Electrospun NiPd Nanoparticles Supported on Polymer Membrane Nanofibers as an Efficient Catalyst for NaBH4 Dehydrogenation
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Experimental Work
2.3. Chemical Reduction of Electrospun Nanofiber Mats
2.4. Characterization
2.5. NaBH4 Hydrolysis Using Prepared Catalysts
3. Results and Discussion
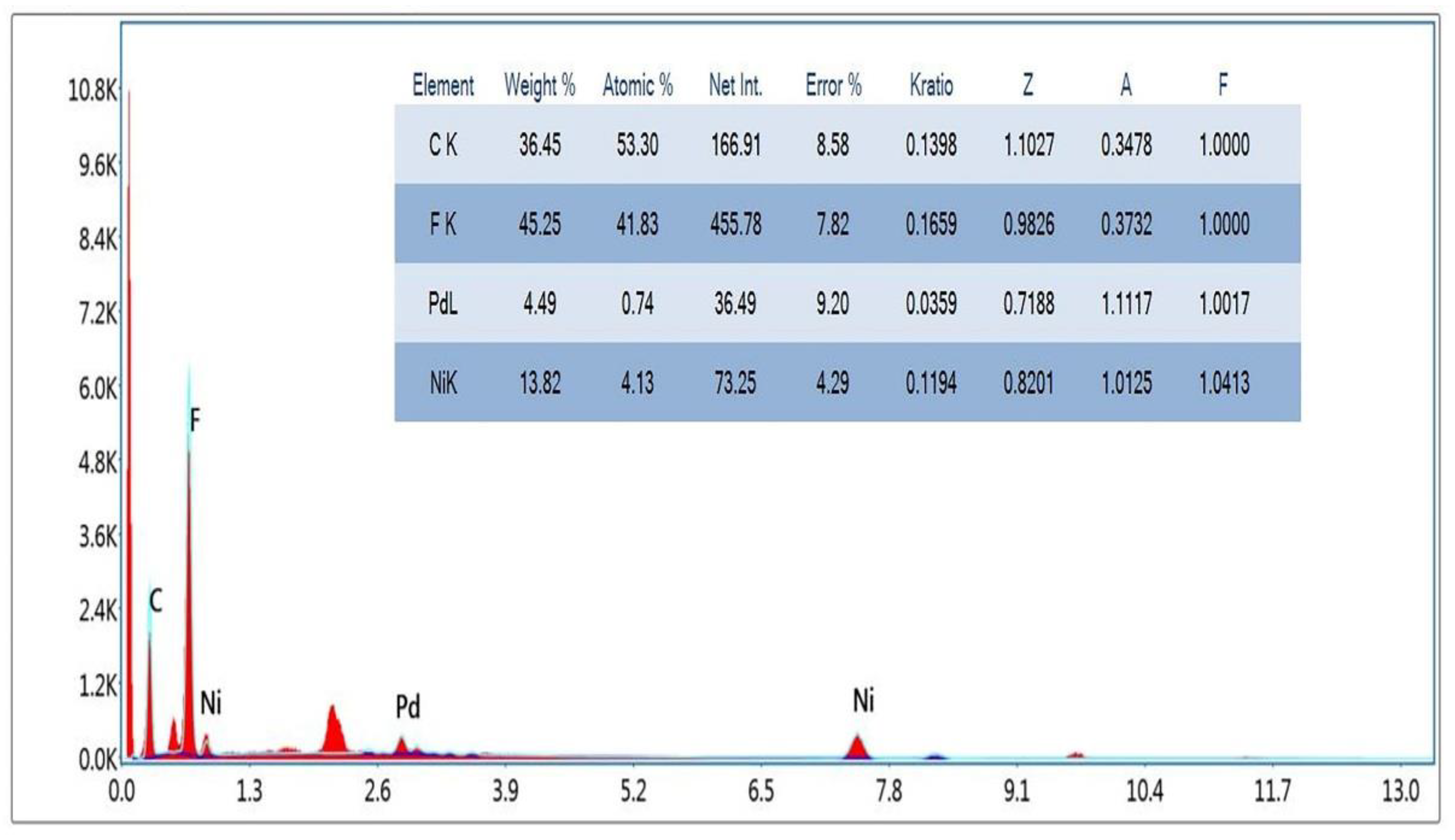
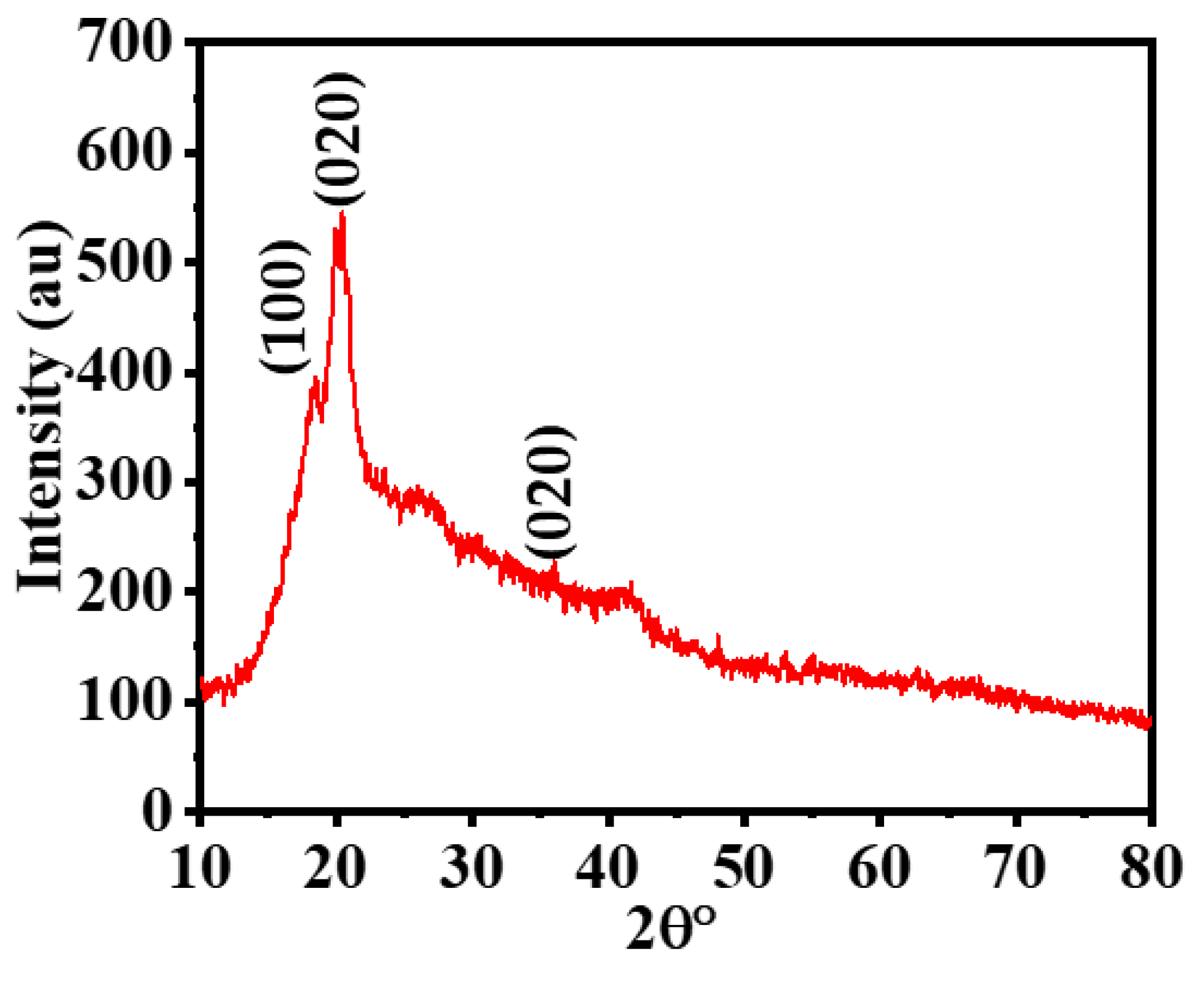
3.1. Hybrid-Membrane Characterization
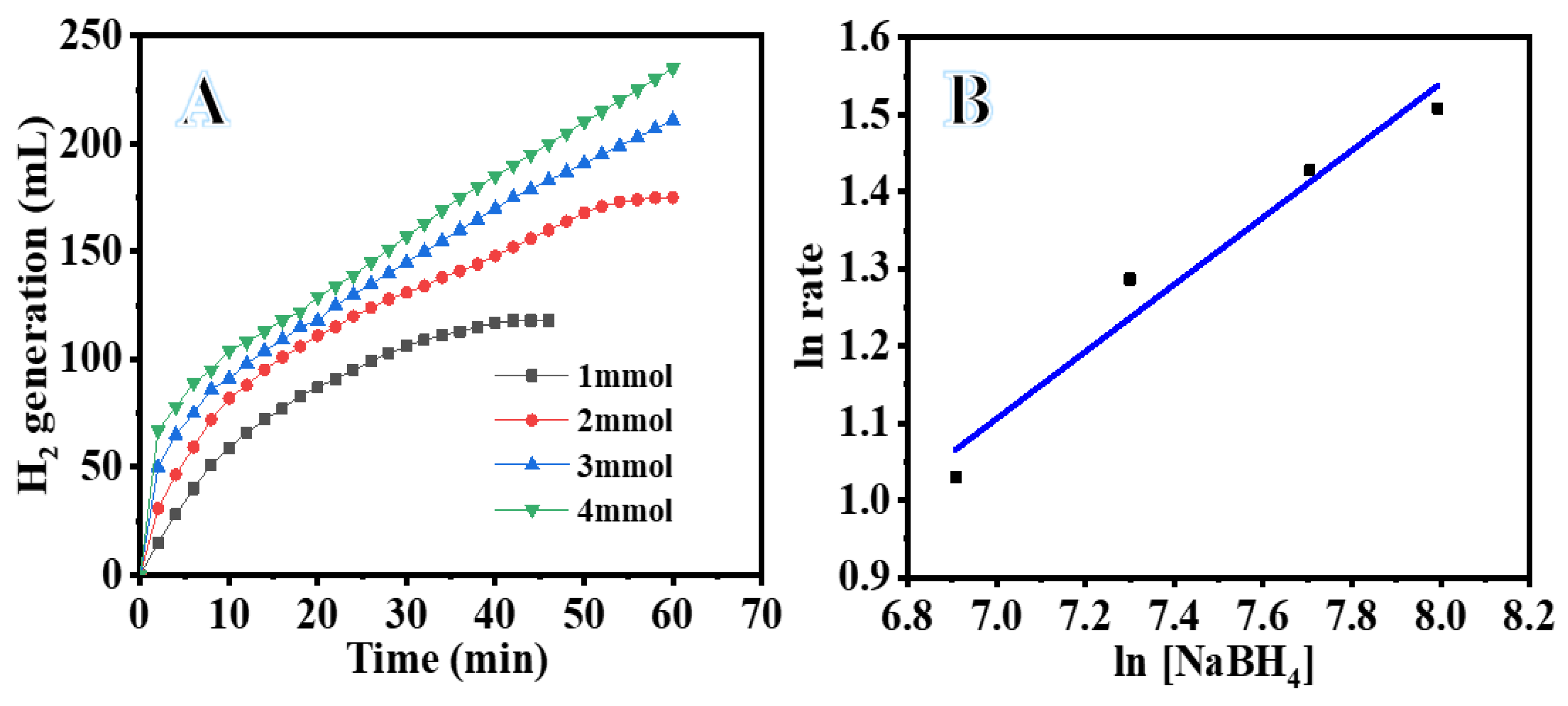
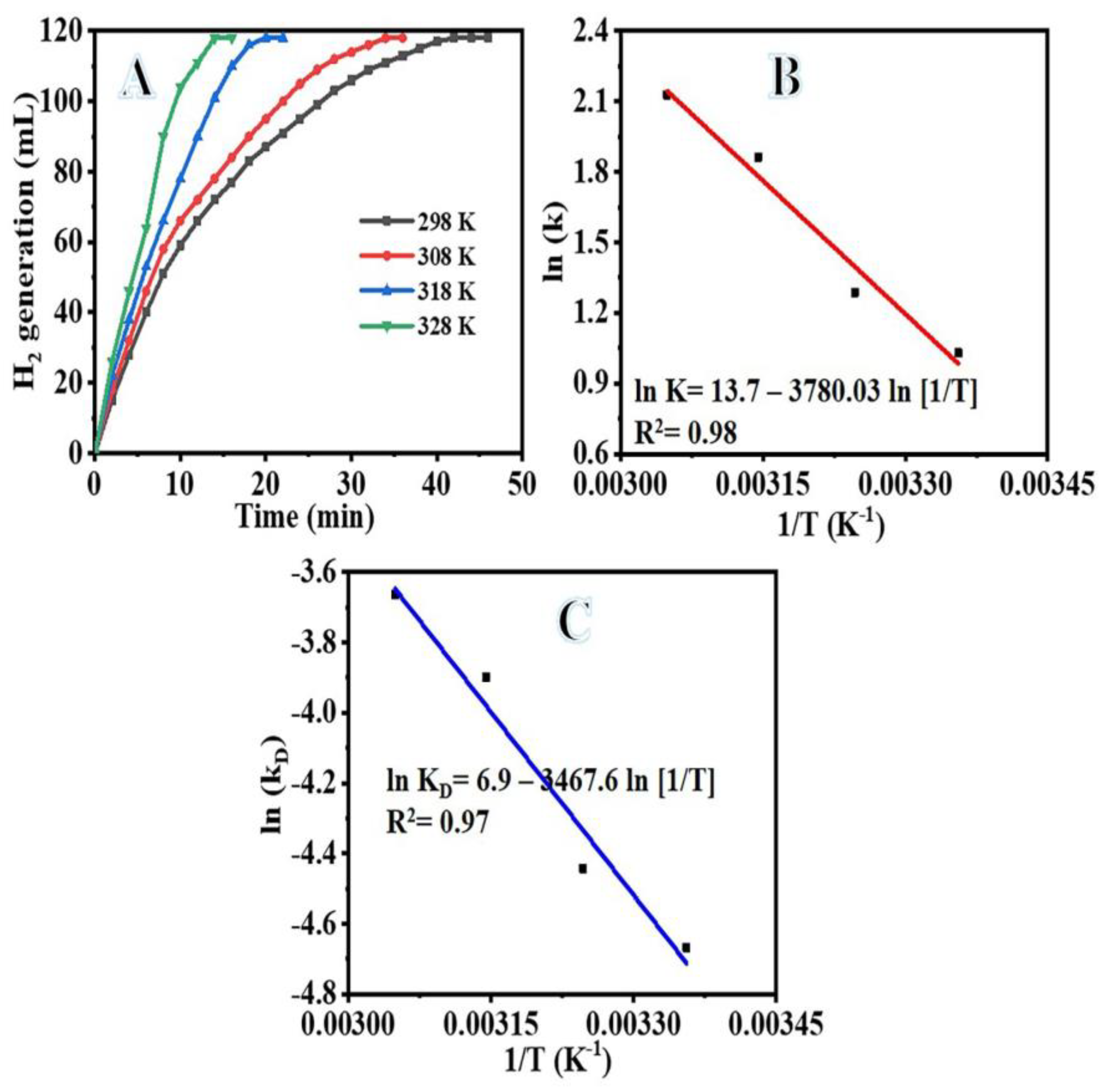
3.2. Catalysis Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, F.; Zhang, A.; Zhang, J.; Yang, L.; Zhang, F.; Li, R.; Dong, H. Hydrogen generation from sodium borohydride hydrolysis promoted by MOF-derived carbon supported cobalt catalysts. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127033. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. A review on hydrogen generation from the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2021, 46, 726–765. [Google Scholar] [CrossRef]
- Yousef, A.; Brooks, R.M.; El-Halwany, M.; Obaid, M.; El-Newehy, M.H.; Al-Deyab, S.S.; Barakat, N.A. A novel and chemical stable Co–B nanoflakes-like structure supported over titanium dioxide nanofibers used as catalyst for hydrogen generation from ammonia borane complex. Int. J. Hydrogen Energy 2016, 41, 285–293. [Google Scholar] [CrossRef]
- Dinc, M.; Metin, Ö.; Özkar, S. Water soluble polymer stabilized iron (0) nanoclusters: A cost-effective and magnetically recoverable catalyst in hydrogen generation from the hydrolysis of sodium borohydride and ammonia borane. Catal. Today 2012, 183, 10–16. [Google Scholar] [CrossRef]
- Demirci, U.B. Ammonia borane, a material with exceptional properties for chemical hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 9978–10013. [Google Scholar] [CrossRef]
- Tayeh, T.; Awad, A.S.; Nakhl, M.; Zakhour, M.; Silvain, J.F.; Bobet, J.L. Production of hydrogen from magnesium hydrides hydrolysis. Int. J. Hydrogen Energy 2014, 39, 3109–3117. [Google Scholar] [CrossRef]
- Xie, X.; Ni, C.; Wang, B.; Zhang, Y.; Zhao, X.; Liu, L.; Wang, B.; Du, W. Recent advances in hydrogen generation process via hydrolysis of Mg-based materials: A short review. J. Alloys Compd. 2020, 816, 152634. [Google Scholar] [CrossRef]
- Cai, H.; Lu, P.; Dong, J. Robust nickel–polymer nanocomposite particles for hydrogen generation from sodium borohydride. Fuel 2016, 166, 297–301. [Google Scholar] [CrossRef]
- Chen, Y.; Kim, H. Ni/Ag/silica nanocomposite catalysts for hydrogen generation from hydrolysis of NaBH4 solution. Mater. Lett. 2008, 62, 1451–1454. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Wang, Y.; Kim, H. Preparation of porous PVDF-NiB capsules as catalytic adsorbents for hydrogen generation from sodium borohydride. Fuel Process. Technol. 2011, 92, 1368–1373. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, J.; Ma, Q.; Li, C.; Liu, Y.; Li, B.; Liu, Z. Polyvinylpyrrolidone stabilized-Ru nanoclusters loaded onto reduced graphene oxide as high active catalyst for hydrogen evolution. J. Nanopart. Res. 2017, 19, 227. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Su, C.-C.; Wang, S.-L.; Lu, M.-C. Development of Al2O3 carrier-Ru composite catalyst for hydrogen generation from alkaline NaBH4 hydrolysis. Energy 2012, 46, 242–247. [Google Scholar] [CrossRef]
