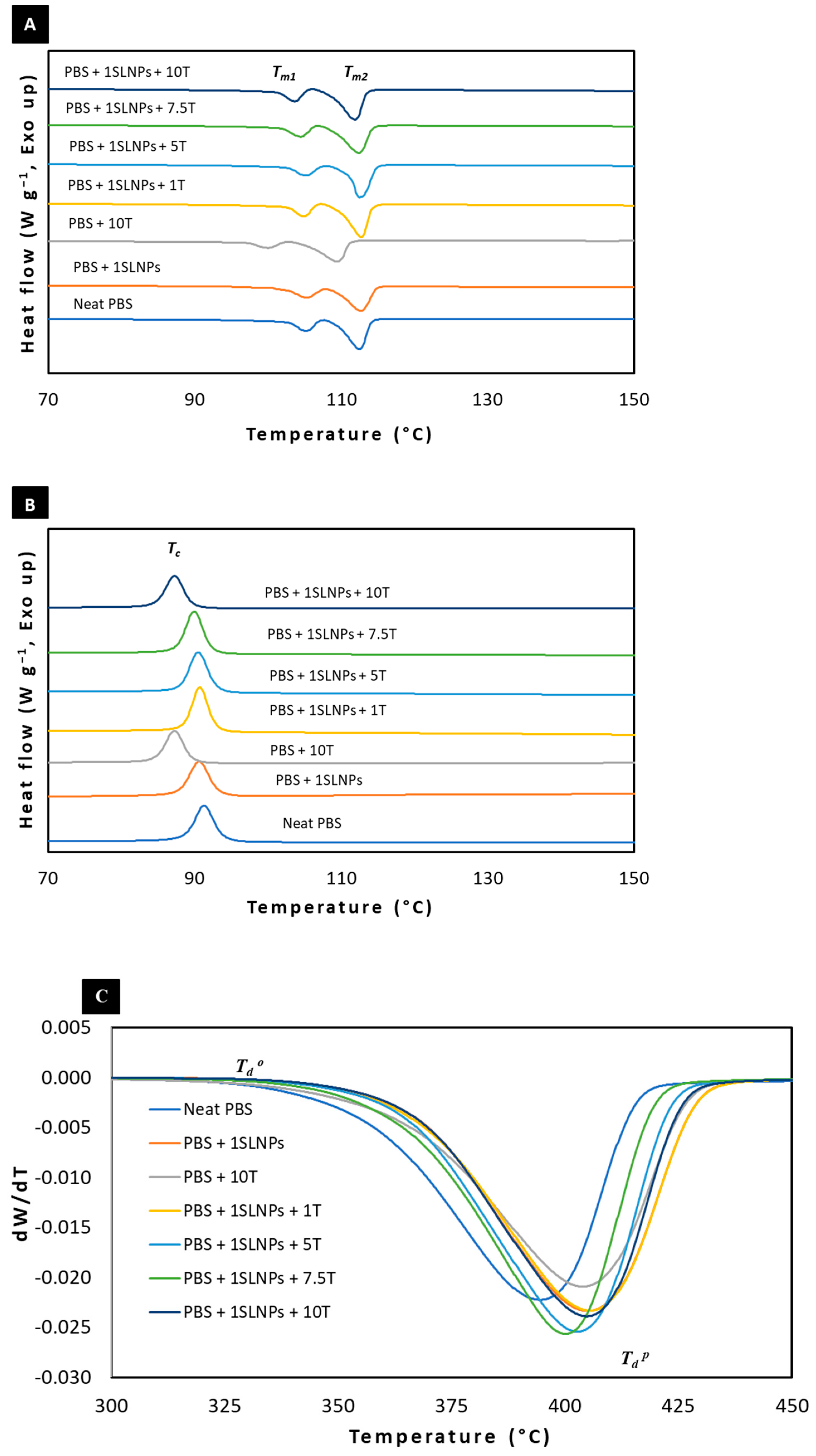
2.4.3. Thermal Properties
2.4.4. Mechanical Properties
2.4.5. Barrier Properties
2.5. Investigation of Antimicrobial Activities of SLNPs
2.5.1. Isolation of Pure Culture of L. theobromae
2.5.2. Antimicrobial Activities of SR-Lignin and SLNPs In Vitro
2.5.3. Antimicrobial Activities of SLNPs Incorporated into PBS Composite Films In Vitro
2.5.4. Antimicrobial Activities of PBS Composite Films In Vivo
2.6. Statistical Analysis
3. Results
3.1. Physicochemical Characterization of PBS Composite Films
3.1.1. Morphology
3.1.2. FTIR Analysis
3.1.3. Thermal Properties
3.1.4. Mechanical Properties
3.1.5. Barrier Properties
3.2. Investigation of Antimicrobial Activities of SLNPs
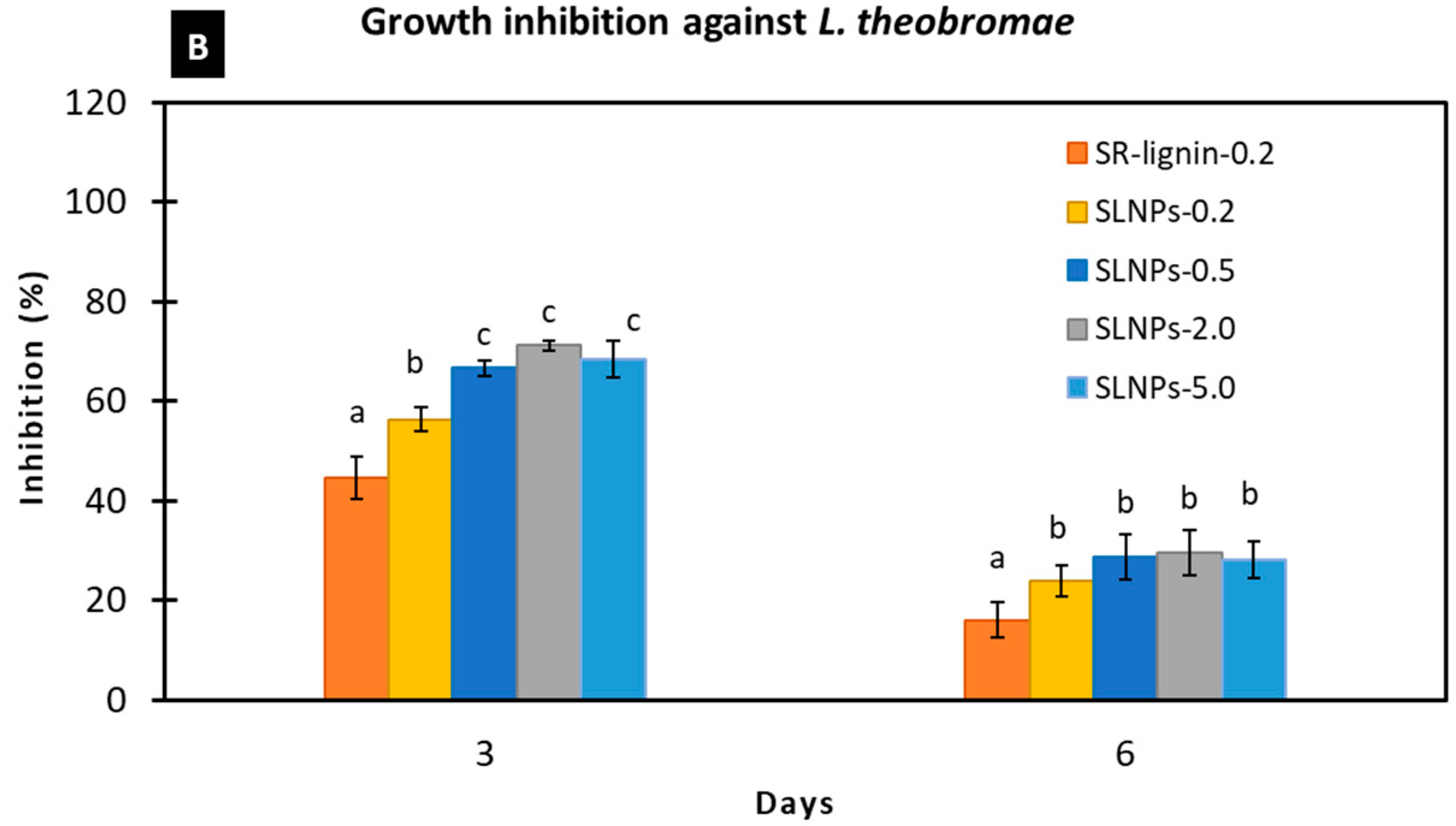
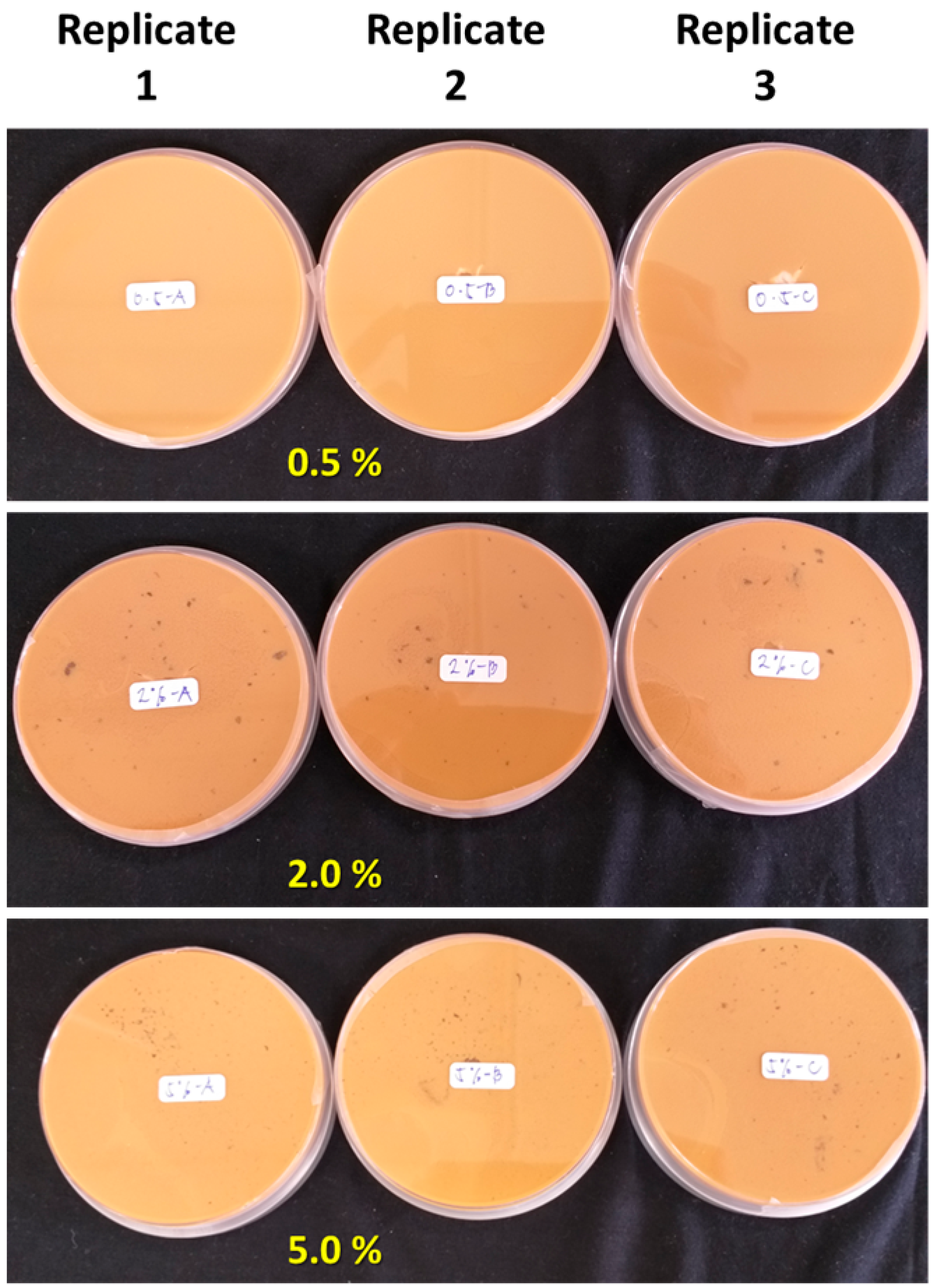
3.2.1. Antimicrobial Activities of SR-Lignin and SLNPs In Vitro
3.2.2. Antimicrobial Activities of SLNPs Incorporated into PBS Composite Films In Vitro
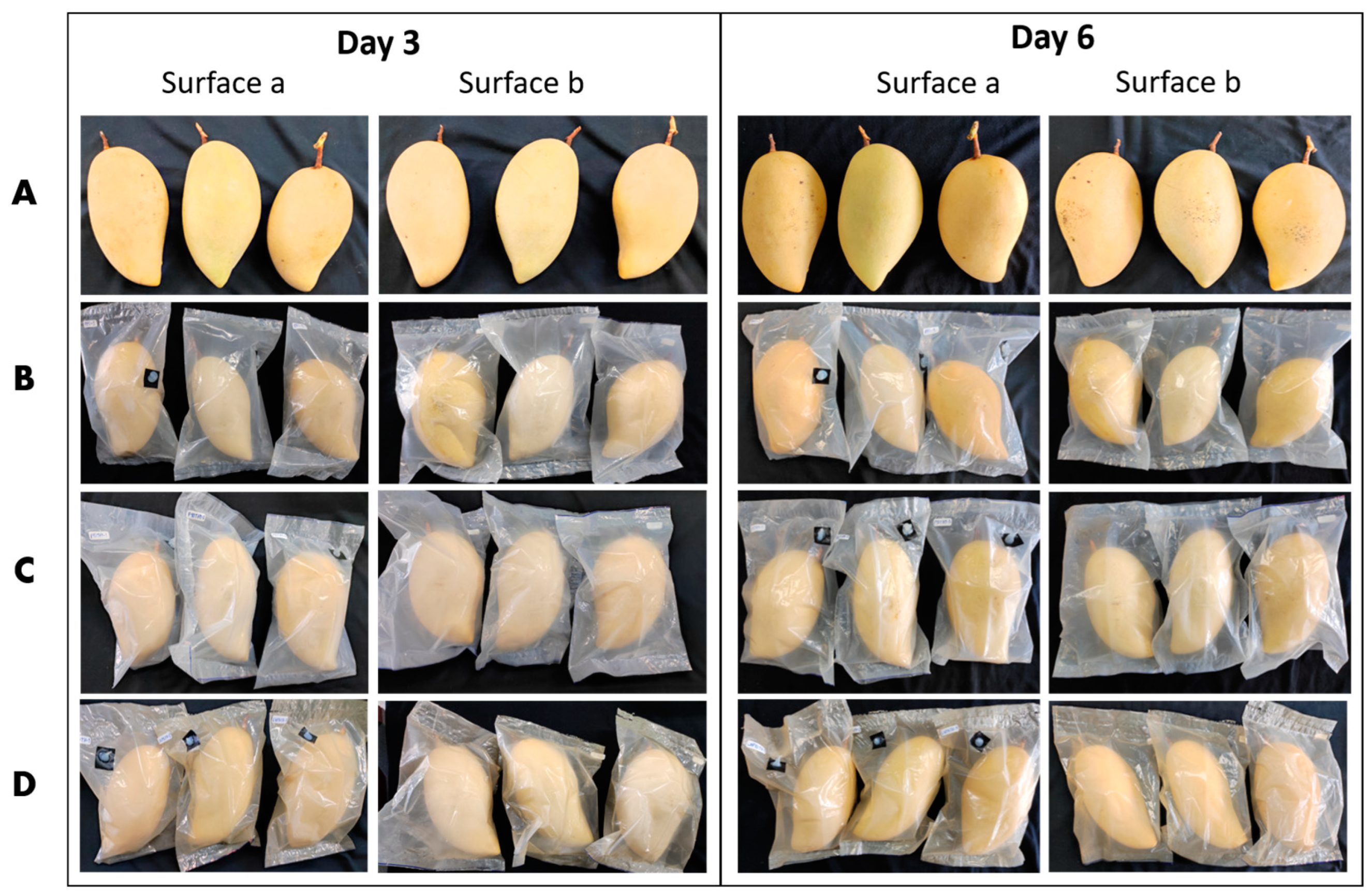
3.2.3. Antimicrobial Activities of PBS Composite Films In Vivo
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sikora, J.W.; Majewski, Ł.; Puszka, A. Modern biodegradable plastics—Processing and properties part II. Materials 2021, 14, 2523. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.M.; Martins, J.R.; Sanvezzo, P.B.; Macedo, J.V.; Branciforti, M.C.; Halley, P.; Botaro, V.R.; Brienzo, M. Advantages and disadvantages of bioplastics production from starch and lignocellulosic components. Polymers 2021, 13, 2484. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.; Vivekanandhan, S.; Mohanty, A.; Denault, J. Nanotechnologies for Agricultural Bioproducts. In Comprehensive Biotechnology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 119–127. [Google Scholar] [CrossRef]
- Brunner, G. Processing of Biomass with Hydrothermal and Supercritical Water. In Supercritical Fluid Science and Technology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 395–509. [Google Scholar] [CrossRef]
- Ahmad, U.M.; Ji, N.; Li, H.; Wu, Q.; Song, C.; Liu, Q.; Ma, D.; Lu, X. Can lignin be transformed into agrochemicals? Recent advances in the agricultural applications of lignin. Ind. Crop. Prod. 2021, 170, 113646. [Google Scholar] [CrossRef]
- Vinardell, M.P.; Mitjans, M. Lignins and Their Derivatives with Beneficial Effects on Human Health. Int. J. Mol. Sci. 2017, 18, 1219. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Fu, S.; Gan, L. Lignin-modified thermoplastic materials. In Lignin Chemistry and Applications; Huang, J., Fu, S., Gan, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–161. [Google Scholar]
- Rudnik, E. Compostable polymer properties and packaging applications. In Plastic films in food packaging; Ebnesajjad, S., Ed.; William Andrew Publishing: Norwich, NY, USA, 2013; pp. 217–248. [Google Scholar]
- Jiang, L.; Zhang, J. Biodegradable and biobased polymers. In Applied plastics engineering handbook, 2nd ed.; Kutz, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 127–143. [Google Scholar]
- Aliotta, L.; Seggiani, M.; Lazzeri, A.; Giganate, V.; Cinelli, P. A brief review of Poly(Butylene Succinate) (PBS) and Its Main Copolymers: Synthesis, Blends, Composites, Biodegradability, and Applications. Polymers 2022, 14, 844. [Google Scholar] [CrossRef]
