Stimuli-Responsive and Antibacterial Cellulose-Chitosan Hydrogels Containing Polydiacetylene Nanosheets
Abstract
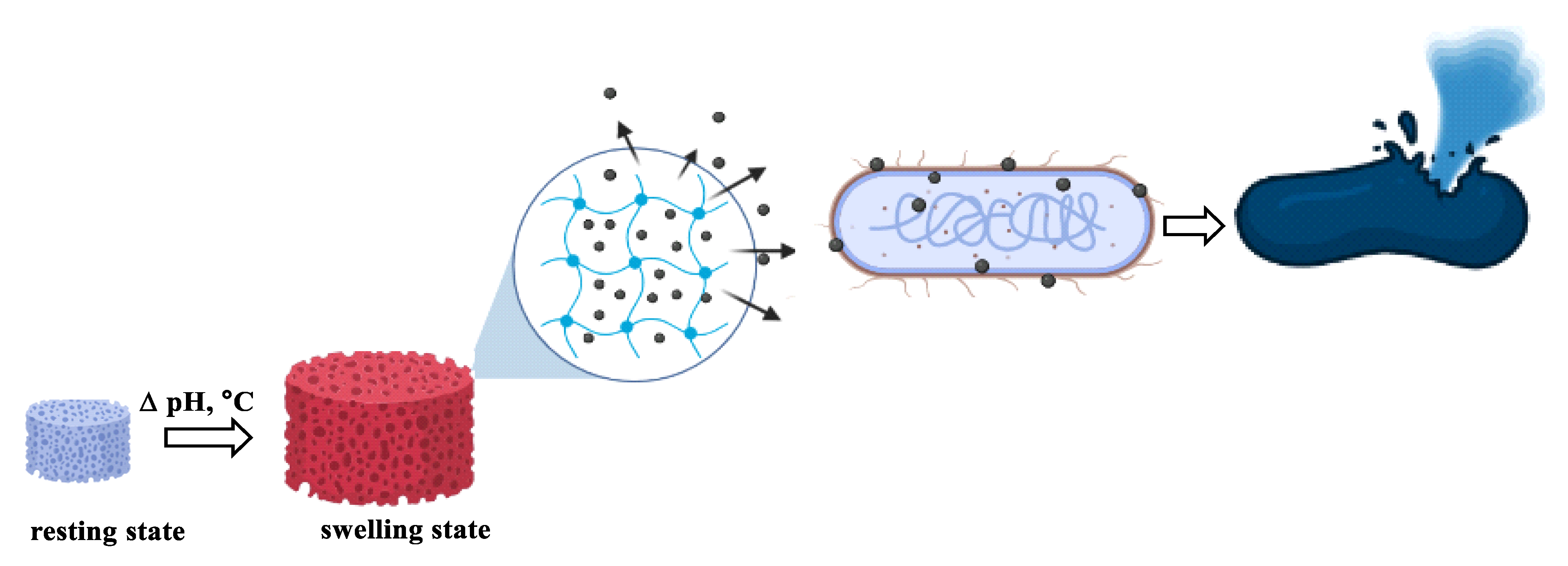
1. Introduction
2. Materials and Methods
2.1. Materials
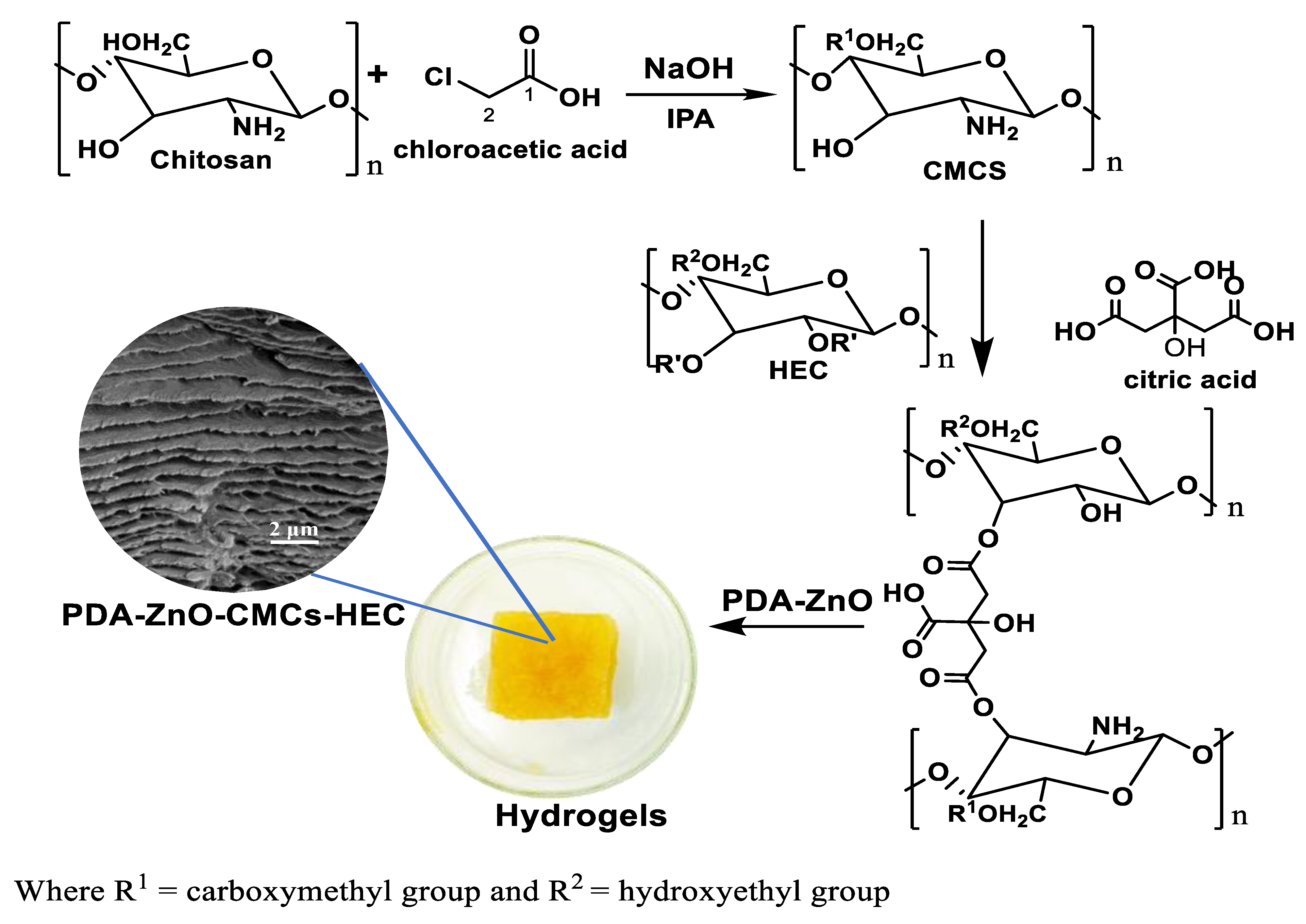
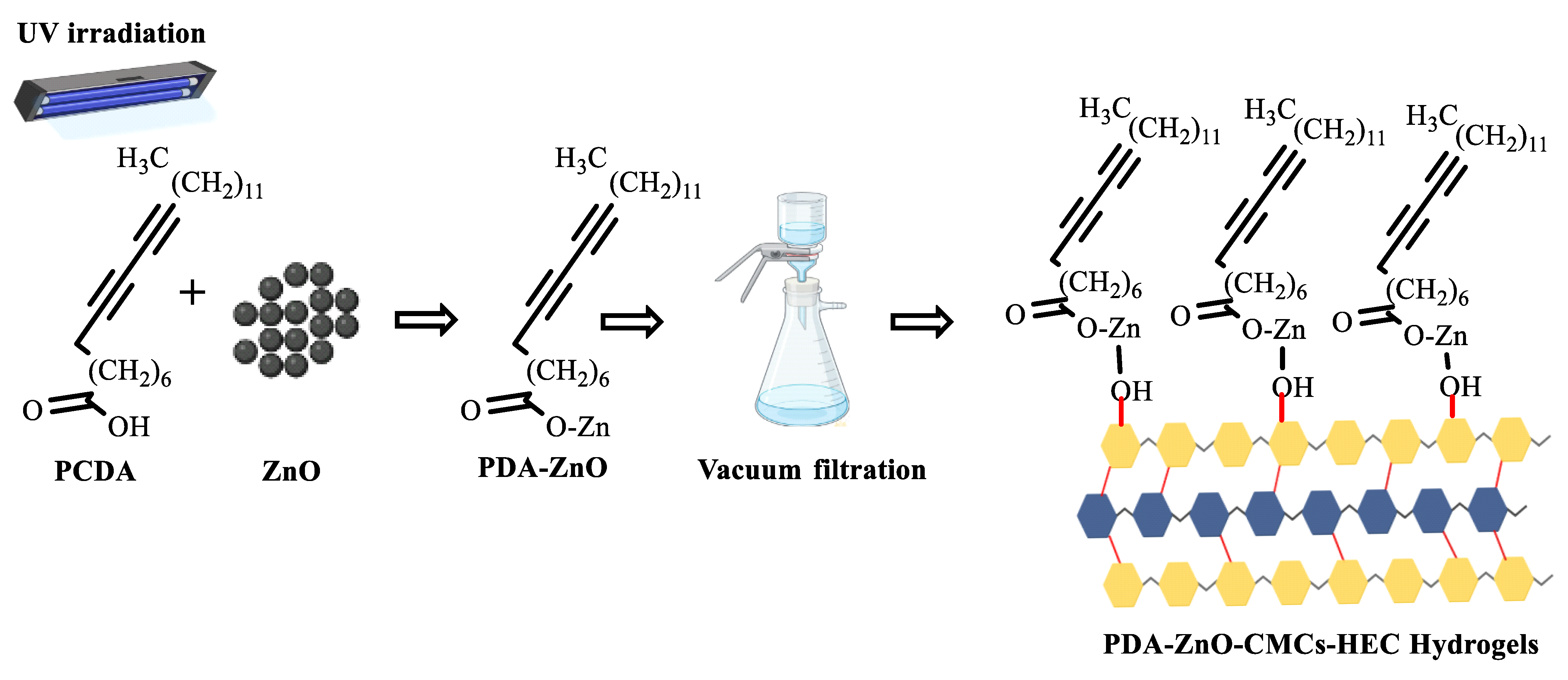
2.2. Synthesis of CMCs-HEC Hydrogels
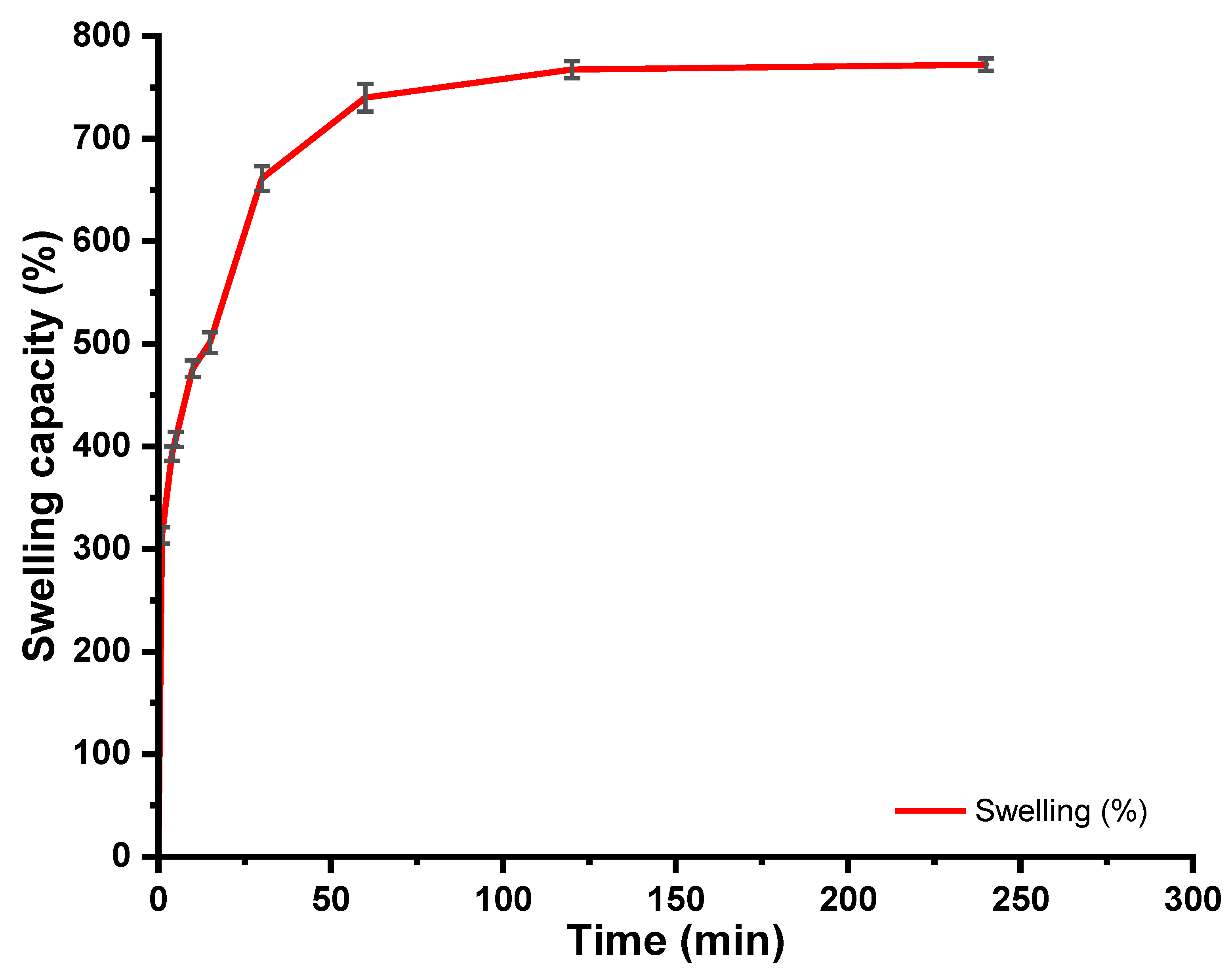
2.3. Swelling Measurements
2.4. Colorimetric Response to pH Change
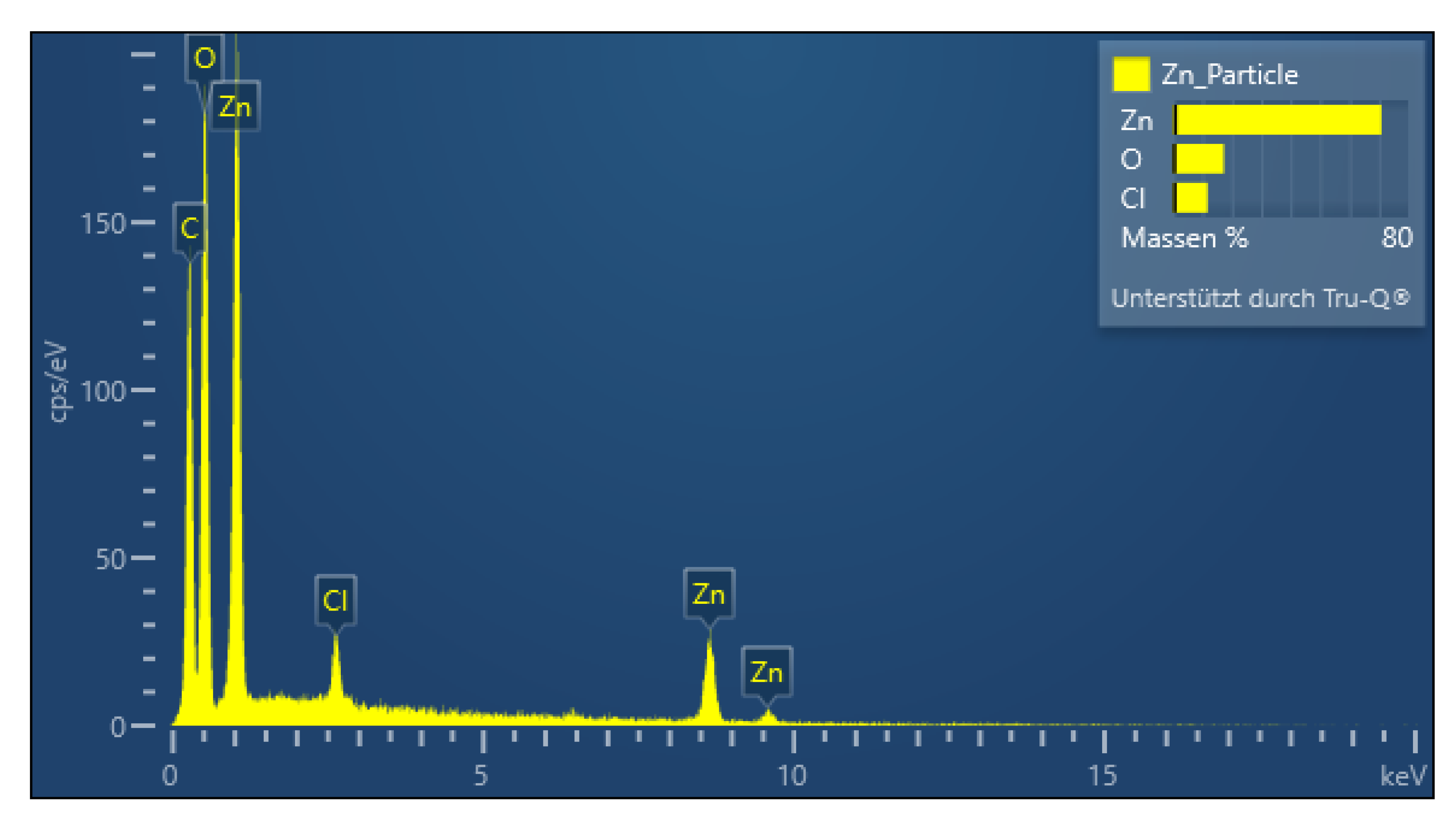
2.5. Characterization of PDA-ZnO-CMCs-HEC
2.6. Zinc Ion Release Experiments
3. Killing Curve Assay
4. Statistical Analysis
5. Results and Discussions
5.1. Swelling Behaviour of CMC-HEC Hydrogels
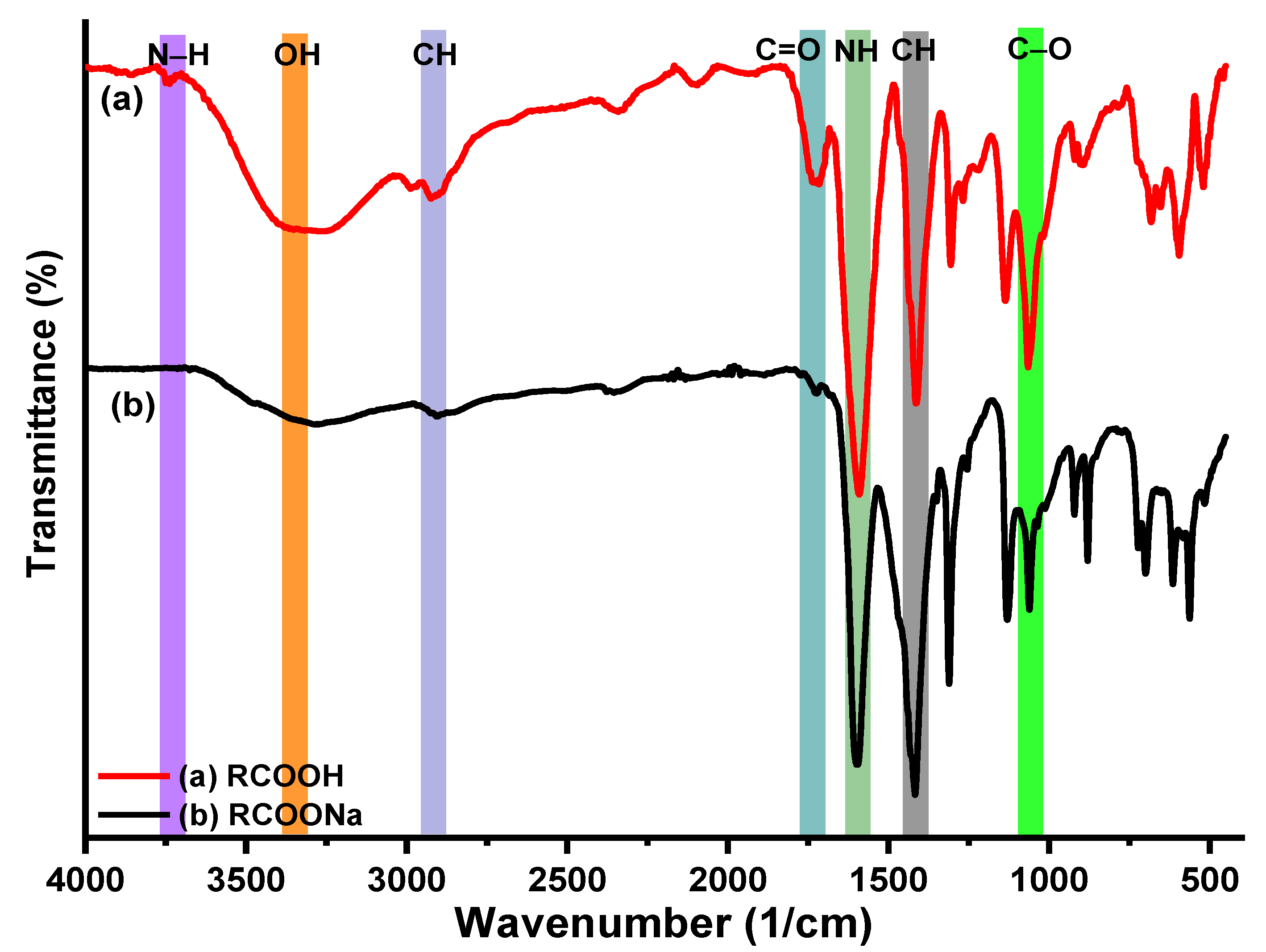
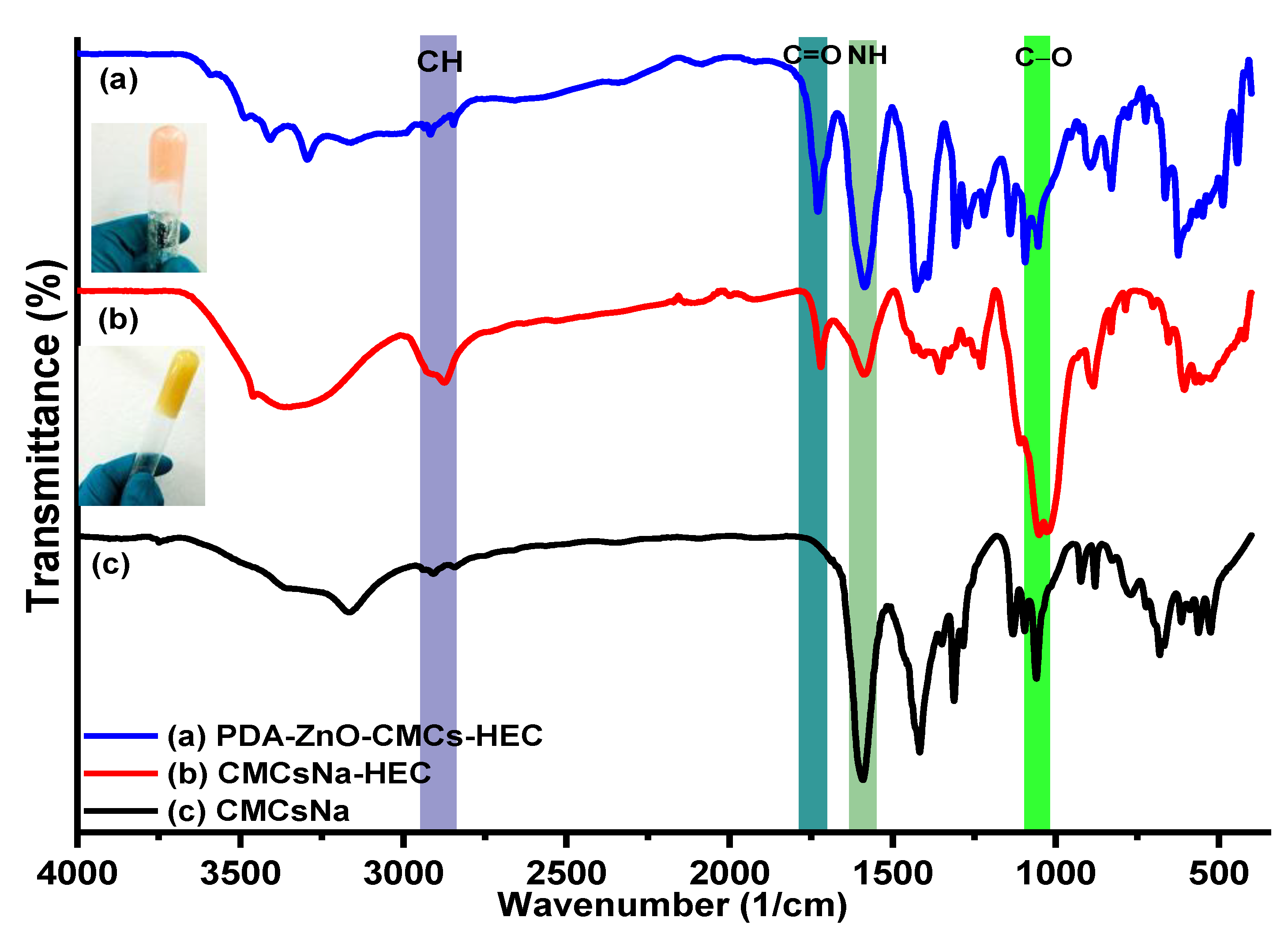
5.2. IR Results of CMCs, CMCs-HEC Hydrogels
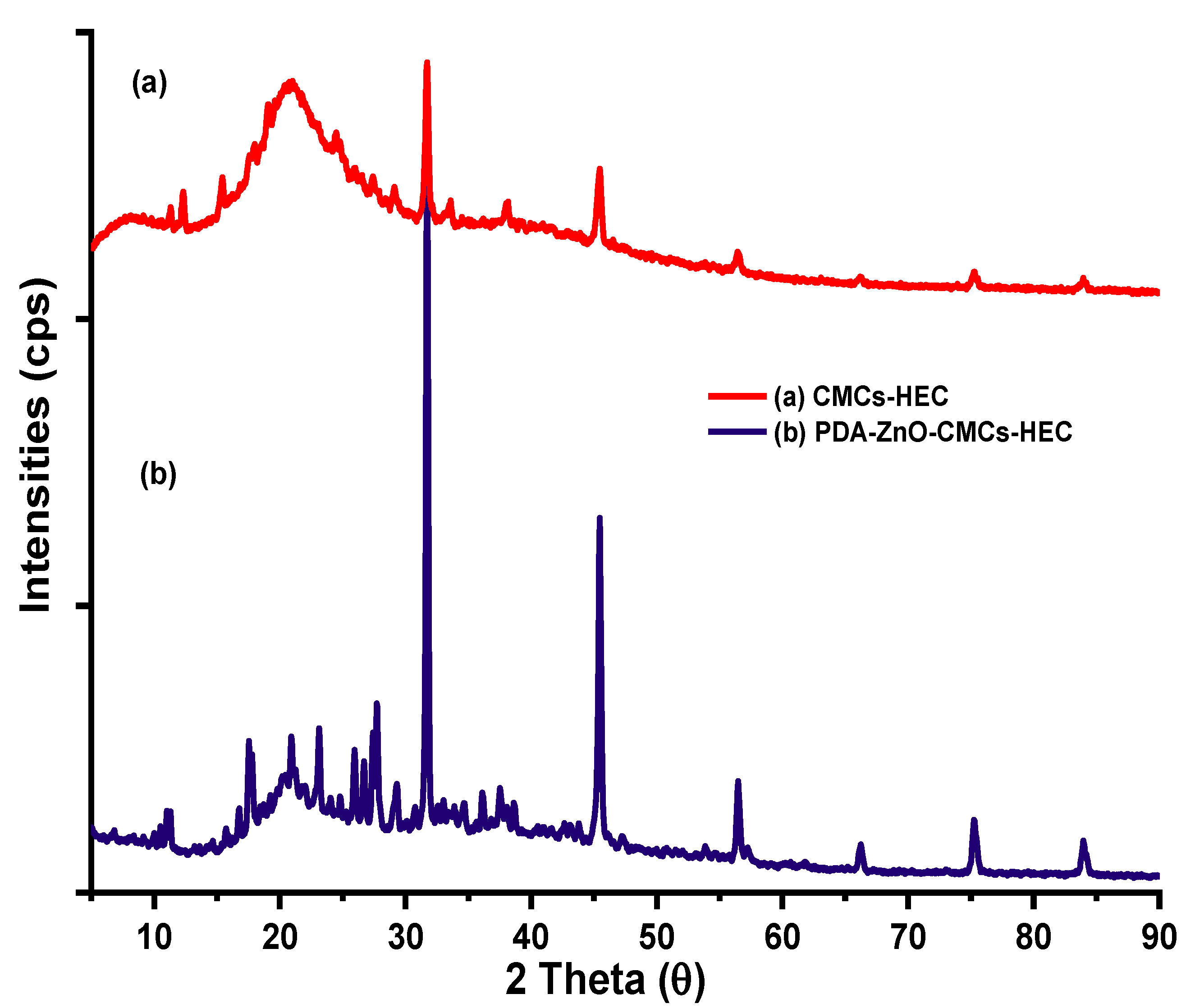
5.3. XRD Results of CMCS-HEC Composite
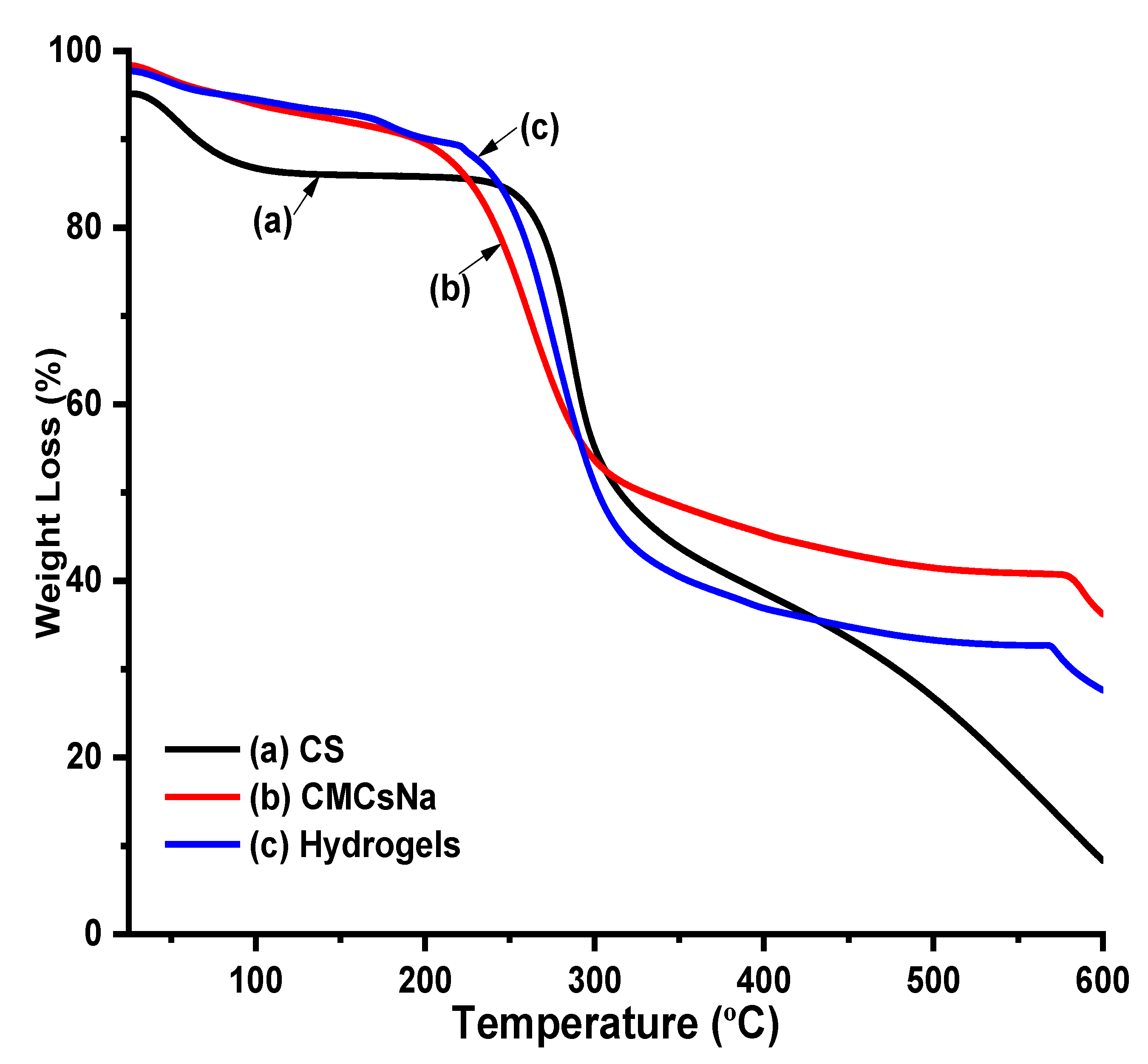
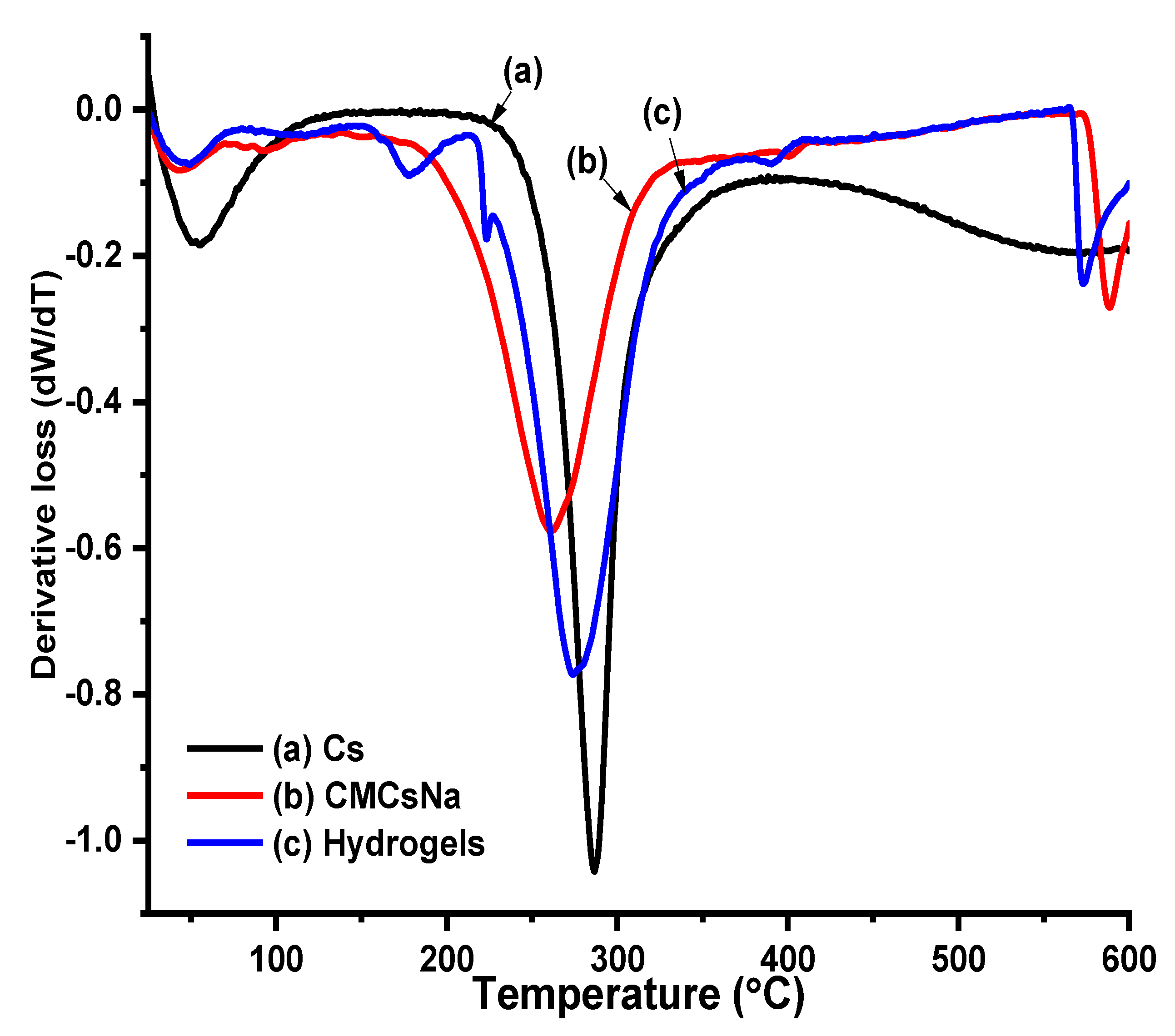
5.4. Thermal Profile of Cs, CMCS, and Hydrogels
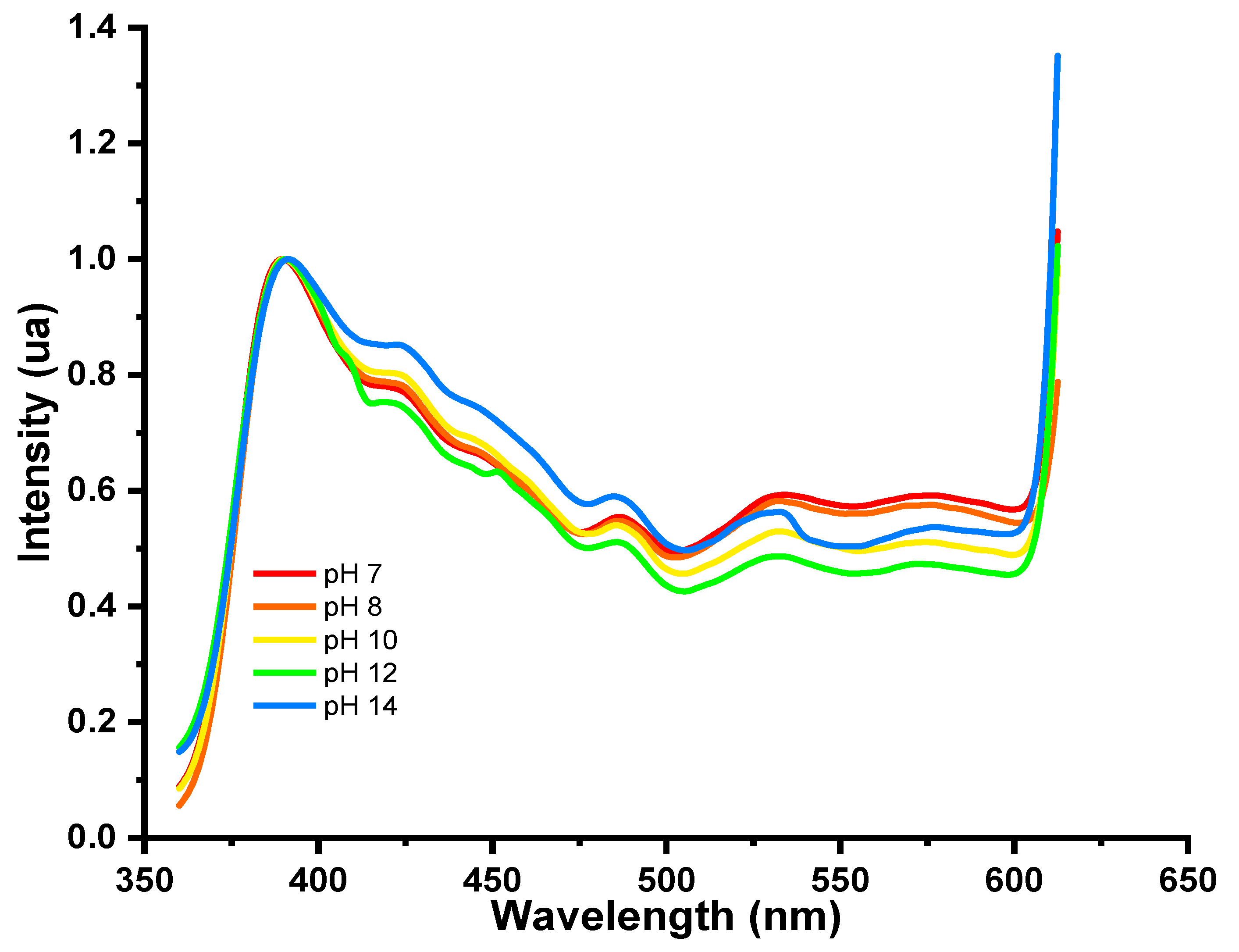
5.5. UV-Vis Spectra of Polydiacetylene
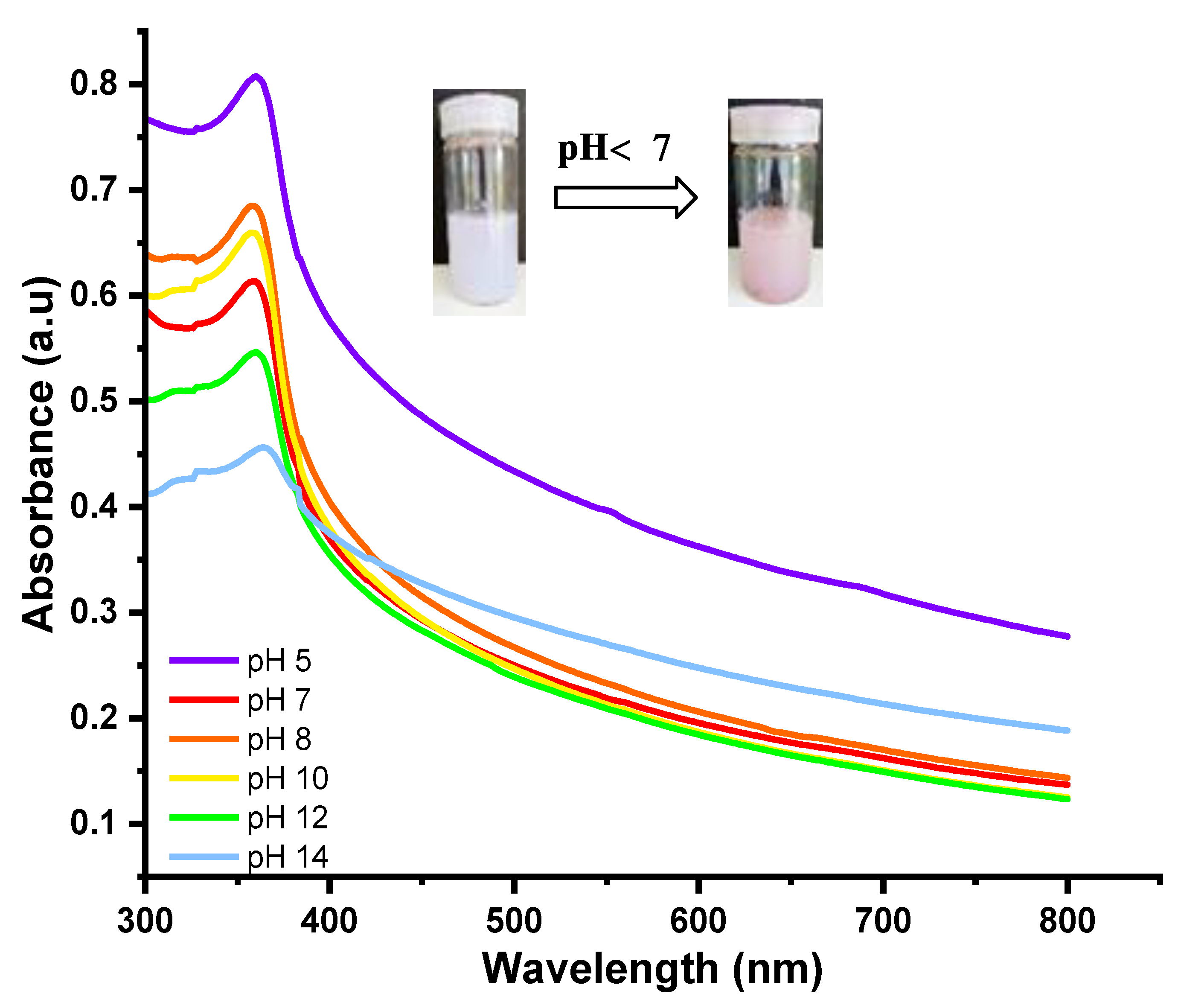
5.6. Colorimetric Response of the Hydrogels to pH
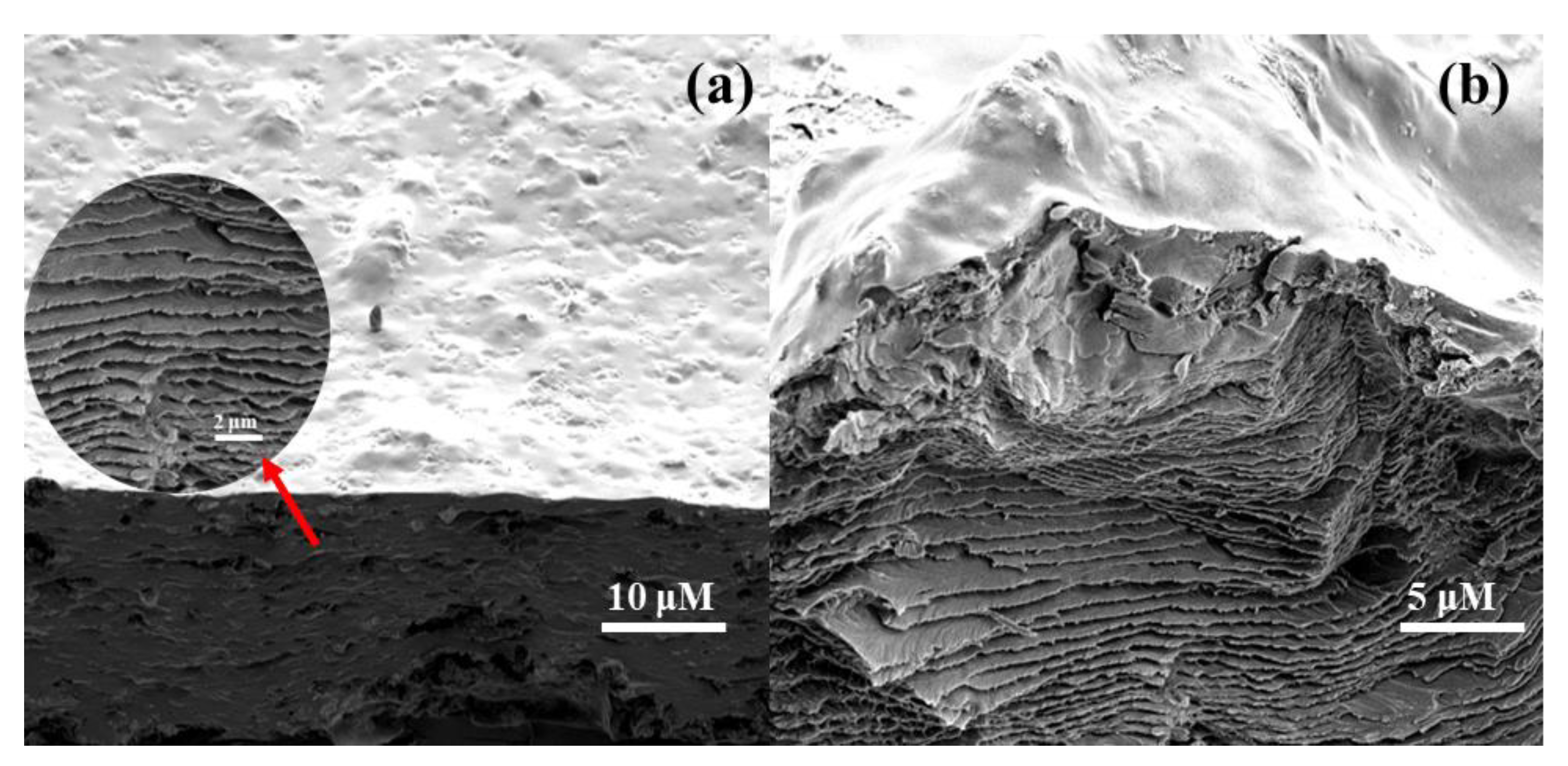
5.7. SEM Micrographs
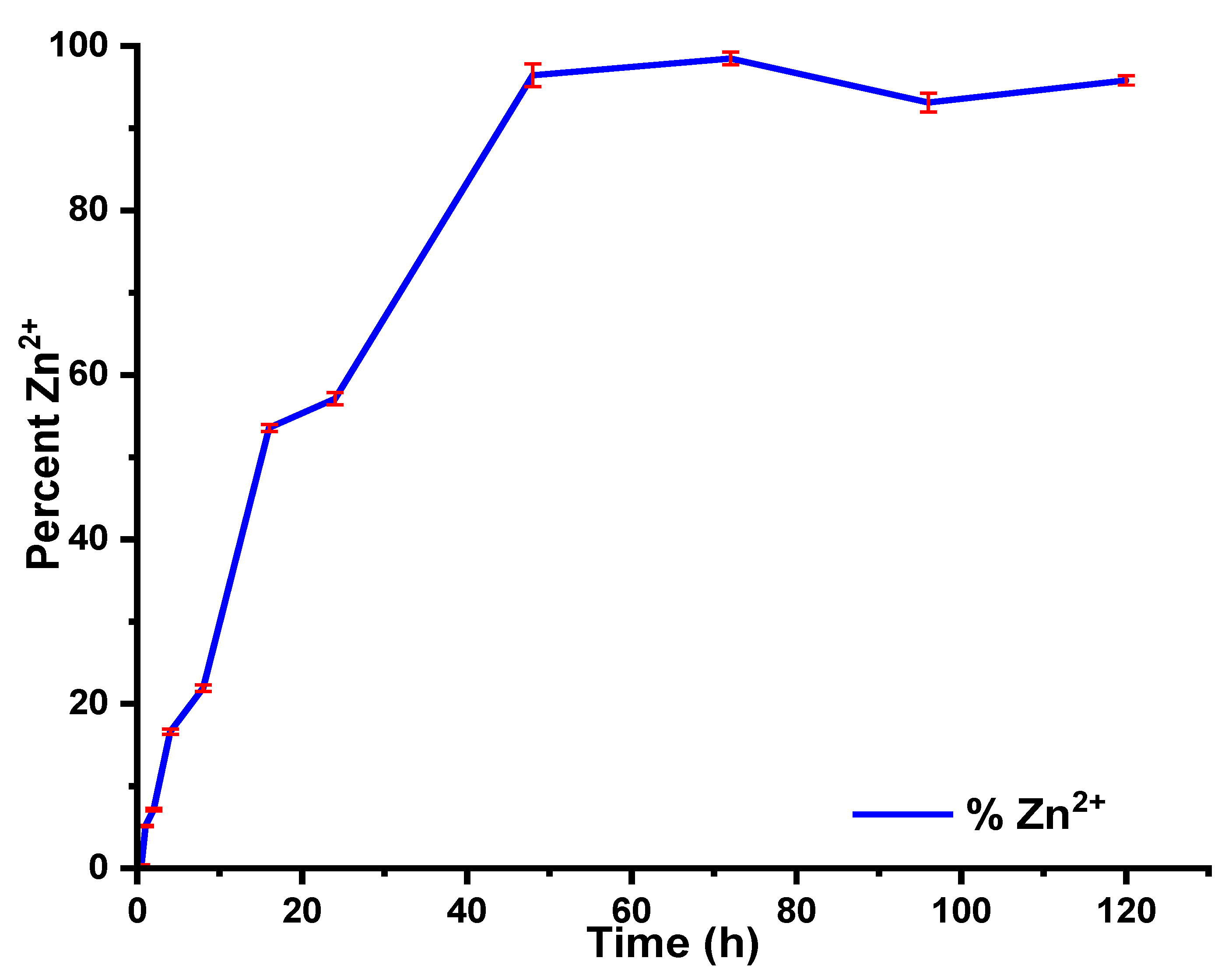
5.8. Kinetics of Zn2+ Release from the Nanocomposites
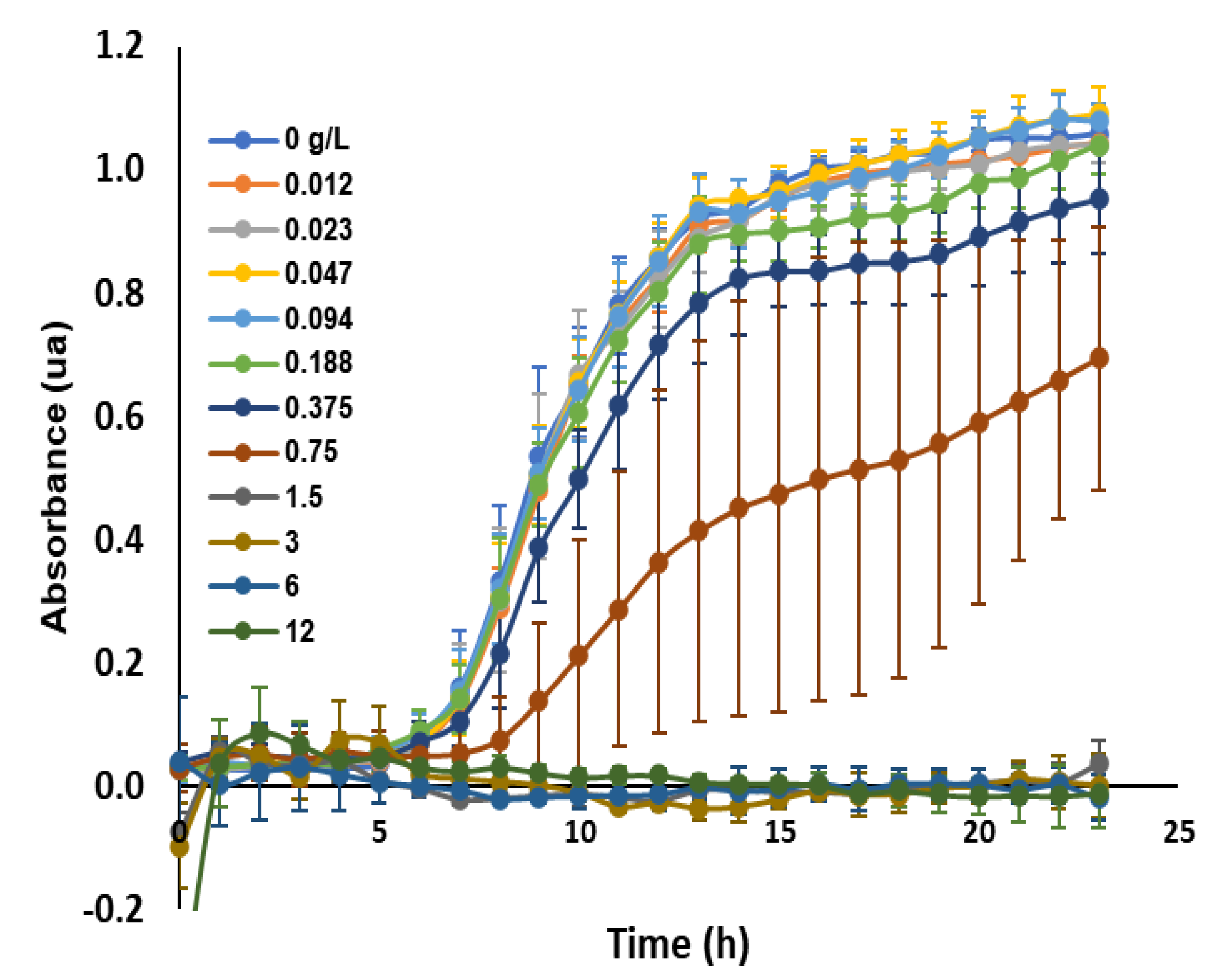
5.9. Antimicrobial Assays
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, H.; Lee, J.; Lee, S.G.; Doyle, P.S. Hydrogel-Based Colorimetric Assay for Multiplexed MicroRNA Detection in a Microfluidic Device. Anal. Chem. 2020, 92, 5750–5755. [Google Scholar] [CrossRef] [PubMed]
- Lebègue, E.; Farre, C.; Jose, C.; Saulnier, J.; Lagarde, F.; Chevalier, Y.; Chaix, C.; Jaffrezic-Renault, N. Responsive polydiacetylene vesicles for biosensing microorganisms. Sensors 2018, 18, 599. [Google Scholar] [CrossRef] [PubMed]