- Ro, G.; Hwang, D.K.; Kim, Y. Hydrogen generation using Pt/Ni bimetallic nanoparticles supported on Fe3O4@SiO2@TiO2 multi-shell microspheres. J. Ind. Eng. Chem. 2019, 79, 364–369. [Google Scholar] [CrossRef]
- Wu, C.; Guo, J.; Zhang, J.; Zhao, Y.; Tian, J.; Isimjan, T.T.; Yang, X. Palladium nanoclusters decorated partially decomposed porous ZIF-67 polyhedron with ultrahigh catalytic activity and stability on hydrogen generation. Renew. Energy 2019, 136, 1064–1070. [Google Scholar] [CrossRef]
- Yue, C.; Yang, P.; Wang, J.; Zhao, X.; Wang, Y.; Yang, L. Facile synthesis and characterization of nano-Pd loaded NiCo microfibers as stable catalysts for hydrogen generation from sodium borohydride. Chem. Phys. Lett. 2020, 743, 137170. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Wu, Z.-J.; Zheng, X.-C.; Peng, Z.-K.; Liu, P. Chitosan-reduced graphene oxide hybrids encapsulated Pd (0) nanocatalysts for H2 generation from ammonia borane. Int. J. Hydrogen Energy 2019, 44, 23610–23619. [Google Scholar] [CrossRef]
- Huff, C.; Long, J.M.; Heyman, A.; Abdel-Fattah, T.M. Palladium nanoparticle multiwalled carbon nanotube composite as catalyst for hydrogen production by the hydrolysis of sodium borohydride. ACS Appl. Energy Mater. 2018, 1, 4635–4640. [Google Scholar] [CrossRef]
- Zhao, W.; Li, W.; Lu, L.; Li, F.; Zhang, H.; Zhang, S. Preparation of Colloidal Pd/Ni Bimetallic Nanoparticle Catalysts and Their Catalytic Activity for Hydrogen Generation from Hydrolysis Reaction of Sodium Borohydride. Rare Met. Mater. Eng. 2016, 45, 3160–3166. [Google Scholar]
- Patel, N.; Miotello, A. Progress in Co–B related catalyst for hydrogen production by hydrolysis of boron-hydrides: A review and the perspectives to substitute noble metals. Int. J. Hydrogen Energy 2015, 40, 1429–1464. [Google Scholar] [CrossRef]
- Dönmez, F.; Ayas, N. Synthesis of Ni/TiO2 catalyst by sol-gel method for hydrogen production from sodium borohydride. Int. J. Hydrogen Energy 2021, 46, 29314–29322. [Google Scholar] [CrossRef]
- Kiren, B.; Ayas, N. Nickel modified dolomite in the hydrogen generation from sodium borohydride hydrolysis. Int. J. Hydrogen Energy 2022, 47, 19702–19717. [Google Scholar] [CrossRef]
- Kassem, A.A.; Abdelhamid, H.N.; Fouad, D.M.; Ibrahim, S.A. Metal-organic frameworks (MOFs) and MOFs-derived CuO@ C for hydrogen generation from sodium borohydride. Int. J. Hydrogen Energy 2019, 44, 31230–31238. [Google Scholar] [CrossRef]
- Hashimi, A.S.; Nohan, M.A.N.M.; Chin, S.X.; Khiew, P.S.; Zakaria, S.; Chia, C.H. Copper nanowires as highly efficient and recyclable catalyst for rapid hydrogen generation from hydrolysis of sodium borohydride. Nanomaterials 2020, 10, 1153. [Google Scholar] [CrossRef]