- Tachibana, Y.; Masuda, T.; Funabashi, M.; Kunioka, M. Chemical Synthesis of Fully Biomass-Based Poly(butylene succinate) from Inedible-Biomass-Based Furfural and Evaluation of Its Biomass Carbon Ratio. Biomacromolecules 2010, 11, 2760–2765. [Google Scholar] [CrossRef]
- Soccio, M.; Dominici, F.; Quattrosoldi, S.; Luzi, F.; Munari, A.; Torre, L.; Lotti, N.; Puglia, D. PBS-Based Green Copolymer as an Efficient Compatibilizer in Thermoplastic Inedible Wheat Flour/Poly(butylene succinate) Blends. Biomacromolecules 2020, 21, 3254–3269. [Google Scholar] [CrossRef]
- Jordá-Reolid, M.; Ibáñez-García, A.; Catani, L.; Martínez-García, A. Development of Blends to Improve Flexibility of Biodegradable Polymers. Polymers 2022, 14, 5223. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Infurna, G.; Baiamonte, M.; D’Anna, F. Natural Compounds as Sustainable Additives for Biopolymers. Polymers 2020, 12, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pivsa-Art, S.; Pivsa-Art, W. Eco-friendly bamboo fiber-reinforced poly(butylene succinate) biocomposites. Polym. Compos. 2021, 42, 1752–1759. [Google Scholar] [CrossRef]
- Saffian, H.A.; Hyun-Joong, K.; Tahir, P.; Ibrahim, N.A.; Lee, S.H.; Lee, C.H. Effect of Lignin Modification on Properties of Kenaf Core Fiber Reinforced Poly(Butylene Succinate) Biocomposites. Materials 2019, 12, 4043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domínguez-Robles, J.; Larrañeta, E.; Fong, M.L.; Martin, N.K.; Irwin, N.J.; Mutjé, P.; Tarrés, Q.; Delgado-Aguilar, M. Lignin/poly(butylene succinate) composites with antioxidant and antibacterial properties for potential biomedical applications. Int. J. Biol. Macromol. 2019, 145, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Mtibe, A.; Hlekelele, L.; Kleyi, P.E.; Muniyasamy, S.; Nomadolo, N.E.; Ofosu, O.; Ojijo, V.; John, M.J. Fabrication of a Polybutylene Succinate (PBS)/Polybutylene Adipate-Co-Terephthalate (PBAT)-Based Hybrid System Reinforced with Lignin and Zinc Nanoparticles for Potential Biomedical Applications. Polymers 2022, 14, 5065. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G. Lignin: A renewable raw material. In Encyclopedia of Renewable and Sustainable Materials; Hashmi, S., Choudhury, I.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 5, pp. 102–118. [Google Scholar]
- Basbasan, A.J.; Hararak, B.; Winotapun, C.; Wanmolee, W.; Leelaphiwat, P.; Boonruang, K.; Chinsirikul, W.; Chonhenchob, V. Emerging challenges on viability and commercialization of lignin in biobased polymers for food packaging: A review. Food Packag. Shelf Life 2022, 34, 100969. [Google Scholar] [CrossRef]
- Liu, R.; Smeds, A.; Tirri, T.; Zhang, H.; Willför, S.; Xu, C. Influence of Carbohydrates Covalently Bonded with Lignin on Solvent Fractionation, Thermal Properties, and Nanoparticle Formation of Lignin. ACS Sustain. Chem. Eng. 2022, 10, 14588–14599. [Google Scholar] [CrossRef]
- Alzagameem, A.; Klein, S.E.; Bergs, M.; Do, X.T.; Korte, I.; Dohlen, S.; Hüwe, C.; Kreyenschmidt, J.; Kamm, B.; Larkins, M.; et al. Antimicrobial Activity of Lignin and Lignin-Derived Cellulose and Chitosan Composites against Selected Pathogenic and Spoilage Microorganisms. Polymers 2019, 11, 670. [Google Scholar] [CrossRef] [Green Version]