- Al Sulaiman, D.; Shapiro, S.J.; Gomez-Marquez, J.; Doyle, P.S. High-Resolution Patterning of Hydrogel Sensing Motifs within Fibrous Substrates for Sensitive and Multiplexed Detection of Biomarkers. ACS Sens. 2021, 6, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.J.; Dendukuri, D.; Doyle, P.S. Design of Hydrogel Particle Morphology for Rapid Bioassays. Anal. Chem. 2018, 90, 13572–13579. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Lu, F.; Liu, Y.; Yang, S.; Wang, F.; Ji, X.; He, Z. Glow-type chemiluminescent hydrogels for point-of-care testing (POCT) of cholesterol. Analyst 2021, 146, 4775–4780. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cao, L.; Bilal, M.; Cao, C.; Zhao, P.; Zhang, H.; Huang, Q. Multifunctional manganese-based carboxymethyl chitosan hydrogels for pH-triggered pesticide release and enhanced fungicidal activity. Carbohydr. Polym. 2021, 262, 117933. [Google Scholar] [CrossRef]
- Yapor, J.P.; Alharby, A.; Gentry-Weeks, C.; Reynolds, M.M.; Alam, A.K.M.M.; Li, Y.V. Polydiacetylene Nanofiber Composites as a Colorimetric Sensor Responding to Escherichia coli and pH. ACS Omega 2017, 2, 7334–7342. [Google Scholar] [CrossRef]
- Suhail, M.; Wu, P.C.; Minhas, M.U. Development and characterization of pH-sensitive chondroitin sulfate-co-poly(acrylic acid) hydrogels for controlled release of diclofenac sodium. J. Saudi Chem. Soc. 2021, 25, 101212. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Cellulose-based hydrogels as sustained drug-delivery systems. Materials 2020, 13, 5270. [Google Scholar] [CrossRef]
- Cao, J.; Wu, P.; Cheng, Q.; He, C.; Chen, Y.; Zhou, J. Ultrafast Fabrication of Self-Healing and Injectable Carboxymethyl Chitosan Hydrogel Dressing for Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 24095–24105. [Google Scholar] [CrossRef]
- Chandran, R.; Mohd Tohit, E.R.; Stanslas, J.; Tuan Mahmood, T.M.; Salim, N. Factors influencing the swelling behaviour of polymethyl vinyl ether-co-maleic acid hydrogels crosslinked by polyethylene glycol. J. Drug Deliv. Sci. Technol. 2022, 68, 103080. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef]