- Liao, J.; Huang, H. Magnetic sensitive Hericium erinaceus residue chitin/Cu hydrogel nanocomposites for H2 generation by catalyzing NaBH4 hydrolysis. Carbohydr. Polym. 2020, 229, 115426. [Google Scholar] [CrossRef]
- Liu, W.; Cai, H.; Lu, P.; Xu, Q.; Zhongfu, Y.; Dong, J. Polymer hydrogel supported Pd–Ni–B nanoclusters as robust catalysts for hydrogen production from hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2013, 38, 9206–9216. [Google Scholar] [CrossRef]
- Du, Y.; Wang, K.; Zhai, Q.; Chen, A.; Xi, Z.; Yan, J.; Kang, X.; Chen, M.; Yuan, X.; Zhu, M. Alloyed palladium-nickel hollow nanospheres with interatomic charge polarization for improved hydrolytic dehydrogenation of ammonia borane. Int. J. Hydrogen Energy 2018, 43, 283–292. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, Y.; Liu, Q.; Zhou, M.; Mi, G.; Du, X. Hierarchically alloyed Pd–Cu microarchitecture with tunable shapes: Morphological engineering, and catalysis for hydrogen evolution reaction of ammonia borane. Int. J. Hydrogen Energy 2019, 44, 30226–30236. [Google Scholar] [CrossRef]
- Cai, H.-K.; Jiang, Z.-Y.; Xu, S.; Xu, Y.; Lu, P.; Dong, J. Polymer Hydrogel Supported Ni/Pd Alloys for Hydrogen Gas Production from Hydrolysis of Dimethylamine Borane with a Long Recyclable Lifetime. Polymers 2022, 14, 4647. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, W.; Li, W.; Xiong, X.; Wang, Y.; Cheng, K.; Kang, J.; Zhang, Q.; Wang, Y. In-situ confinement of ultrasmall palladium nanoparticles in silicalite-1 for methane combustion with excellent activity and hydrothermal stability. Appl. Catal. B Environ. 2020, 276, 119142. [Google Scholar] [CrossRef]
- Al-Thabaiti, S.A.; Khan, Z.; Malik, M.A. Bimetallic Ag-Ni nanoparticles as an effective catalyst for hydrogen generation from hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2019, 44, 16452–16466. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Daneshvari-Esfahlan, V.; Wolf, S.; Hacker, V. Novel Bimetallic Pd–X (X= Ni, Co) Nanoparticles Assembled on N-Doped Reduced Graphene Oxide as an Anode Catalyst for Highly Efficient Direct Sodium Borohydride–Hydrogen Peroxide Fuel Cells. ACS Appl. Energy Mater. 2021, 4, 6025–6039. [Google Scholar] [CrossRef]
- Ye, K.; Ma, X.; Cang, R.; Wang, G.; Cheng, K.; Wang, G.; Cao, D. Nickel nanowires decorated with ultra-low palladium loading as an effective electrocatalyst for NaBH 4 oxidation. Catal. Sci. Technol. 2017, 7, 1991–1995. [Google Scholar] [CrossRef]
- Huang, W.; Xu, F.; Liu, X. Superior hydrogen generation from sodium borohydride hydrolysis catalyzed by the bimetallic Co–Ru/C nanocomposite. Int. J. Hydrogen Energy 2021, 46, 25376–25384. [Google Scholar] [CrossRef]
- Paksoy, A.; Kurtoğlu, S.F.; Dizaji, A.K.; Altıntaş, Z.; Khoshsima, S.; Uzun, A.; Balcı, Ö. Nanocrystalline cobalt–nickel–boron (metal boride) catalysts for efficient hydrogen production from the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2021, 46, 7974–7988. [Google Scholar] [CrossRef]
- Chen, C.-W.; Chen, C.-Y.; Huang, Y.-H. Method of preparing Ru-immobilized polymer-supported catalyst for hydrogen generation from NaBH4 solution. Int. J. Hydrogen Energy 2009, 34, 2164–2173. [Google Scholar] [CrossRef]