- Morena, A.G.; Bassegoda, A.; Natan, M.; Jacobi, G.; Banin, E.; Tzanov, T. Antibacterial Properties and Mechanisms of Action of Sonoenzymatically Synthesized Lignin-Based Nanoparticles. ACS Appl. Mater. Interfaces 2022. [Google Scholar] [CrossRef]
- Yun, J.; Wei, L.; Li, W.; Gong, D.; Qin, H.; Feng, X.; Li, G.; Ling, Z.; Wang, P.; Yin, B. Isolating High Antimicrobial Ability Lignin From Bamboo Kraft Lignin by Organosolv Fractionation. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef]
- Gregorova, A.; Redik, S.; Sedlarik, V.; Stelzer, F. Lignin-containing polyethylene films with antibacterial activity. In Proceedings of the 3rd International Conference on Thomson Reuters of NANOCON, Brno, Czech Republic, 21–23 September 2011. [Google Scholar]
- Dong, X.; Dong, M.; Lu, Y.; Turley, A.; Jin, T.; Wu, C. Antimicrobial and antioxidant activities of lignin from residue of corn stover to ethanol production. Ind. Crop. Prod. 2011, 34, 1629–1634. [Google Scholar] [CrossRef]
- Lobo, F.; Franco, A.; Fernandes, E.; Reis, R. An Overview of the Antimicrobial Properties of Lignocellulosic Materials. Molecules 2021, 26, 1749. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonruang, K.; Kerddonfag, N.; Chinsirikul, W.; Mitcham, E.J.; Chonhenchob, V. Antifungal effect of poly(lactic acid) films containing thymol and R-(-)-carvone against anthracnose pathogens isolated from avocado and citrus. Food Control. 2017, 78, 85–93. [Google Scholar] [CrossRef]
- Zadeh, E.M.; O’Keefe, S.F.; Kim, Y.-T. Utilization of Lignin in Biopolymeric Packaging Films. ACS Omega 2018, 3, 7388–7398. [Google Scholar] [CrossRef] [Green Version]
- Hararak, B.; Wanmolee, W.; Wijaranakul, P.; Prakymoramas, N.; Winotapun, C.; Kraithong, W.; Nakason, K. Physicochemical properties of lignin nanoparticles from softwood and their potential application in sustainable pre-harvest bagging as transparent UV-shielding films. Int. J. Biol. Macromol. 2023, 229, 575–588. [Google Scholar] [CrossRef]
- Suwanamornlert, P.; Kerddonfag, N.; Sane, A.; Chinsirikul, W.; Zhou, W.; Chonhenchob, V. Poly (lactic acid)/poly (butylene-succinate-co-adipate)(PLA/PBSA) blend films containing thymol as alternative to synthetic preservatives for active packaging of bread. Food Packaging and Shelf Life 2020, 25, 100515. [Google Scholar] [CrossRef]
- Boonruang, K.; Chinsirikul, W.; Hararak, B.; Kerddonfag, N.; Chonhenchob, V. Antifungal Poly(lactic acid) Films Containing Thymol and Carvone. MATEC Web Conf. 2016, 67, 6107. [Google Scholar] [CrossRef]
- Yang, W.; Owczarek, J.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.; Kenny, J.; Torre, L.; Puglia, D. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Ind. Crop. Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]
- Platnieks, O.; Gaidukovs, S.; Barkane, A.; Sereda, A.; Gaidukova, G.; Grase, L.; Thakur, V.; Filipova, I.; Fridrihsone, V.; Skute, M.; et al. Bio-Based Poly(butylene succinate)/Microcrystalline Cellulose/Nanofibrillated Cellulose-Based Sustainable Polymer Composites: Thermo-Mechanical and Biodegradation Studies. Polymers 2020, 12, 1472. [Google Scholar] [CrossRef]