- Wang, T.; Chen, L.; Shen, T.; Wu, D. Preparation and properties of a novel thermo-sensitive hydrogel based on chitosan/hydroxypropyl methylcellulose/glycerol. Int. J. Biol. Macromol. 2016, 93, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Liu, L.; He, G.; Zhang, T.; Yang, M.; Cai, J.; Fan, L.; Tao, S. Preparation and properties of carboxymethyl chitosan/oxidized hydroxyethyl cellulose hydrogel. Int. J. Biol. Macromol. 2020, 162, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Akar, E.; Altinişik, A.; Seki, Y. Preparation of pH- and ionic-strength responsive biodegradable fumaric acid crosslinked carboxymethyl cellulose. Carbohydr. Polym. 2012, 90, 1634–1641. [Google Scholar] [CrossRef]
- Lin, G.; Chen, X.; Zhou, H.; Zhou, X.; Xu, H.; Chen, H. Elaboration of a feather keratin/carboxymethyl cellulose complex exhibiting pH sensitivity for sustained pesticide release. J. Appl. Polym. Sci. 2019, 136, 47160. [Google Scholar] [CrossRef]
- Singh, P.; Baisthakur, P.; Yemul, O.S. Synthesis, characterization and application of crosslinked alginate as green packaging material. Heliyon 2020, 6, e03026. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, Y.; Liu, B.; Chen, Z.; Nian, G.; Qu, S.; Yang, W. 3D Printing Method for Tough Multifunctional Particle-Based Double-Network Hydrogels. ACS Appl. Mater. Interfaces 2021, 13, 13714–13723. [Google Scholar] [CrossRef]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural Hydrogel-Based Bio-Inks for 3D Bioprinting in Tissue Engineering: A Review. Gels 2022, 8, 179. [Google Scholar] [CrossRef]
- Tsirigotis-Maniecka, M. Alginate-, Carboxymethyl Cellulose-, and κCarrageenan-Based Microparticles as Storage Vehicles for Cranberry Extract. Molecules 2020, 25, 3998. [Google Scholar] [CrossRef]
- Tavakoli, S.; Kharaziha, M.; Nemati, S.; Kalateh, A. Nanocomposite hydrogel based on carrageenan-coated starch/cellulose nanofibers as a hemorrhage control material. Carbohydr. Polym. 2021, 251, 117013. [Google Scholar] [CrossRef]
- Barrett-Catton, E.; Ross, M.L.; Asuri, P. Multifunctional hydrogel nanocomposites for biomedical applications. Polymers 2021, 13, 856. [Google Scholar] [CrossRef]