- Metin, Ö.; Özkar, S. Synthesis and characterization of poly (N-vinyl-2-pyrrolidone)-stabilized water-soluble nickel (0) nanoclusters as catalyst for hydrogen generation from the hydrolysis of sodium borohydride. J. Mol. Catal. A Chem. 2008, 295, 39–46. [Google Scholar] [CrossRef]
- Metin, O.; Ozkar, S. Hydrogen generation from the hydrolysis of ammonia-borane and sodium borohydride using water-soluble polymer-stabilized cobalt (0) nanoclusters catalyst. Energy Fuels 2009, 23, 3517–3526. [Google Scholar] [CrossRef]
- Metin, Ö.; Şahin, Ş.; Özkar, S. Water-soluble poly (4-styrenesulfonic acid-co-maleic acid) stabilized ruthenium (0) and palladium (0) nanoclusters as highly active catalysts in hydrogen generation from the hydrolysis of ammonia–borane. Int. J. Hydrogen Energy 2009, 34, 6304–6313. [Google Scholar] [CrossRef]
- Malvadkar, N.A.; Sekeroglu, K.; Dressick, W.J.; Demirel, M.C. Catalytic activity of cobalt on nanotextured polymer films for hydrogen production. J. Power Sources 2011, 196, 8553–8560. [Google Scholar] [CrossRef]
- Rakap, M.; Özkar, S. Intrazeolite cobalt (0) nanoclusters as low-cost and reusable catalyst for hydrogen generation from the hydrolysis of sodium borohydride. Appl. Catal. B Environ. 2009, 91, 21–29. [Google Scholar] [CrossRef]
- Zahmakiran, M.; Özkar, S. Intrazeolite ruthenium (0) nanoclusters: A superb catalyst for the hydrogenation of benzene and the hydrolysis of sodium borohydride. Langmuir 2008, 24, 7065–7067. [Google Scholar] [CrossRef] [PubMed]
- Zahmakiran, M.; Özkar, S. Zeolite-confined ruthenium (0) nanoclusters catalyst: Record catalytic activity, reusability, and lifetime in hydrogen generation from the hydrolysis of sodium borohydride. Langmuir 2009, 25, 2667–2678. [Google Scholar] [CrossRef] [PubMed]
- Saka, C.; Eygi, M.S.; Balbay, A. CoB doped acid modified zeolite catalyst for enhanced hydrogen release from sodium borohydride hydrolysis. Int. J. Hydrogen Energy 2020, 45, 15086–15099. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Dehydrogenation of sodium borohydride using cobalt embedded zeolitic imidazolate frameworks. J. Solid State Chem. 2021, 297, 122034. [Google Scholar] [CrossRef]
- Abutaleb, A.; Zouli, N.; El-Halwany, M.; Ubaidullah, M.; Yousef, A. Graphitic nanofibers supported NiMn bimetallic nanoalloys as catalysts for H2 generation from ammonia borane. Int. J. Hydrogen Energy 2021, 46, 35248–35260. [Google Scholar] [CrossRef]
- Brooks, R.; Maafa, I.M.; Al-Enizi, A.; El-Halwany, M.; Ubaidullah, M.; Yousef, A. Electrospun bimetallic nicr nanoparticles@ carbon nanofibers as an efficient catalyst for hydrogen generation from ammonia borane. Nanomaterials 2019, 9, 1082. [Google Scholar] [CrossRef] [Green Version]
- Al-Enizi, A.M.; Nafady, A.; El-Halwany, M.; Brooks, R.M.; Abutaleb, A.; Yousef, A. Electrospun carbon nanofiber-encapsulated NiS nanoparticles as an efficient catalyst for hydrogen production from hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2019, 44, 21716–21725. [Google Scholar] [CrossRef]