- Khan, M.; Chonhenchob, V.; Huang, C.; Suwanamornlert, P. Antifungal Activity of Propyl Disulfide from Neem (Azadirachta indica) in Vapor and Agar Diffusion Assays against Anthracnose Pathogens (Colletotrichum gloeosporioides and Colletotrichum acutatum) in Mango Fruit. Microorganisms 2021, 9, 839. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2015, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaguer, M.P.; Lopez-Carballo, G.; Catala, R.; Gavara, R.; Hernandez-Munoz, P. Antifungal properties of gliadin films incorporating cinnamaldehyde and application in active food packaging of bread and cheese spread foodstuffs. Int. J. Food Microbiol. 2013, 166, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Phua, Y.J. Reactive processing of maleic anhydride-grafted poly(butylene succinate) and the compatibilizing effect on poly(butylene succinate) nanocomposites. Express Polym. Lett. 2013, 7, 340–354. [Google Scholar] [CrossRef]
- Saffian, H.; Yamaguchi, M.; Ariffin, H.; Abdan, K.; Kassim, N.; Lee, S.; Lee, C.; Shafi, A.; Alias, A.H. Thermal, Physical and Mechanical Properties of Poly(Butylene Succinate)/Kenaf Core Fibers Composites Reinforced with Esterified Lignin. Polymers 2021, 13, 2359. [Google Scholar] [CrossRef]
- Valderrama, A.C.S.; Rojas De, G.C. Traceability of Active Compounds of Essential Oils in Antimicrobial Food Packaging Using a Chemometric Method by ATR-FTIR. Am. J. Anal. Chem. 2017, 8, 726–741. [Google Scholar] [CrossRef] [Green Version]
- Mohamad, N.; Mazlan, M.M.; Tawakkal, I.S.M.A.; Talib, R.A.; Kian, L.K.; Jawaid, M. Characterization of Active Polybutylene Succinate Films Filled Essential Oils for Food Packaging Application. J. Polym. Environ. 2021, 30, 585–596. [Google Scholar] [CrossRef]
- Zarei, M.; El Fray, M. Synthesis of Hydrophilic Poly(butylene succinate-butylene dilinoleate) (PBS-DLS) Copolymers Containing Poly(Ethylene Glycol) (PEG) of Variable Molecular Weights. Polymers 2021, 13, 3177. [Google Scholar] [CrossRef]
- Rajkumar, P.; Selvaraj, S.; Suganya, R.; Velmurugan, D.; Gunasekaran, S.; Kumaresan, S. Vibrational and electronic spectral analysis of thymol an isomer of carvacrol isolated from Trachyspermum ammi seed: A combined experimental and theoretical study. Chem. Data Collect. 2018, 15-16, 10–31. [Google Scholar] [CrossRef]
- Sahoo, S.; Misra, M.; Mohanty, A.K. Enhanced properties of lignin-based biodegradable polymer composites using injection moulding process. Compos. Part A-Appl. Sci. Manuf. 2011, 42, 1710–1718. [Google Scholar] [CrossRef]
- Sen, S.; Patil, S.; Argyropoulos, D. Thermal properties of lignin in copolymers, blends, and composites: A review. Green Chem. 2015, 17, 4862–4887. [Google Scholar] [CrossRef]
- Pereira, L.A.S.; Silva, P.D.C.E.; Pagnossa, J.P.; Miranda, K.W.E.; Medeiros, E.S.; Piccoli, R.H.; de Oliveira, J.E. Antimicrobial zein coatings plasticized with garlic and thyme essential oils. Braz. J. Food Technol. 2019, 22. [Google Scholar] [CrossRef]