- Mokhtari, H.; Tavakoli, S.; Safarpour, F.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Ramakrishna, S.; Berto, F. Recent advances in chemically-modified and hybrid carrageenan-based platforms for drug delivery, wound healing, and tissue engineering. Polymers 2021, 13, 1744. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Wang, Z.; Jiang, R.; Gai, W.; Li, Q.; Lv, S.; Zhi, C. Energy-dissipative dual-crosslinked hydrogels for dynamically super-tough sensors. Sci. China Mater. 2021, 64, 2764–2776. [Google Scholar] [CrossRef]
- Chu, W.; Nie, M.; Ke, X.; Luo, J.; Li, J. Recent Advances in Injectable Dual Crosslinking Hydrogels for Biomedical Applications. Macromol. Biosci. 2021, 21, 2100109. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Luna, V.H.; González-Reynoso, O. Encapsulation of biological agents in hydrogels for therapeutic applications. Gels 2018, 4, 61. [Google Scholar] [CrossRef]
- Budianto, E.; Amalia, A. Swelling behavior and mechanical properties of Chitosan-Poly(N-vinyl-pyrrolidone) hydrogels. J. Polym. Eng. 2020, 40, 551–560. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, W.; Ai, K.; Liu, J. Highly Sensitive Polydiacetylene Ensembles for Biosensing and Bioimaging. Front. Chem. 2020, 8, 565782. [Google Scholar] [CrossRef]
- Saenjaiban, A.; Singtisan, T.; Suppakul, P.; Jantanasakulwong, K.; Punyodom, W.; Rachtanapun, P. Novel color change film as a time–temperature indicator using polydiacetylene/silver nanoparticles embedded in carboxymethyl cellulose. Polymers 2020, 12, 2306. [Google Scholar] [CrossRef]
- Tang, J.; Weston, M.; Kuchel, R.P.; Lisi, F.; Liang, K.; Chandrawati, R. Fabrication of polydiacetylene particles using a solvent injection method. Mater. Adv. 2020, 1, 1745–1752. [Google Scholar] [CrossRef]
- Sawayama, J.; Okitsu, T.; Nakamata, A.; Kawahara, Y.; Takeuchi, S. Hydrogel Glucose Sensor with In Vivo Stable Fluorescence Intensity Relying on Antioxidant Enzymes for Continuous Glucose Monitoring. iScience 2020, 23, 101243. [Google Scholar] [CrossRef]
- Kim, C.; Lee, K. Polydiacetylene (PDA) Liposome-Based Immunosensor for the Detection of Exosomes. Biomacromolecules 2019, 20, 3392–3398. [Google Scholar] [CrossRef] [PubMed]