- Li, T.; Xiang, C.; Chu, H.; Xu, F.; Sun, L.; Zou, Y.; Zhang, J. Catalytic effect of highly dispersed ultrafine Ru nanoparticles on a TiO2-Ti3C2 support: Hydrolysis of sodium borohydride for H2 generation. J. Alloys Compd. 2022, 906, 164380. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.; Khalil, K.A.; Unnithan, A.R.; Panthi, G.; Pant, B.; Kim, H.Y. Photocatalytic release of hydrogen from ammonia borane-complex using Ni (0)-doped TiO2/C electrospun nanofibers. Colloids Surf. A Physicochem. Eng. Asp. 2012, 410, 59–65. [Google Scholar] [CrossRef]
- Erat, N.; Bozkurt, G.; Özer, A. Co/CuO–NiO–Al2O3 catalyst for hydrogen generation from hydrolysis of NaBH4. Int. J. Hydrogen Energy 2022, 47, 24255–24267. [Google Scholar] [CrossRef]
- Kılınç, D.; Şahi, Ö.; Saka, C. Salicylaldimine-Ni complex supported on Al2O3: Highly efficient catalyst for hydrogen production from hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2018, 43, 251–261. [Google Scholar] [CrossRef]
- Dai, P.; Zhao, X.; Xu, D.; Wang, C.; Tao, X.; Liu, X.; Gao, J. Preparation, characterization, and properties of Pt/Al2O3/cordierite monolith catalyst for hydrogen generation from hydrolysis of sodium borohydride in a flow reactor. Int. J. Hydrogen Energy 2019, 44, 28463–28470. [Google Scholar] [CrossRef]
- Aydın, K.; Kulaklı, B.N.; Filiz, B.C.; Alligier, D.; Demirci, U.B.; Figen, A.K. Closing the hydrogen cycle with the couple sodium borohydride-methanol, via the formation of sodium tetramethoxyborate and sodium metaborate. Int. J. Energy Res. 2020, 44, 11405–11416. [Google Scholar] [CrossRef]
- Lo, C.T.; Karan, K.; Davis, B.R. Kinetic studies of reaction between sodium borohydride and methanol, water, and their mixtures. Ind. Eng. Chem. Res. 2007, 46, 5478–5484. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, G.; Zhang, L.; Wang, W.; Miao, W.; Chen, K.; Cheng, L.; Li, Y.; Han, S. Ultrafine cobalt nanoparticles supported on carbon nanospheres for hydrolysis of sodium borohydride. J. Renew. Energy 2020, 162, 345–354. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Yousef, A.; Shaikh, S.F.; Pandit, B.; El-Halwany, M.M. Electrospun Nickel Nanoparticles@ Poly (vinylidene fluoride-hexafluoropropylene) Nanofibers as Effective and Reusable Catalyst for H2 Generation from Sodium Borohydride. Arab. J. Chem. 2022, 15, 104207. [Google Scholar] [CrossRef]
- Raghavan, P.; Zhao, X.; Kim, J.K.; Manuel, J.; Chauhan, G.S.; Ahn, J.H.; Nah, C. Ionic conductivity and electrochemical properties of nanocomposite polymer electrolytes based on electrospun poly (vinylidene fluoride-co-hexafluoropropylene) with nano-sized ceramic fillers. Electrochim. Acta 2008, 54, 228–234. [Google Scholar] [CrossRef]
- Mališ, J.; Mazúr, P.; Schauer, J.; Paidar, M.; Bouzek, K. Polymer-supported 1-butyl-3-methylimidazolium trifluoromethanesulfonate and 1-ethylimidazolium trifluoromethanesulfonate as electrolytes for the high temperature PEM-type fuel cell. Int. J. Hydrogen Energy 2013, 38, 4697–4704. [Google Scholar] [CrossRef]