- Javidi, Z.; Hosseini, S.F.; Rezaei, M. Development of flexible bactericidal films based on poly(lactic acid) and essential oil and its effectiveness to reduce microbial growth of refrigerated rainbow trout. Lwt 2016, 72, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Balani, K.; Verma, V.; Agarwal, A.; Narayan, R. Physical, thermal, and mechanical properties of polymers. In Biosurfaces: A Materials Science and Engineering Perspective; Balani, K., Verma, V., Agarwal, A., Narayan, R., Eds.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2015; pp. 329–344. [Google Scholar]
- Celebi, H.; Gunes, E. Combined effect of a plasticizer and carvacrol and thymol on the mechanical, thermal, morphological properties of poly(lactic acid). J. Appl. Polym. Sci. 2017, 135, 45895. [Google Scholar] [CrossRef]
- Berlin, H. Investigation of Polymers with Differential Scanning Calorimetry. Available online: https://polymerscience.physik.hu-berlin.de/docs/manuals/DSC.pdf (accessed on 27 November 2022).
- Basu, P. Biomass gasification, pyrolysis and torrefaction: Practical design and theory. In Biomass Gasification, Pyrolysis and Torrefaction, 3rd ed.; Basu, P., Ed.; Academic press: Cambridge, MA, USA, 2018; pp. 479–495. [Google Scholar]
- Król-Morkisz, K.; Pielichowska, K. Thermal decomposition of polymer nanocomposites with functionalized nanoparticles. In Polymer composites with functionalized nanoparticles; Pielichowski, K., Majka, T.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 405–435. [Google Scholar]
- Mariën, H.; Peeters, L.; Harumashi, T.; Rubens, M.; Vendamme, R.; Vleeschouwers, R.; Vanbroekhoven, K. Improving the Thermal Stability of MS Polymers with Lignin Fractions. Adhes. Adhes. 2022, 19, 30–33. [Google Scholar] [CrossRef]
- Mousavioun, P.; Doherty, W.O.S.; George, G. Thermal stability and miscibility of poly(hydroxybutyrate) and soda lignin blends. Ind. Crops Prod. 2010, 32, 656–661. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. Physiochemical properties and degradation. In Microplastic Pollutants; Elsevier: Amsterdam, The Netherlands, 2017; pp. 57–100. [Google Scholar] [CrossRef]
- Weihua, K.; He, Y.; Asakawa, N.; Inoue, Y. Effect of lignin particles as a nucleating agent on crystallization of poly(3-hydroxybutyrate). J. Appl. Polym. Sci. 2004, 94, 2466–2474. [Google Scholar] [CrossRef]
- Purnama, P.; Kim, S.H. Biodegradable blends of stereocomplex polylactide and lignin by supercritical carbon dioxide-solvent system. Macromol. Res. 2013, 22, 74–78. [Google Scholar] [CrossRef]
- Petchwattana, N.; Naknaen, P. Utilization of thymol as an antimicrobial agent for biodegradable poly(butylene succinate). Mater. Chem. Phys. 2015, 163, 369–375. [Google Scholar] [CrossRef]
- Salamon, D. Advanced ceramics. In Advanced ceramics for dentistry; Shen, J.Z., Kosmač, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 103–122. [Google Scholar]
- Mariana, M.; Alfatah, T.; Abdul Khalil, H.P.S.; Yahya, E.B.; Olaiya, N.; Nuryawan, A.; Mistar, E.; Abdullah, C.; Abdulmadjid, S.; Ismail, H. A current advancement on the role of lignin as sustainable reinforcement material in biopolymeric blends. J. Mater. Res. Technol. 2021, 15, 2287–2316. [Google Scholar] [CrossRef]
- Chantapet, P.; Kunanopparat, T.; Menut, P.; Siriwattanayotin, S. Extrusion Processing of Wheat Gluten Bioplastic: Effect of the Addition of Kraft Lignin. J. Polym. Environ. 2012, 21, 864–873. [Google Scholar] [CrossRef]