- Weston, M.; Tjandra, A.D.; Chandrawati, R. Tuning chromatic response, sensitivity, and specificity of polydiacetylene-based sensors. Polym. Chem. 2020, 11, 166–183. [Google Scholar] [CrossRef]
- Kimani, P.K.; Kareru, P.G.; Madivoli, S.E.; Kairigo, P.K.; Maina, E.G. Comparative Study of Carboxymethyl Cellulose Synthesis from Selected Kenyan Biomass. Chem. Sci. Int. J. 2016, 17, 1–8. [Google Scholar] [CrossRef]
- Seki, Y.; Altinisik, A.; Demircioğlu, B.; Tetik, C. Carboxymethylcellulose (CMC)-hydroxyethylcellulose (HEC) based hydrogels: Synthesis and characterization. Cellulose 2014, 21, 1689–1698. [Google Scholar] [CrossRef]
- Durpekova, S.; Filatova, K.; Cisar, J.; Ronzova, A.; Kutalkova, E.; Sedlarik, V. A Novel Hydrogel Based on Renewable Materials for Agricultural Application. Int. J. Polym. Sci. 2020, 2020, 8363418. [Google Scholar] [CrossRef]
- Muslim, T.; Rahman, M.H.; Begum, H.A.; Rahman, M.A. Chitosan and Carboxymethyl Chitosan from Fish Scales of Labeo rohita. Dhaka Univ. J. Sci. 2013, 61, 145–148. [Google Scholar] [CrossRef]
- Wen, X.; Bao, D.; Chen, M.; Zhang, A.; Liu, C.; Sun, R. Preparation of CMC/HEC Crosslinked Hydrogels for Drug Delivery. BioResources 2015, 10, 8339–8351. [Google Scholar] [CrossRef]
- Hassan, M.A.; Tamer, T.M.; Omer, A.M.; Baset, W.M.A.; Abbas, E.; Mohy-Eldin, M.S. Therapeutic potential of two formulated novel chitosan derivatives with prominent antimicrobial activities against virulent microorganisms and safe profiles toward fibroblast cells. Int. J. Pharm. 2023, 634, 122649. [Google Scholar] [CrossRef]
- Li, Q.; Ren, S.; Peng, Y.; Lv, Y.; Wang, W.; Wang, Z.; Gao, Z. A Colorimetric Strip for Rapid Detection and Real-Time Monitoring of Histamine in Fish Based on Self-Assembled Polydiacetylene Vesicles. Anal. Chem. 2020, 92, 1611–1617. [Google Scholar] [CrossRef]
- Madivoli, E.S.; Kareru, P.G.; Gachanja, A.N.; Mugo, S.M.; Makhanu, D.S. Synthesis and characterization of dialdehyde cellulose nanofibers from O. sativa husks. SN Appl. Sci. 2019, 1, 723. [Google Scholar] [CrossRef]