- Vijayakumar, E.; Subramania, A.; Fei, Z.; Dyson, P.J. High-performance dye-sensitized solar cell based on an electrospun poly(vinylidene fluoride-co-hexafluoropropylene)/cobalt sulfide nanocomposite membrane electrolyte. RSC Adv. 2015, 5, 52026–52032. [Google Scholar] [CrossRef]
- Zhang, P.; Li, R.; Huang, J.; Liu, B.; Zhou, M.; Wen, B.; Xia, Y.; Okada, S. Flexible poly(vinylidene fluoride-co-hexafluoropropylene)-based gel polymer electrolyte for high-performance lithium-ion batteries. RSC Adv. 2021, 11, 11943–11951. [Google Scholar] [CrossRef]
- Tian, X.; Jiang, X. Poly(vinylidene fluoride-co-hexafluoropropene) (PVDF-HFP) membranes for ethyl acetate removal from water. J. Hazard. Mater. 2008, 153, 128–135. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Bakenov, Z.; Gosselink, D.; Chen, P. Poly (vinylidene fluoride-co-hexafluoropropylene)/poly (methylmethacrylate)/nanoclay composite gel polymer electrolyte for lithium/sulfur batteries. J. Solid State Electrochem. 2014, 18, 1111–1116. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Y.; Wang, Y. Preparation of hollow poly (vinylidene fluoride) capsules containing nickel catalyst for hydrogen storage and production. Int. J. Energy Res. 2015, 39, 634–642. [Google Scholar] [CrossRef]
- Kang, H.-C.; Chen, Y.; Arthur, E.E.; Kim, H. Microstructural control of catalyst-loaded PVDF microcapsule membrane for hydrogen generation by NaBH4 hydrolysis. Int. J. Hydrogen Energy 2014, 39, 15656–15664. [Google Scholar] [CrossRef]
- Stephan, A.M.; Nahm, K.S.; Kulandainathan, M.A.; Ravi, G.; Wilson, J.J. Poly (vinylidene fluoride-hexafluoropropylene)(PVdF-HFP) based composite electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 1728–1734. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Guan, H.; Zhao, Y.; Yang, J.-H.; Zhang, B. Preparation of bimetallic Cu-Co nanocatalysts on poly (diallyldimethylammonium chloride) functionalized halloysite nanotubes for hydrolytic dehydrogenation of ammonia borane. Appl. Surf. Sci. 2018, 427, 106–113. [Google Scholar] [CrossRef]
- Yao, Q.; Lu, Z.-H.; Wang, Y.; Chen, X.; Feng, G. Synergetic catalysis of non-noble bimetallic Cu–Co nanoparticles embedded in SiO2 nanospheres in hydrolytic dehydrogenation of ammonia borane. J. Phys. Chem. C 2015, 119, 14167–14174. [Google Scholar] [CrossRef]
- Subramanian, N.D.; Balaji, G.; Kumar, C.S.S.R.; Spivey, J.J. Development of cobalt–copper nanoparticles as catalysts for higher alcohol synthesis from syngas. Catal. Today 2009, 147, 100–106. [Google Scholar] [CrossRef]
- Singh, S.K.; Iizuka, Y.; Xu, Q. Nickel-palladium nanoparticle catalyzed hydrogen generation from hydrous hydrazine for chemical hydrogen storage. Int. J. Hydrogen Energy 2011, 36, 11794–11801. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Zhang, Z.; Wang, S.; Williams, N.; Cheng, Y.; Luo, S.; Gu, J. rGO supported PdNi-CeO2 nanocomposite as an efficient catalyst for hydrogen evolution from the hydrolysis of NH3BH3. Int. J. Hydrogen Energy 2018, 43, 18745–18753. [Google Scholar] [CrossRef]