- Sakunkittiyut, Y.; Kunanopparat, T.; Menut, P.; Siriwattanayotin, S. Effect of kraft lignin on protein aggregation, functional, and rheological properties of fish protein-based material. J. Appl. Polym. Sci. 2012, 127, 1703–1710. [Google Scholar] [CrossRef]
- Bhat, R.; Abdullah, N.; Din, R.H.; Tay, G.-S. Producing novel sago starch based food packaging films by incorporating lignin isolated from oil palm black liquor waste. J. Food Eng. 2013, 119, 707–713. [Google Scholar] [CrossRef]
- Shankar, S.; Reddy, J.P.; Rhim, J.-W. Effect of lignin on water vapor barrier, mechanical, and structural properties of agar/lignin composite films. Int. J. Biol. Macromol. 2015, 81, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Gomide, R.A.C.; De Oliveira, A.C.S.; Luvizaro, L.B.; Yoshida, M.I.; De Oliveira, C.R.; Borges, S.V. Biopolymeric films based on whey protein isolate/lignin microparticles for waste recovery. J. Food Process. Eng. 2020, 44. [Google Scholar] [CrossRef]
- Lian, H.; Wei, W.; Wang, D.; Jia, L.; Yang, X. Effect of thymol on physical properties, antimicrobial properties and fresh-keeping application of cherry tomato of starch/PBAT extrusion blowing films. Food Sci. Technol. 2022, 42. [Google Scholar] [CrossRef]
- Aadil, K.R.; Prajapati, D.; Jha, H. Improvement of physcio-chemical and functional properties of alginate film by Acacia lignin. Food Packag. Shelf Life 2016, 10, 25–33. [Google Scholar] [CrossRef]
- Izaguirre, N.; Gordobil, O.; Robles, E.; Labidi, J. Enhancement of UV absorbance and mechanical properties of chitosan films by the incorporation of solvolytically fractionated lignins. Int. J. Biol. Macromol. 2020, 155, 447–455. [Google Scholar] [CrossRef]
- Zhou, S.-J.; Wang, H.-M.; Xiong, S.-J.; Sun, J.-M.; Wang, Y.-Y.; Yu, S.; Sun, Z.; Wen, J.-L.; Yuan, T.-Q. Technical Lignin Valorization in Biodegradable Polyester-Based Plastics (BPPs). ACS Sustain. Chem. Eng. 2021, 9, 12017–12042. [Google Scholar] [CrossRef]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Echegoyen Sanz, Y.E.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the Processing and Properties of Polymer Nanocomposites and Nanocoatings and Their Applications in the Packaging, Automotive and Solar Energy Fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [Green Version]
- Abdan, K.B.; Yong, S.C.; Chiang, E.C.W.; Talib, R.A.; Hui, T.C.; Hao, L.C. Barrier properties, antimicrobial and antifungal activities of chitin and chitosan-based IPNs, gels, blends, composites, and nanocomposites. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 175–227. [Google Scholar] [CrossRef]
- Kovalcik, A.; Machovsky, M.; Kozakova, Z.; Koller, M. Designing packaging materials with viscoelastic and gas barrier properties by optimized processing of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with lignin. React. Funct. Polym. 2015, 94, 25–34. [Google Scholar] [CrossRef]