- Kian, L.K.; Jawaid, M.; Ariffin, H.; Alothman, O.Y. Isolation and characterization of microcrystalline cellulose from roselle fibers. Int. J. Biol. Macromol. 2017, 103, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Otenda, B.V.; Kareru, P.G.; Madivoli, E.S.; Salim, A.M.; Gichuki, J.; Wanakai, S.I. Starch-Hibiscus-Cellulose Nanofibrils Composite Films as a Model Antimicrobial Food Packaging Material. J. Nat. Fibers 2022, 19, 12371–12384. [Google Scholar] [CrossRef]
- Lin, C.C.; Lee, M.H.; Chi, M.H.; Chen, C.J.; Lin, H.Y. Preparation of Zinc Oxide Nanoparticles Containing Spray and Barrier Films for Potential Photoprotection on Wound Healing. ACS Omega 2019, 4, 1801–1809. [Google Scholar] [CrossRef]
- Priebe, M.; Widmer, J.; Suhartha Löwa, N.; Abram, S.L.; Mottas, I.; Woischnig, A.K.; Brunetto, P.S.; Khanna, N.; Bourquin, C.; Fromm, K.M. Antimicrobial silver-filled silica nanorattles with low immunotoxicity in dendritic cells. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hérault, N.; Wagner, J.; Abram, S.L.; Widmer, J.; Horvath, L.; Vanhecke, D.; Bourquin, C.; Fromm, K.M. Silver-containing titanium dioxide nanocapsules for combating multidrug-resistant bacteria. Int. J. Nanomed. 2020, 15, 1267–1281. [Google Scholar] [CrossRef] [PubMed]
- Mali, K.K.; Dhawale, S.C.; Dias, R.J.; Dhane, N.S.; Ghorpade, V.S. Citric acid crosslinked carboxymethyl cellulose-based composite hydrogel films for drug delivery. Indian J. Pharm. Sci. 2018, 80, 657–667. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Ho, H.-O.; Liu, D.-Z.; Siow, W.-S.; Sheu, M.-T. Swelling/Floating Capability and Drug Release Characterizations of Gastroretentive Drug Delivery System Based on a Combination of Hydroxyethyl Cellulose and Sodium Carboxymethyl Cellulose. PLoS ONE 2015, 10, e0116914. [Google Scholar] [CrossRef] [PubMed]
- Barbucci, R.; Magnani, A.; Consumi, M. Swelling Behavior of Carboxymethylcellulose Hydrogels in Relation to Cross-Linking, pH, and Charge Density. Macromolecules 2000, 33, 7475–7480. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Astrini, N.; Anah, L.; Haryono, A. Crosslinking Parameter on the Preparation of Cellulose Based Hydrogel with Divynilsulfone. Procedia Chem. 2012, 4, 275–281. [Google Scholar] [CrossRef]