- Fang, R.; Yang, Z.; Wang, Z.; Ran, J.; Yan, Y.; Zhang, L. Novel non-noble metal catalyst with high efficiency and synergetic photocatalytic hydrolysis of ammonia borane and mechanism investigation. Energy 2022, 244, 123187. [Google Scholar] [CrossRef]
- Al-shaikh, H.; Lasri, J.; Knight, J.G.; Al-Goul, S.T. Palladium mesoporous nanoparticles Pd NPs@[KIT-6] and Pd NPs@[KIT-6]-PEG-imid as efficient heterogeneous catalysts for H2 production from NaBH4 hydrolysis. Fuel 2022, 325, 124962. [Google Scholar] [CrossRef]
- Singh, A.K.; Xu, Q. Synergistic catalysis over bimetallic alloy nanoparticles. ChemCatChem 2013, 5, 652–676. [Google Scholar] [CrossRef]
- Lu, P.; Teranishi, T.; Asakura, K.; Miyake, M.; Toshima, N. Polymer-protected Ni/Pd bimetallic nano-clusters: Preparation, characterization and catalysis for hydrogenation of nitrobenzene. J. Phys. Chem. B 1999, 103, 9673–9682. [Google Scholar] [CrossRef]
- Fuku, K.; Sakano, T.; Kamegawa, T.; Mori, K.; Yamashita, H. Enhanced hydrogenation activity of nano-sized Pd–Ni bimetal particles on Ti-containing mesoporous silica prepared by a photo-assisted deposition method. J. Mater. Chem. 2012, 22, 16243–16247. [Google Scholar] [CrossRef]
- Patel, N.; Fernandes, R.; Miotello, A. Promoting effect of transition metal-doped Co–B alloy catalysts for hydrogen production by hydrolysis of alkaline NaBH4 solution. J. Catal. 2010, 271, 315–324. [Google Scholar] [CrossRef]
- Tonbul, Y.; Akbayrak, S.; Özkar, S. Palladium (0) nanoparticles supported on ceria: Highly active and reusable catalyst in hydrogen generation from the hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2016, 41, 11154–11162. [Google Scholar] [CrossRef]
- Yen, H.; Seo, Y.; Kaliaguine, S.; Kleitz, F. Role of metal–support interactions, particle size, and metal–metal synergy in CuNi nanocatalysts for H2 generation. ACS Catal. 2015, 5, 5505–5511. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Goodman, D.W. The nature of the metal-metal bond in bimetallic surfaces. Science 1992, 257, 897–903. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Hiyoshi, N.; Sato, O.; Osada, M.; Shirai, M. Lignin gasification over charcoal-supported palladium and nickel bimetal catalysts in supercritical water. Chem. Lett. 2010, 39, 1251–1253. [Google Scholar] [CrossRef]
- Ozay, O.; Aktas, N.; Inger, E.; Sahiner, N. Hydrogel assisted nickel nanoparticle synthesis and their use in hydrogen production from sodium boron hydride. Int. J. Hydrogen Energy 2011, 36, 1998–2006. [Google Scholar] [CrossRef]
- Kaufman, C.M.; Sen, B. Hydrogen generation by hydrolysis of sodium tetrahydroborate: Effects of acids and transition metals and their salts. J. Chem. Soc. Dalton Trans. 1985, 2, 307–313. [Google Scholar] [CrossRef]
- Patel, N.; Patton, B.; Zanchetta, C.; Fernandes, R.; Guella, G.; Kale, A.; Miotello, A. Pd-C powder and thin film catalysts for hydrogen production by hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2008, 33, 287–292. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Y.; Liu, X.; Zhang, Y.J. Preparation of polyvinylidene fluoride–nickel hollow fiber catalytic membranes for hydrogen generation from sodium borohydride. Fuel 2015, 140, 685–692. [Google Scholar] [CrossRef]