- Othman, S.H.; Nordin, N.; Azman, N.A.A.; Tawakkal, I.S.M.A.; Basha, R.K. Effects of nanocellulose fiber and thymol on mechanical, thermal, and barrier properties of corn starch films. Int. J. Biol. Macromol. 2021, 183, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-W.; Nair, S.S.; Chen, H.; Yan, N.; Farnood, R.; Li, F.-Y. Thermally stable, enhanced water barrier, high strength starch bio-composite reinforced with lignin containing cellulose nanofibrils. Carbohydr. Polym. 2019, 230, 115626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Terrasson, V.; Guénin, E. Lignin Nanoparticles and Their Nanocomposites. Nanomaterials 2021, 11, 1336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Lasiodiplodia theobromae in Citrus Fruit (Diplodia Stem-End Rot). In Postharvest Decay: Control Strategies; Bautista-Baños, S., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 309–335. [Google Scholar]
- Chen, K.; Qiu, X.; Yang, D.; Qian, Y. Amino acid-functionalized polyampholytes as natural broad-spectrum antimicrobial agents for high-efficient personal protection. Green Chem. 2020, 22, 6357–6371. [Google Scholar] [CrossRef]
- Spasojevic, D.; Zmejkoski, D.; Glamoclija, J.; Nikolic, M.; Sokovic, M.; Milosevic, V.; Jaric, I.; Stojanovic, M.; Marinkovic, E.; Barisani-Asenbauer, T.; et al. Lignin model compound in alginate hydrogel: A strong antimicrobial agent with high potential in wound treatment. Int. J. Antimicrob. Agents 2016, 48, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, N.; Karsani, S.A.; Alias, S.A. Fungal survival under temperature stress: A proteomic perspective. Peerj 2020, 8, e10423. [Google Scholar] [CrossRef]
- Mensah-Attipoe, J.; Toyinbo, O. Fungal growth and aerosolization from various conditions and materials. In Fungal infection; de Loreto, É.S., Tondolo, J.S.M., Eds.; IntechOpen: London, UK, 2019; pp. 1–10. [Google Scholar]
- Zikeli, F.; Vinciguerra, V.; Sennato, S.; Mugnozza, G.S.; Romagnoli, M. Preparation of Lignin Nanoparticles with Entrapped Essential Oil as a Bio-Based Biocide Delivery System. ACS Omega 2019, 5, 358–368. [Google Scholar] [CrossRef]
- Kamle, M.; Kumar, P. Colletotrichum gloeosporioides: Pathogen of Anthracnose Disease in Mango (Mangifera indica L.). In Current Trends in Plant Disease Diagnostics and Management Practices; Fungal Biology: Berlin/Heidelberg, Germany, 2016; pp. 207–219. [Google Scholar]
- Galsurker, O.; Diskin, S.; Duanis-Assaf, D.; Doron-Faigenboim, A.; Maurer, D.; Feygenberg, O.; Alkan, N. Harvesting Mango Fruit with a Short Stem-End Altered Endophytic Microbiome and Reduce Stem-End Rot. Microorganisms 2020, 8, 558. [Google Scholar] [CrossRef]
- Dannemiller, K.C.; Weschler, C.J.; Peccia, J. Fungal and bacterial growth in floor dust at elevated relative humidity levels. Indoor Air 2016, 27, 354–363. [Google Scholar] [CrossRef]




| Formulations | PBS (w/w) | SLNPs (w/w) | Thymol (w/w) |
|---|---|---|---|
| PBS | 100 | - | - |
| PBS + 10T | 90 | - | 10 |
| PBS + 1SLNPs | 99 | 1 | - |
| PBS + 1SLNPs + 1T | 98 | 1 | 1 |
| PBS + 1SLNPs + 5T | 94 | 1 | 5 |
| PBS + 1SLNPs + 7.5T | 91.5 | 1 | 7.5 |
| PBS + 1SLNPs + 10T | 89 | 1 | 10 |
| Film Samples | Tg (°C) | Tm1 (°C) | Tm2 (°C) | Tc (°C) | Tdo (°C) | Tdp (°C) | Xc (%) |
|---|---|---|---|---|---|---|---|
| Neat PBS | −39.1 | 105.2 | 112.5 | 91.3 | 354.1 | 394.5 | 36.1 |
| PBS + 1SLNPs | −35.7 | 105.2 | 112.7 | 90.8 | 364.7 | 405.0 | 38.5 |
| PBS + 10T | −43.7 | 100.0 | 109.5 | 87.5 | 361.7 | 404.0 | 35.4 |