- Raucci, M.G.; Alvarez-Perez, M.A.; Demitri, C.; Giugliano, D.; De Benedictis, V.; Sannino, A.; Ambrosio, L. Effect of citric acid crosslinking cellulose-based hydrogels on osteogenic differentiation. J. Biomed. Mater. Res. Part A 2015, 103, 2045–2056. [Google Scholar] [CrossRef] [PubMed]
- Sim, P.; Strudwick, X.L.; Song, Y.; Cowin, A.J.; Garg, S. Influence of Acidic pH on Wound Healing In Vivo: A Novel Perspective for Wound Treatment. Int. J. Mol. Sci. 2022, 23, 13655. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Feng, P.; Chen, Z.; Xie, Z.; Li, H.; Peng, X.F.; Mi, H.Y.; Liu, Y. Highly Stretchable, Self-Healable, Freezing-Tolerant, and Transparent Polyacrylic Acid/Nanochitin Composite Hydrogel for Self-Powered Multifunctional Sensors. ACS Sustain. Chem. Eng. 2021, 9, 9209–9220. [Google Scholar] [CrossRef]
- Cao, J.; You, J.; Zhang, L.; Zhou, J. Homogeneous synthesis and characterization of chitosan ethers prepared in aqueous alkali/urea solutions. Carbohydr. Polym. 2018, 185, 138–144. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, W.; Zhang, H.; Zheng, W.; Zhou, Q. Carboxymethyl chitosan-based hydrogels containing fibroblast growth factors for triggering diabetic wound healing. Carbohydr. Polym. 2022, 287, 119336. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001; ISBN 978-0-470-09307-8. [Google Scholar]
- Madivoli, E.S.; Kareru, P.G.; Gichuki, J.; Elbagoury, M.M. Cellulose nanofibrils and silver nanoparticles enhances the mechanical and antimicrobial properties of polyvinyl alcohol nanocomposite film. Sci. Rep. 2022, 12, 19005. [Google Scholar] [CrossRef]
- Kim, J.; Moon, B.S.; Hwang, E.; Shaban, S.; Lee, W.; Pyun, D.G.; Lee, D.H.; Kim, D.H. Solid-state colorimetric polydiacetylene liposome biosensor sensitized by gold nanoparticles. Analyst 2021, 146, 1682–1688. [Google Scholar] [CrossRef]
- Abreu, F.D.; Campana-Filho, S.P. Preparation and characterization of carboxymethylchitosan. Polimeros 2005, 15, 79–83. [Google Scholar] [CrossRef]