- Chinnappan, A.; Kim, H.; Baskar, C.; Hwang, I.T. Hydrogen generation from the hydrolysis of sodium borohydride with new pyridinium dicationic salts containing transition metal complexes. Int. J. Hydrogen Energy 2012, 37, 10240–10248. [Google Scholar] [CrossRef]
- Chinnappan, A.; Kim, H. Nanocatalyst: Electrospun nanofibers of PVDF–Dicationic tetrachloronickelate (II) anion and their effect on hydrogen generation from the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2012, 37, 18851–18859. [Google Scholar] [CrossRef]
- Yang, K.; Yao, Q.; Huang, W.; Chen, X.; Lu, Z.-H. Enhanced catalytic activity of NiM (M = Cr, Mo, W) nanoparticles for hydrogen evolution from ammonia borane and hydrazine borane. Int. J. Hydrogen Energy 2017, 42, 6840–6850. [Google Scholar] [CrossRef]






| Ni | Pd | Ni95Pd5 | Ni90Pd10 | Ni85Pd15 | Ni80Pd20 | Ni75Pd25 | Ni70Pd30 | |
|---|---|---|---|---|---|---|---|---|
| Volume (mL) | 87 | 78 | 83 | 90 | 94 | 108 | 117 | 101 |
| Yield% | 72.5 | 65 | 69.2 | 75 | 78.3 | 90 | 98 | 84.2 |
| Rate (mL min−1) | 2.29 | 2.05 | 2.18 | 2.37 | 2.47 | 2.84 | 3.03 | 2.66 |
| Catalyst (gm) | ||||
|---|---|---|---|---|
| 0.1 | 0.15 | 0.2 | 0.25 | |
| Volume (mL) | 118 | 118 | 118 | 118 |
| Yield% | 98.3 | 98.3 | 98.3 | 98.3 |
| Reaction time (min) | 42 | 32 | 22 | 16 |
| Rate (mL min−1) | 2.81 | 3.69 | 5.36 | 7.38 |
| Catalytic Material | Ea (kJ mol−1) | Ref. |
|---|---|---|
| Ni | 42.28 | [83] |
| Ni | 71 | [84] |
| Raney Ni | 63 | [84] |
| Ni(0) | 51.4 | [76] |
| Ni-Ag | 16.2 | [31] |
| Pd/C powder | 28 | [85] |
| Pd-Ni-B | 31.1 | [26] |
| Pd NPs@ [KIT-6]-PEG-imid | 35.7 | [74] |
| Ni-hollow PVDF capsules | 49.3 | [65] |
| Ni-PVDF hollow fiber | 55.3 | [86] |
| ([C6(mpy)2][NiCl4]2− | 56.4 | [87] |
| PVDF-[C6(mpy)2][NiCl4]2− | 44.6 | [88] |
| NiPd@PVDF-HFP | 31.43 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zouli, N.; Maafa, I.M.; Abutaleb, A.; Yousef, A.; El-Halwany, M.M. Electrospun NiPd Nanoparticles Supported on Polymer Membrane Nanofibers as an Efficient Catalyst for NaBH4 Dehydrogenation. Polymers 2023, 15, 1083. https://doi.org/10.3390/polym15051083
Zouli N, Maafa IM, Abutaleb A, Yousef A, El-Halwany MM. Electrospun NiPd Nanoparticles Supported on Polymer Membrane Nanofibers as an Efficient Catalyst for NaBH4 Dehydrogenation. Polymers. 2023; 15(5):1083. https://doi.org/10.3390/polym15051083
Chicago/Turabian StyleZouli, Nasser, Ibrahim M. Maafa, Ahmed Abutaleb, Ayman Yousef, and M. M. El-Halwany. 2023. "Electrospun NiPd Nanoparticles Supported on Polymer Membrane Nanofibers as an Efficient Catalyst for NaBH4 Dehydrogenation" Polymers 15, no. 5: 1083. https://doi.org/10.3390/polym15051083
APA StyleZouli, N., Maafa, I. M., Abutaleb, A., Yousef, A., & El-Halwany, M. M. (2023). Electrospun NiPd Nanoparticles Supported on Polymer Membrane Nanofibers as an Efficient Catalyst for NaBH4 Dehydrogenation. Polymers, 15(5), 1083. https://doi.org/10.3390/polym15051083