| PBS + 1SLNPs + 1T | −33.2 | 104.8 | 112.8 | 90.3 | 364.6 | 405.5 | 34.1 |
| PBS + 1SLNPs + 5T | −34.3 | 105.2 | 112.5 | 90.2 | 362.7 | 402.8 | 35.9 |
| PBS + 1SLNPs + 7.5T | −40.9 | 104.5 | 112.5 | 90.0 | 362.9 | 400.2 | 37.0 |
| PBS + 1SLNPs + 10T | −42.1 | 103.7 | 111.8 | 89.7 | 365.2 | 404.8 | 32.2 |
| Film Samples | YM (MPa) | TS (MPa) | EB (%) |
|---|---|---|---|
| Neat PBS | 593.4 ± 84.2 bc | 34.3 ± 1.4 bc | 11.6 ± 0.8 a |
| PBS + 1SLNPs | 601.9 ± 57.9 c | 35.6 ± 1.8 c | 10.3 ± 0.8 a |
| PBS + 10T | 491.9 ± 62.8 ab | 28.3 ± 2.5 a | 11.9 ± 0.4 a |
| PBS + 1SLNP + 1T | 589.1 ± 63.9 bc | 34.9 ± 1.2 bc | 11.6 ± 0.9 a |
| PBS + 1SLNP + 5T | 445.3 ± 97.3 a | 34.0 ± 7.1 bc | 11.6 ± 2.1 a |
| PBS + 1SLNP + 7.5T | 466.5 ± 62.2 a | 34.9 ± 3.2 bc | 11.2 ± 1.7 a |
| PBS + 1SLNP + 10T | 486.6 ± 104.4 ab | 30.0 ± 4.6 ab | 10.6 ± 0.6 a |
| Film Samples | Thickness (µm) | Oxygen Permeability (cm3 m h−1 m−2 atm−1) | Water Vapor Permeability (g m h−1 m−2 atm−1) |
|---|---|---|---|
| Neat PBS | 29.8 ± 0.8 a | 2.28 × 10−3 ± 0.0002 b | 1.54 × 10−3 ± 0.0001 d |
| PBS + 1SLNPs | 30.0 ± 1.6 a | 1.59 × 10−3 ± 0.0002 a | 1.41 × 10−3 ± 0.0001 cd |
| PBS + 10T | 31.8 ± 4.9 a | 2.37 × 10−3 ± 0.0002 bc | 1.14 × 10−3 ± 0.0001 ab |
| PBS + 1SLNP + 1T | 30.2 ± 2.7 a | 2.40 × 10−3 ± 0.0004 bc | 1.28 × 10−3 ± 0.0001 bc |
| PBS + 1SLNP + 5T | 30.4 ± 2.7 a | 2.08 × 10−3 ± 0.0001 b | 1.15 × 10−3 ± 0.0002 ab |
| PBS + 1SLNP + 7.5T | 33.4 ± 1.5 a | 2.56 × 10−3 ± 0.0005 bc | 1.28 × 10−3 ± 0.0003 ab |
| PBS + 1SLNP + 10T | 33.4 ± 2.4 a | 2.84 × 10−3 ± 0.0004 c | 1.09 × 10−3 ± 0.0000 ab |
| Treatment | Decay Area (cm2) |
|---|---|
| CONTROL | 16.0 ± 2.1 c |
| Neat PBS | 17.2 ± 0.3 c |
| PBS + 10T | 5.2 ± 0.7 b |
| PBS + 1SLNPs + 10T | 1.1 ± 0.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basbasan, A.J.; Hararak, B.; Winotapun, C.; Wanmolee, W.; Chinsirikul, W.; Leelaphiwat, P.; Chonhenchob, V.; Boonruang, K. Lignin Nanoparticles for Enhancing Physicochemical and Antimicrobial Properties of Polybutylene Succinate/Thymol Composite Film for Active Packaging. Polymers 2023, 15, 989. https://doi.org/10.3390/polym15040989
Basbasan AJ, Hararak B, Winotapun C, Wanmolee W, Chinsirikul W, Leelaphiwat P, Chonhenchob V, Boonruang K. Lignin Nanoparticles for Enhancing Physicochemical and Antimicrobial Properties of Polybutylene Succinate/Thymol Composite Film for Active Packaging. Polymers. 2023; 15(4):989. https://doi.org/10.3390/polym15040989
Chicago/Turabian StyleBasbasan, Angel Jr, Bongkot Hararak, Charinee Winotapun, Wanwitoo Wanmolee, Wannee Chinsirikul, Pattarin Leelaphiwat, Vanee Chonhenchob, and Kanchana Boonruang. 2023. "Lignin Nanoparticles for Enhancing Physicochemical and Antimicrobial Properties of Polybutylene Succinate/Thymol Composite Film for Active Packaging" Polymers 15, no. 4: 989. https://doi.org/10.3390/polym15040989
APA StyleBasbasan, A. J., Hararak, B., Winotapun, C., Wanmolee, W., Chinsirikul, W., Leelaphiwat, P., Chonhenchob, V., & Boonruang, K. (2023). Lignin Nanoparticles for Enhancing Physicochemical and Antimicrobial Properties of Polybutylene Succinate/Thymol Composite Film for Active Packaging. Polymers, 15(4), 989. https://doi.org/10.3390/polym15040989