- Tian, X.; Wu, S.; Zhang, Q.; Zou, G. Colorimetric sensor for fine differentiation of organic solvents based on only one kind of polydiacetylene coated on polymer optical fiber. IEEE Sens. J. 2012, 12, 1946–1949. [Google Scholar] [CrossRef]
- Jang, S.; Son, S.U.; Kang, B.; Lim, J.; Seo, S.B.; Kang, T.; Jung, J.; Seo, S.; Lim, E.-K. Polydiacetylene-Based Hydrogel Beads as Colorimetric Sensors for the Detection of Biogenic Amines in Spoiled Food. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Liu, G.; Ding, Z.; Yuan, Q.; Xie, H.; Gu, Z. Multi-Layered Hydrogels for Biomedical Applications. Front. Chem. 2018, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Xu, J.; Xue, Y.; Zhang, X.; Feng, M.; Wang, C.; Yao, W.; Wang, J.; He, M. Research progress in the multilayer hydrogels. Gels 2021, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Wang, Z.; Hu, Q. Chitosan Hydrogel Structure Modulated by Metal Ions. Sci. Rep. 2016, 6, 36005. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madivoli, E.S.; Schwarte, J.V.; Kareru, P.G.; Gachanja, A.N.; Fromm, K.M. Stimuli-Responsive and Antibacterial Cellulose-Chitosan Hydrogels Containing Polydiacetylene Nanosheets. Polymers 2023, 15, 1062. https://doi.org/10.3390/polym15051062
Madivoli ES, Schwarte JV, Kareru PG, Gachanja AN, Fromm KM. Stimuli-Responsive and Antibacterial Cellulose-Chitosan Hydrogels Containing Polydiacetylene Nanosheets. Polymers. 2023; 15(5):1062. https://doi.org/10.3390/polym15051062
Chicago/Turabian StyleMadivoli, Edwin Shigwenya, Justine Veronique Schwarte, Patrick Gachoki Kareru, Anthony Ngure Gachanja, and Katharina M. Fromm. 2023. "Stimuli-Responsive and Antibacterial Cellulose-Chitosan Hydrogels Containing Polydiacetylene Nanosheets" Polymers 15, no. 5: 1062. https://doi.org/10.3390/polym15051062
APA StyleMadivoli, E. S., Schwarte, J. V., Kareru, P. G., Gachanja, A. N., & Fromm, K. M. (2023). Stimuli-Responsive and Antibacterial Cellulose-Chitosan Hydrogels Containing Polydiacetylene Nanosheets. Polymers, 15(5), 1062. https://doi.org/10.3390/polym15051062






